Abstract
Adenovirus (AdV) is a non-enveloped virus with a double-stranded DNA genome. Bovine AdV is a member of the family Adenoviridaein and is responsible for adenovirus infection. In this study, BAV304a recombinant adenovirus was modified to carry a green fluorescence marker and subsequently added to fresh maturation medium or microinjected into the cytoplasm of bovine oocytes. Adenovirus infection in bovine oocytes and cumulus cells was observed by fluorescence microscopy and validated by polymerase chain reaction. Further analysis indicated that adenoviral infection had no significant effect on the first polar body extrusion and oocyte cleavage ratio during bovine oocyte maturation. Our data indicate that the zona pellucida plays a vital role in bovine oocytes resisting BAV304a adenovirus and also suggest that oocytes protect themselves from non-self substances.
Key words
adenovirus; bovine oocytes; cumulus-oocyte complexes; zona pellucida
Introduction
Adenovirus (AdV) is a non-enveloped virus with a linear double-stranded genome . AdV has strong infectivity in various mammalian cells and can express proteins in most mammalian cells and tissues. AdV infects almost all cell types, including some anti-AdV-infected lymphoma cells. Previous research using model systems for the non-proliferation of gene expression showed that cells and primary cells could be transformed by AdV. These experimental results were compared directly.
AdV was first discovered in cultivated healthy glandular tissue in 1953 [1]. Currently, more than one hundred types of AdV have been identified with the ability to infect humans, mammals, and birds. AdV can infect cells of the respiratory tract, eye conjunctiva, gastrointestinal tract, urinary tract, and other organs. Among these organs, the respiratory tract is the main target of AdV. Following the proportion of respiratory syncytial virus, the proportion of infantile viral pneumonia caused by AdV is very high. Furthermore, gland viral pneumonia caused by AdV infection is one of the most serious types of infantile pneumonia. AdV can also induce malignant tumours and alter cell function, and therefore can be used to study the mechanism of tumourigenesis in animals [2-4]. AdV is also used for in vitro gene transduction, in vivo vaccination, gene therapy, and in other fields.
The zona pellucida is a protective outer layer surrounding oocytes and acts as a “coat” that is composed primarily of glycoproteins secreted by oocytes or other cells. This layer protects fragile oocytes and embryos from external damage and prevents the entry of sperm cells [5].
Recently, AdV infection in somatic cells has been intensively investigated [6-8]. However, these studies only focus on somatic cells, as AdV infection in oocytes has been rarely reported, and AdV infection in bovine oocytes has never been reported. In this experiment, the first polar body discharge and blastocyst rates of bovine oocytes indicated that AdVs are able to infect bovine oocytes.
Materials and methods
Reagents
Medium 199 and insulin–transferrin–selenium were purchased from Gibco BRL (Gaithersburg, MD, USA). Sodium pyruvate, Bovine Serum Albumin (BSA), EGF, β -estradiol, L-glutamine, uracil, sodium bicarbonate, HEPES, mineral oil, ionomycin, hyaluronidase, cytochalasin B, 6-dimethylaminopurine (6-DMAP) were procured from Sigma-Aldrich (Saint Louis, MO, USA). Cleavage medium and serum protein substitute were obtained from Sage Biopharma (Bedminster, NJ, USA). Foetal bovine serum was purchased from Hyclone. Cells direct kit and Super Script III cell direct cDNA synthesis system were procured from Invitrogen (USA).
