PKP: Penetrating Keratoplasty; ALK: Anterior Lamellar Keratoplasty; DALK: Deep Anterior Lamellar Keratoplasty; PLK: Posterior Lamellar Keratoplasty; DLEK: Deep Lamellar Endothelial Keratoplasty; DSEK: Descemet Stripping Endothelial Keratoplasty; DSAEK: Descemet stripping automated endothelial keratoplasty; SALK: Superficial Anterior Lamellar Keratoplasty; DMEK: Descemet membrane endothelial keratoplasty; M-K medium : McCarey-Kaufman medium
Corneal blindness is responsible for approximately 10% of the cases of blindness worldwide [1]. More importantly, in most developing countries corneal diseases represent the second leading cause of blindness [2]. Corneal transplantation or keratoplasty is the only effective treatment for most of the disorders leading to irreversible corneal damage and eventually to corneal blindness. The indications of keratoplasty have substantially changed since the era of the first corneal transplantation in 1905. The initial indication was the treatment of irreversible corneal scaring and haziness as a consequence of various infectious insults: bacterial, fungal, trachomal and viral (herpes simplex). In many countries of the developing world these infectious diseases remain indeed one of the most important reasons for corneal blindness and subsequent keratoplasty [3,4]. In the western countries these initial indications have been replaced by the bullous keratopathy and the graft failure after previous transplantation attempts [5].
New indications, like keratoconus and Fuchs’ endothelial dystrophy have also emerged in the more recent years, especially after 2000. These diseases are now the main indications for corneal transplantation, in Germany as well as in other developed countries. Finally, other corneal diseases such as xerophthalmia, the iridocorneal endothelial syndrome, ocular trauma, the corneal ulcer and other corneal dystrophies are indeed rarer, but important indications for a corneal transplantation [6-10].
A full thickness or a partial keratoplasty can be performed depending mainly on the indication for corneal transplantation (Figure 1; Table 1).
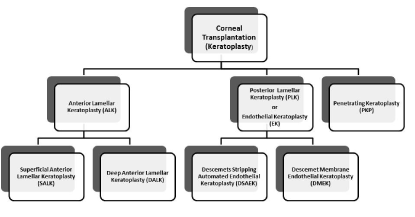
Figure 1. The various techniques of keratoplasty
Table 1. Keratoplasty: Techniques and indications
Method |
Advantages |
Disadvantages |
The most common
indications |
PKP (Penetrating
Keratoplasty) |
Long time experience,
Standardized technique,
Optimal method for low risk corneal diseases |
Postoperative astigmatismus,
Prologed visual rehabilitation,
Need for long term care,
Allograft rejection |
Graft failure
Keratoconus
Infection
Bullous keratopathy
Corneal scar
Fuchs’ dystrophy |
DALK (Deep Anterior Lamellar Keratoplasty) |
Faster visual rehabilitation,
Lower rejection rate |
Flat learning curve
More demanding in technical equipment
Irregularities in the interface |
Keratoconus
Infection
Corneal Scar |
DSAEK (Descemet stripping with automated endothelial keratoplasty) |
Faster visual rehabilitation
without
postoperative astigmatismus,
Lower rejection rate |
Flat learning curve
More demanding in technical equipment
Irregularities in the interface |
Graft Failure
Infection
Bullous Keratopathy
Corneal Scar
Fuchs’ Dystrophy
Non- Fuchs’ Dystrophy |
DMEK (Descemet membrane endothelial keratoplasty) |
The fastest visual rehabilitation |
The same as DSAEK but less demanding in equipment and more demanding in operating skills |
Bullous Keratopathy
Fuchs’ Dystrophy
Non- Fuchs’ Dystrophy |
1. Penetrating keratoplasty (PKP) or full thickness corneal transplantation has been the gold standard treatment for many corneal diseases for over a century. In PKP all the layers of the cornea are transplanted. The central part of the patient's cornea is cut out circularly with a trephine or with the use of a femtosecond laser and replaced with the donors’ cornea. The donor cornea is sewn together at the edges with the limbus of the patient cornea. This is the method of choice in the cases of profound corneal defects due to extensive stromal scarring, opacities with an uncertain status of the endothelium or significant posterior corneal involvement, corneal ectasia (such as keratoconus and pellucid marginal degeneration, especially if there is a history of hydrops), combined stromal and epithelial disease (such as Peters anomaly), and infectious or non-infectious corneal ulcerations or perforations [11]. Although PKP in a low-risk corneal disease has the best outcome regarding graft survival rate among all transplantations, clinicians became from the beginning aware of some undesirable postoperative consequences of PKP. These include: high astigmatism induced by the placement of corneal sutures causing prolonged visual rehabilitation despite the presence of completely clear corneal graft, unpredictable refractive outcome, post keratoplasty glaucoma, increased vulnerability to eye trauma and infection for many years after surgery or even expulsive bleeding during the operation [12-14]. There is also a higher risk of allograft rejection compared with other keratoplasty types [15]. Given the high incidence of complications, the question of replacing only the diseased part of the cornea avoiding the damage to the structural integrity of the globe came rather early in the forefront. Nevertheless, the development of this type of keratoplasty, the so called ``lamellar keratoplasty``, was delayed for a few decades due to technical difficulties. The various techniques of lamellar keratoplasty available nowadays can be divided in two broad categories: those dealing with the replacement of the anterior corneal layers (anterior lamellar keratoplasty-ALK) and those replacing only the posterior part of the cornea i.e. the Descemet’s membrane and the endothelium (posterior lamellar keratoplasty -PLK or endothelial keratoplasty-EK).
2. Anterior lamellar keratoplasty (ALK)
Superficial anterior lamellar keratoplasty (SALK): In the SALK only the epithelium and the anterior stroma are transplanted. Stromal opacities located in the anterior stroma, which may be caused by anterior stromal dystrophy, degeneration, infection, chronic inflammation or previous refractive surgery resulting in corneal scarring, are the main indications of SALK. This operation can be performed with a microkeratome or with a femtosecond laser. If the femtosecond laser is used to create the lamellar cut, then the procedure is called femtosecond-laser assisted anterior lamellar keratoplasty (FALK). SALK is associated with a significantly lower risk of rejection and of intraoperative complications. However, these grafts often heal with pronounced scarring, which limits the vision after surgery [16,17].
Deep anterior lamellar keratoplasty (DALK): In the DALK the goal is to transplant the whole stroma and epithelium of the patient and preserve only the Descemet’s membrane and the healthy endothelium. The main indications for DALK are: deep stromal opacities which may be caused by herpetic or other infectious scars, chronic inflammation with scarring after corneal burns and keratoconus [18]. The preservation of host endothelium leads to a reduced incidence of graft rejection, to a faster visual rehabilitation and to a reduced risk of intraoperative and postoperative complications, compared to PKP [19]. However, DALK is technically demanding and may be complicated with perforations of Descemt’s membrane and consequent need for PKP. Moreover, a rejection of the stroma, with neovascularization and vision loss, can occur more often than in DALK [20,21].
3. Posterior lamellar keratoplasty (PLK) or Endothelial keratoplasty (EK)
Descemet stripping automated endothelial keratoplasty (DSAEK): DSAEK is a procedure that involves the selective removal of the Descement membrane and endothelium, followed by transplantation of donor corneal endothelium in addition to donor corneal stroma. A microkeratome is used to prepare the donor tissue. A tunneled corneoscleral incision is created in the diseased eye, the recipient endothelium and Descemet's membrane is removed, the graft is folded and inserted with non-coapting forceps and an air bubble is placed in the anterior chamber to support graft adherence [22]. The procedure is used to treat corneal edema in the setting of endothelial dystrophies (such as Fuchs corneal dystrophy and posterior polymorphous corneal dystrophy), pseudophakic bullous keratopathy, iridocorneal endothelial syndrome, endothelial failure in the setting of prior intraocular surgery or of a previous PKP graft, and other causes of corneal endothelial dysfunction [23,24]. Advantages of DSAEK over PKP are numerous: corneal astigmatism is much lower as compared to PKP due to the lack of sutures. Therefore, visual recovery is fast, and most patients have usable vision within 6 weeks after operation, and some of them have excellent vision at just 1 week, especially with ultra-thin DSAEK grafts [25]. The two major complications following DSAEK surgery are graft dislocation and primary graft failure [26].
Descemet membrane endothelial keratoplasty (DMEK): This is again a partial-thickness cornea transplant procedure that involves selective removal of the patient's Descemet's membrane and endothelium, followed by transplantation of donor corneal endothelium and Descemet's membrane without additional stromal tissue from the donor. A clear corneal incision is created, the recipient endothelium and Descemet's membrane are removed, and the graft is loaded into an inserter. After injecting the tissue into the anterior chamber, the surgeon orients and unscrolls the graft, and a bubble of 20% sulfur hexafluoride is placed in the anterior chamber to support graft adherence [27]. The indications as well as the outcome are similar to those for DSAEK. On the other hand, the patients after DMEK obtain better visual acuity in a quicker time frame and the graft rejection rates are much lower. In the disadvantages of the method one should consider the technical difficulties and the reported high rates of graft detachment [28,29].