Cumulus–oocyte complex (COC) collection
Bovine ovaries were collected and transported to our laboratory in a Thermos flask with saline solution (0.9% NaCl, 100 IU/mL penicillin, and 100 mg/mL streptomycin) at 20°C to 24°C within 6-8 hours after the animals were slaughtered in a local slaughterhouse. The ovaries were washed thrice with the same saline solution pre-warmed to 37°C ; cumulus–oocyte complexes (COCs) were then collected from follicles with diameter ranging from 2 to 6 mm by using a 10-mL syringe and a 12-ga sterile needle. Follicular fluids were pooled in a sterile centrifuge tube; the collected COCs were washed thrice with COC wash solution (Medium 199 containing 10% NBS, 2.2 g/L sodium bicarbonate, 10 mmol/L HEPES; pH 7.2–7.4) pre-warmed to 37°C and subsequently distributed in a 60 mm cell culture dish. The COCs with multiple layers of cumulus cells were selected under a stereomicroscope for IVM.
COC culture in vitro
The COCs were washed thrice in maturation medium at 38.5°C under a humidified atmosphere of 5% CO2 in air and cultured in 500 mL of maturation medium (Medium 199 containing 10% BSA, 0.1 IU/mL human menopausal gonadotropin, 1.0 mg/mL β -estradiol, 50 ng/mL EGF, 50 mg/mL uracil, and 10 mL/mL insulin–transferrin–selenium) for 2 hours. Afterward, the COCs were cultured in fresh maturation medium with recombinant BAV304a AdV that was modified to carry a green fluorescence marker at MOI of 0.1. Zona-free COCs were cultured in the same solution as a control. The maturation medium was equilibrated in a CO2 incubator for at least 2 hours before maturation culture was performed.
Microinjection of BAV304a AdV solution
Oocytes were collected from ovaries, cultured for 2 hours in maturation medium, and gently stripped of the surrounding excessive cumulus cells. Only oocytes with intact cumuli were used. BAV304a recombinant AdV solution was microinjected into the ooplasm of oocytes, and control oocytes were injected with an equal volume of water. The oocytes were then transferred to fresh maturation medium for 20 minutes and washed thrice. Only viable oocytes were cultured in fresh maturation medium for the next 10 hours.
Oocyte imaging
Live cells were observed using a Zeiss microscope. Live oocytes were incubated in maturation medium with BAV304a AdV at MOI of 0.1. Brightfield images of AdV infection were recorded with a charge-coupled device (CCD) camera (Axiocam MRm; Carl Zeiss, Shanghai, China) attached to the Zeiss microscope.
PCR validation
Total RNA was extracted from cumulus cells and blastocysts from different experimental groups by directly adding oocytes to 10 μL of resuspension buffer and later treating with 1 μL of RNaseout for 10 minutes at 75°C . The cDNA template was obtained by reverse transcription. Double PCR method was used to detect AdV in the extract, and the PCR products were examined by agarose gel electrophoresis.
Statistical analysis
Data from at least three replicates per experiment were analysed by chi-square tests. Differences of P < 0.05 (*) and P < 0.01 (**) were considered to be significant and extremely significant, respectively.
Results
By co-incubation, BAV304a AdV infects cumulus cells, but does not infect oocytes
First, the COCs were co-incubated in fresh maturation medium with BAV304a AdV solution. Green fluorescence was observed in the cumulus cells 7 hours after the cells were cultured in vitro, but no fluorescence was detected in the oocytes (Supplementary material). After 20 hours, the COCs completed meiosis I and extruded the first polar body. At this time, the observed fluorescence signal was stronger in cumulus cells (Figure 1A, Figure 1B). Fluorescence disappeared when the oocytes were separated from the surrounding cumulus cells using 0.3% hyaluronidase (Figure 1C, Figure 1D). The rate of the first polar body extrusion (PB1E) in the experimental group and the control group were investigated and compared. Green fluorescence was still not found in oocytes that were moved from the cleavage medium after being stimulated to undergo parthenogenetic development (Figure 1E, Figure 1F). In addition, the cleavage rate in the experimental group was lower, but this finding was not significantly different from the control group (Table 1).
Table 1. The oocyte maturation of each group.
Group |
PB1E rate (%) |
4 to 8-cell stage embryo rate (%) |
The normal control group
|
82 |
40 |
Adenovirus culture group
|
77 |
38 |
Adenovirus injection group |
52 |
11 |
Water injection group |
56 |
13 |
2021 Copyright OAT. All rights reserv
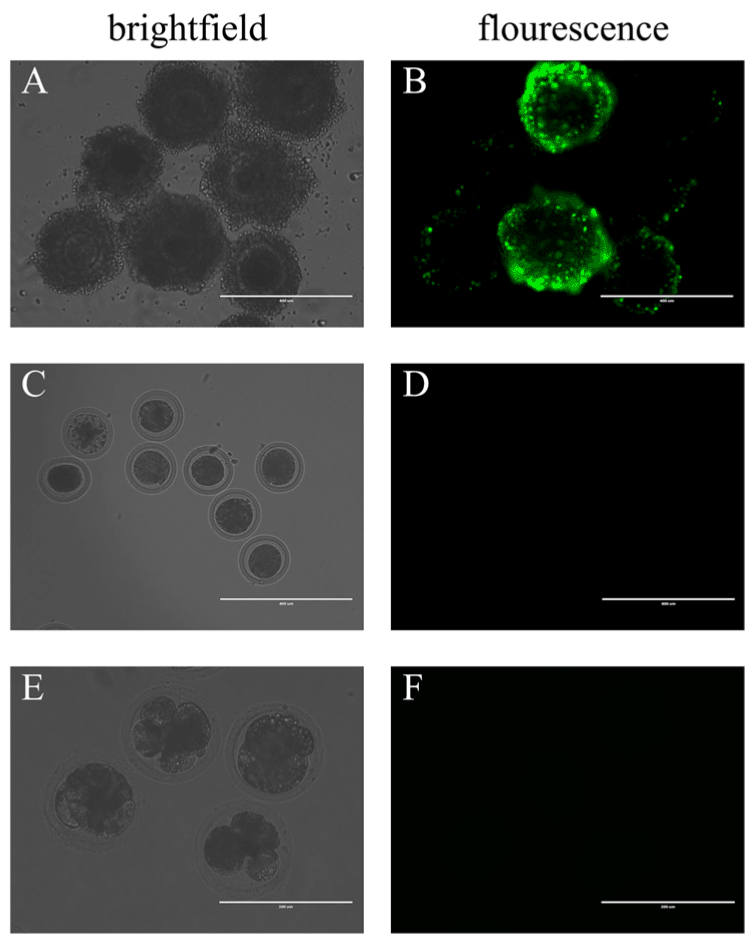
Figure 1. By co-incubation, BAV304a AdV infects cumulus cells but does not infect oocytes.
(A) COCs in brightfield, scale bar = 400 μM. (B) COCs in fluorescence, scale bar = 400 μM. (C) In brightfield, COCs with surrounding cumulus cells removed, scale bar = 400 μM. (D) In fluorescence, COCs with surrounding cumulus cells removed, scale bar = 400 μM. (E) Brightfield of oocytes in multi-cell stage, scale bar = 200 μM. (F) Fluorescence of oocytes in multi-cell stage, scale bar = 200 μM. A total of 100 oocytes were collected from each sample, and experiments were repeated three times.
By co-incubation, Adenovirus infects zona-free oocytes
Considering the zona pellucida blocks BAV304a adenovirus to a certain extent, we next investigated whether the zona pellucida plays a vital role in AdV-infected oocytes. To address this issue, we first removed the cumulus cells. Then, a zona-free procedure was carried out in pronase solution after the COCs were cultured for 2 hours. The naked oocytes were incubated in fresh maturation medium with BAV304a AdV solution. Green fluorescence was detected in a few oocytes after 20 hours (Figure 2A, Figure 2B).