Finally, a new method combining the above two techniques has been recently introduced. The so called ultra-thin DSAEK combines the advantages of DSAEK (easier manipulation with the endothelial graft and consequently decreased endothelial cell loss) with the advantages of DMEK (thin grafts bring better vision) [30].
The preservation of donor corneas
The eye banks: During the initial decades of keratoplasty, eyes form living donors enucleated due to posterior segment pathology, was the only source of corneal graft. Corneas should be immediately transplantated because of concerns about tissue death, making thus the keratoplasty an emergency procedure. Since the surgical techniques were not well developed and antibiotics or immunosuppressive agents were not available, the outcomes were rather limited [31].
The seminal report of Filatov about the feasibility of storing cadaveric corneas in a moist chamber at 40C offered for the first time the opportunity to use preserved tissue and paved the way for the development of the eye banks where corneas could be collected, stored and distributed [32]. Nevertheless, keratoplasty continued for a long time to be performed in an emergency basis since, due to concerns about the short term living of corneal endothelial cells, the grafting was accomplished no later than 48 hours postmortem [33]. The first eye bank named “Eye-Bank for Sight Restoration, Inc”., was founded in New York in 1944 by Townley Paton who after performing many corneal transplants, came to the conclusion that a formal system of eye collection needed to be developed [34]. Moist-chamber storage at 4°C was the preservation method of choice, while research for alternatives (e.g., drying, formalin fixation, freezing, freeze-drying, and liquid paraffin storage) did not produce satisfactory results [35]. In 1961 the Eye Bank Association of America (EBAA) was established, bringing together “lay and professional individuals dedicated to the advancement of worldwide eye banking” [31].
A turning point in the history of eye banking happened in 1974 when McCarey and Kaufman in the U.S. demonstrated that by excising the cornea from the globe and placing it in a tissue culture medium at 4°C, the endothelium could remain viable for several days [36]. McCarey and Kaufman reported the development of a modified tissue medium (McCarey-Kaufman [M-K] medium) in which human corneas with viable endothelium could be preserved at 4°C for at least 4 days. ``MK medium`` containing TC199, Earle's salts, HEPES buffer and gentamicin, remained the standard corneal preservation medium for some 15 years. The application of this method gave the opportunity to keratoplasty to become a scheduled surgery. As underlined by Wilson and Bourne in their 1989 major review on corneal preservation, “this allowed the patient to better plan for the transplant and for the surgery to be performed when a well-trained regular team of operating personnel was available to assist a well-trained surgeon.” [35]. Corneas stored at 4°C in M-K medium remained thin and clear and could be transported in polystyrene containers with ice. This was subsequently superseded by other commercial preparations, such as K-Sol and Optisol, containing osmotic agents to limit corneal tissue swelling, and offering extended preservation times of a week or ten days.
The eye banking concept came to Europe much later compared to the U.S. The first eye bank in Continental Europe was the one established in the Barraquer Eye Center in Barcelona, Spain by Dr. Joaquin Barraquer in 1962 [37]. The European Eye Bank Association was founded in 1989 with the aim as stated “to help provide tissues and cells of optimum quality and safety for transplantation and the treatment of eye diseases, according to the highest medical and scientific standards, and making them available to as many patients in need as possible in an ethical and humanitarian way, in accordance with the Declaration of Helsinki and applicable national and international laws and regulations” [38].
Since the foundation of the first eye bank an enormous progress in both surgical techniques and methods of corneal preservation happened. Keratoplasty became widely accepted as the only effective treatment for corneal blindness. As a consequence, the demand for corneal allografts increased in an exponential way and this has ultimately led to an explosion in the number of eye banks. According to a recent global survey 742 eye banks exist currently worldwide. India has the most banks, followed by the United States and China [38]. Nevertheless, given an estimated global graft demand of 12.7 million, a severe imbalance between supply and demand exists. Moreover, the continuous global population growth (mainly in India, China and Africa) will likely further aggravate this imbalance [39].