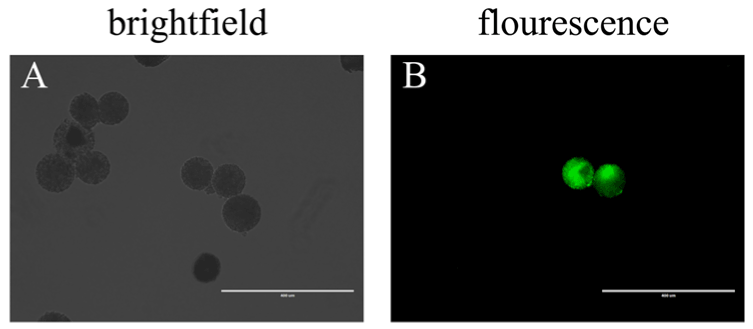
Figure 2. By co-incubation, AdV infects zona-free oocytes (A) Zona-free oocytes in brightfield, scale bar = 400 μM. (B) Zona-free oocytes in fluorescence, scale bar = 400 μM.
By microinjection, BAV304a AdV infects oocytes and cumulus cells
After microinjecting the BAV304a AdV solution, COCs were moved to fresh maturation medium for culture. Green fluorescence appeared in the oocytes and the surrounding cumulus cells after 10 hours. However, the fluorescence intensity in the oocytes was much weaker when compared to the surrounding cumulus cells (Figure 3A, Figure 3B). Fluorescence was also found in oocytes with the surrounding cumulus cells removed (Figure 3C, Figure 3D) and in oocytes in the multi-cell stage (Figure 3E, Figure 3F). The PB1E rate was similar between oocytes injected with the AdV solution and control oocytes injected with water. Mature oocytes from both the experimental and control groups were placed in cleavage medium for several hours after being stimulated to undergo parthenogenetic division, and green fluorescence disappeared in both groups. No significant difference was found between the cleavage ratios of the two groups (Table 1).
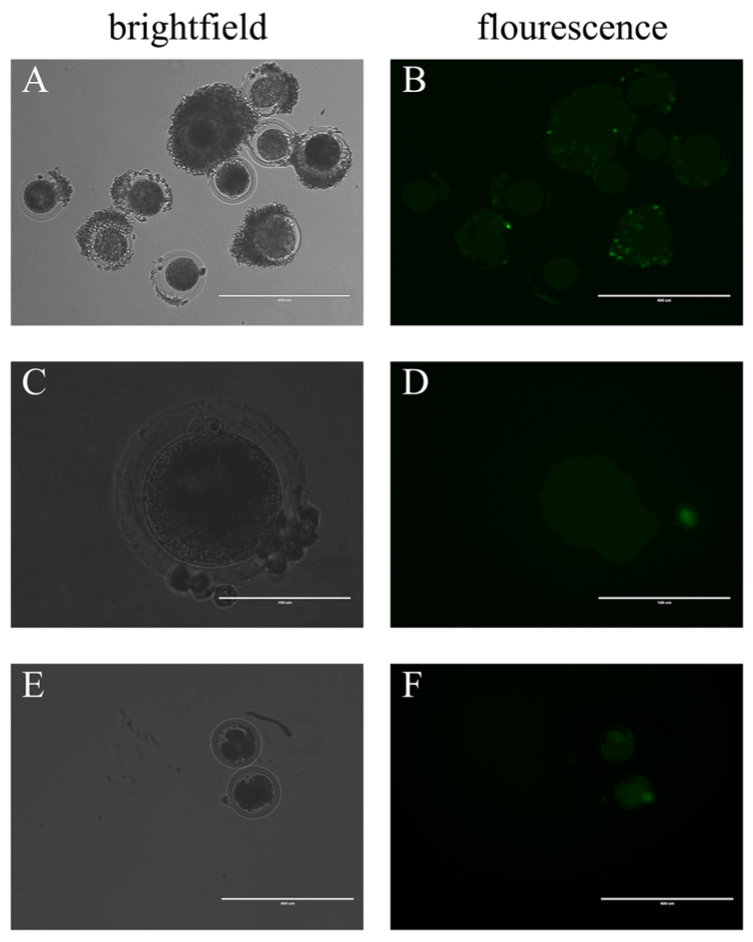
Figure 3. By microinjection, BAV304a AdV infects both cumulus cells and oocytes
(A) COCs in brightfield, scale bar = 400 μM. (B) COCs in fluorescence, scale bar = 400 μM. (C) In brightfield, oocytes with the surrounding cumulus cells removed, scale bar = 100 μM. (D) In fluorescence, oocytes with the surrounding cumulus cells removed, scale bar = 100 μM. (E) Oocytes in multi-cell stage in brightfield, scale bar = 400 μM. (F) Oocytes in multi-cell stage in fluorescence, scale bar = 400 μM. A total of 100 oocytes were collected from each sample, and experiments were repeated three times.
PCR validation
The microinjected, co-cultured, zona-free oocytes and cumulus cells were collected for cDNA extraction. The fluorescence marker gene was found in microinjected cumulus cells, co-cultured cumulus cells, microinjected oocytes, and zona-free oocytes (Figure 4). The following procedure describes PCR validation of cDNA in detail. The first PCR reaction included 0.5 μL of cDNA template, 0.3 μL each of the upstream and downstream primers, 5 μL of 2× Taq Mis, and 3.9 μL of sterile H2O. The initial denaturation occurred at 94°C for 5 min followed by 35 cycles of 94°C for 40 s, 52°C for 30 s, and 72°C for 45 s for amplification. Extension occurred at 72°C for 5 min followed by incubation at 12°C for 10 min. The second PCR reaction included 0.5 μL of PCR product, 0.4 μL each of the upstream and downstream primers, 5 μL of 2× Taq Mis, and 3.7 μL of sterile H2O. The initial denaturation occurred at 94°C for 5 min, followed by 35 cycles of 94°C for 30 s, 51°C for 30 s, and 72°C for 30 s. Extension occured at 72°C for 5 min followed by incubation at 12°C for 10 min. After the reactions were complete, PCR products were analysed by agarose gel electrophoresis.
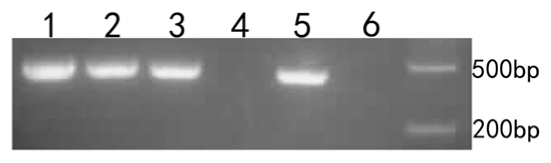
Figure 4. Oocyte PCR validation. D2000 was used as a marker. 1. microinjected cumulus cells. 2. co-cultured cumulus cells. 3. microinjected oocytes. 4. co-cultured oocytes. 5. zona-free oocytes. 6. water.
Discussion
Bovine oocytes exhibit resistance to AdV to some extent, possibly because oocytes can protect themselves from non-self substances. Oocyte resistance is also associated with AdV pathogenicity and the protection provided by the zona pellucida. The zona pellucida is a non-cell layer composed of glycoprotein surrounding the oocytes, and it protects oocytes and prevents entry of heterogeneous sperm, thereby protecting oocytes and embryos from external damage.
In this study, BAV304a AdV was modified to carry a green fluorescence marker. BAV304a AdV was added to fresh maturation medium and this solution was microinjected into the cytoplasm of bovine oocytes. After co-culture, the cumulus cells appeared green, but the oocytes remained unchanged. However, in zona-free cells, adenovirus infected the oocytes and some oocytes appeared green. This finding indicates that the bovine zona pellucida blocked BAV304a adenoviral infection. After microinjection, green fluorescence was observed in both the oocytes and the cumulus cells. This finding indicates that a portion of adenovirus escaped from bovine oocytes and subsequently infected the cumulus cells. This phenomenon may be caused by damage to the zona pellucida. Microinjection is a physical method that may affect the function of the zona pellucida and weaken its resistance to AdV. In addition, these results are confirmed by PCR validation. Overall, these results indicate that the zona pellucida plays a vital role in BAV304a AdV resistance in bovine oocytes.
In another study, mouse oocytes were co-cultured with a Pbr322HBV BAV304a plasmid and evaluated by PCR, FISH, and Southern blot. The results revealed only 36 eggs tested positive for HBV DNA [9]. Studies conducted by Zhang qingjian also indicated that oocytes exhibit some resistance to non-self substances. In our experiments, fluorescence intensity in the oocytes was much weaker compared to the surrounding cumulus cells when AdV was injected into the oocyte cytoplasm. Moreover, by co-incubation, AdV infected a small portion zona-free oocytes. This indicated the oocytes exhibited resistance to AdV to some extent, possibly because oocytes protect themselves from non-self substances [Video].
Bovine oocytes are transcriptionally quiescent from the GV stage to the 8-cells stage. However, green fluorescent protein was expressed in oocytes after BAV304a AdV infection. This finding indicates that BAV304a AdV used the transcription factors in the oocytes to generate AdV mRNA. This result also suggests that the transcription factors were available and that transcriptional quiescence in bovine oocytes is due to DNA stored as chromatin during meiosis.
In our experiments, oocyte complexes co-incubated with bovine oocyte maturation medium and bovine AdV showed no significant difference in the first polar body discharge rate and blastocyst rate compared to normal oocytes. In addition, no significant differences were observed between oocytes microinjected with bovine AdV compared to control oocytes injected with water. Thus, bovine AdV infection does not affect the normal maturation of oocytes and blastocyst formation. The virulence of bovine AdV may also be involved.
Our experimental results indicate that bovine AdV could be used as a basis for further research on the zona pellucida. In addition, these results help to characterise viral infection in oocytes.
References
- Rowe WP, Huebner RJ, Gilmore LK, Parrott RH, Ward TG (1953) Isolation of a cytopathogenic agent from human adenoids undergoing spontaneous degeneration in tissue culture. Proc Soc Exp Biol Med 84: 570-573. [Crossref]
- Baxi MK, Babiuk LA, Mehtali M, Tikoo SK (1999) Transcription map and expression of bovine herpesvirus-1 glycoprotein D in early region 4 of bovine adenovirus-3. Virology 261: 143-152. [Crossref]
- Ma H, Liu Y, Liu S, Xu R, Zheng D (2005) Oral adeno-associated virus-sTRAIL gene therapy suppresses human hepatocellular carcinoma growth in mice. Hepatology 42: 1355-1363. [Crossref]
- Hilleman MR, Werner JH (1954) Recovery of new agent from patients with acute respiratory illness. Proc Soc Exp Biol Med 85: 183-188. [Crossref]
- Bleil JD, Wassarman PM (1980) Structure and function of the zona pellucida: identification and characterization of the proteins of the mouse oocyte's zona pellucida. Dev Biol 76: 185-202. [Crossref]
- Dicks MD, Guzman E, Spencer AJ, Gilbert SC, Charleston B, et al. (2015) The relative magnitude of transgene-specific adaptive immune responses induced by human and chimpanzee adenovirus vectors differs between laboratory animals and a target species. Vaccine 33: 1121-1128. [Crossref]
- Li YK, Fu CZ, Zhang YR, Zan LS (2015) Efficient construction of recombinant adenovirus expression vector of the Qinchuan cattle LYRM1 gene. Genet Mol Res 14: 9469-9477. [Crossref]
- Schutta C, Barrera J, Pisano M, Zsak L, Grubman MJ, et al. (2016) Multiple efficacy studies of an adenovirus-vectored foot-and-mouth disease virus serotype A24 subunit vaccine in cattle using homologous challenge. Vaccine 34: 3214-3220. [Crossref]
- Zhang QJ, Huang TH, Xie QD (2004) Study on transfection of mouse oocytes with recombinant plasmid,pbr322-hbv. Carcinogenesis Teratogenesis & Mutagenesis 16: 324-327.