The various methods of corneal preservation
On the importance of corneal endothelium in corneal transplantation: The human cornea is a transparent avascular structure covering the front part of the eye globe. Its main function is to transmit and focus the light to the retina to generate vision. Its transparency needs to be maintained for optimal vision (Figure 2). The cornea is structured into well-organized layers, and each layer has its own importance in maintaining the viability and transparency of the tissue. From the anterior to the posterior cornea, the human corneal tissue consists of a stratified epithelium, Bowman’s layer, the stroma, Descemet’s membrane, and a mosaic-like patterned monolayer of hexagonal endothelial cells [40]. The epithelium is a self-renewing layer and harbors a resident stem cell population at its periphery. However, the stroma and endothelium are usually quiescent and so far, have not been considered to regenerate [41]. The corneal endothelium that lines the posterior corneal surface is derived from the neural crest during embryologic development [42]. Human endothelial cell density is approximately 6000 cells/mm² during the first month of life [43] but decreases to about 3500 cells / mm² by the age of five years [44]. A further decrease at a slower pace continues throughout life so that at the age of 85 the mean cell density is 2300 cells/mm² [45]. There is evidence that the endothelial cells possess proliferative capacity since in tissue culture they can be induced to divide. In vivo, however, they are arrested in the G1 phase of the cell cycle constructing this way a non-replicable monolayer [46]. The Descemet's membrane is formed from collagen secreted form the endothelial cells. At the time of birth, the Descemet's membrane is approximately 3 μ thick and consists of collagen in a banded pattern. Throughout life, endothelial cells continue to secrete collagen added to the Descemet membrane but in a non-banded pattern. Nevertheless, if the endothelial cells are stressed or damaged, they may secrete collagen added to the posterior part of the Descemet's membrane in a banded fashion [47]. The transparency of the cornea is mainly the result of the ultrathin structure, the crystalline organization and the restriction in the range of distances between adjacent stromal collagen fibrils. These particular properties of the stromal collagen in combination with the extreme thickness of the other corneal layers prevents almost completely light scattering. However, if the cornea swells, disruptions of the spacing among the collagen fibrils ensues with a consequent significant scattering of the light and lose of corneal transparency [48]. The corneal endothelium plays a critical role in the regulation of stromal hydration by behaving as a semi-permeable barrier to the movement of fluids and nutrients. This property is due to the presence of intercellular gap junctions and tight junctions at the apical membrane of endothelial cells. Therefore, fluids and nutrients can leak in the paracellular space and enter the stroma. On the other hand, endothelial cells prevent excessive fluid entrance in the stroma and subsequent corneal swelling by actively pumping ions and drawing this way osmotically water from the stroma to the aqueous humor. Endothelial cell loss or endothelial dysfunction has as a consequence the inability to efficiently pump fluid out of the stroma, resulting in stromal and epithelial edema and eventually in loss of corneal clarity and visual acuity [49,50].
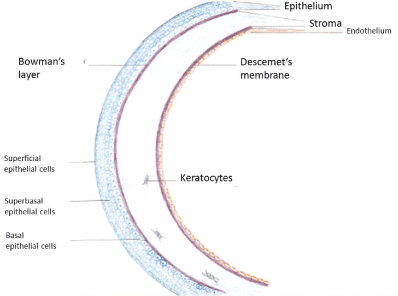
Figure 2. The structure of human cornea
Since endothelial cells cannot actively divide in vivo, the only way to preserve the proper corneal function despite the normal age-related or the accelerated disease- or trauma-related endothelial loss is by expansion of the existing cells. In the case of age-associated decline, the remaining endothelial cells are usually enough to maintain a sufficient barrier and pump function. However, if the density falls below a critical value of 500 to 1000 cells/m², then the function of corneal endothelium becomes compromised and stromal edema ensues [51].
Given the unique role of endothelium in the maintenance of corneal function, it is not surprising that the main goal of corneal graft preservation is to minimize corneal endothelial loss, which independent of the method of preservation reaches 10-30%. This renders up to 40% of preserved donor corneas eventually unsuitable for transplantation aggravating further the problem of graft shortage [50]. This decline in endothelial cell density of the donor corneas is directly related to the length of storage time [52,53] and has been mainly attributed to apoptosis [54]. After transplantation, endothelial loss of corneal grafts continues at a pace faster than that of normal corneas to the point that by 3 years 53% of the preoperative endothelial cell density is lost [55]. This process can end up to a condition called late endothelial failure characterized by graft swelling and haziness unresponsive to the treatment with corticosteroids. Late endothelial failure accounts for the majority of late graft failures [56]. It has been shown that the initial postoperative endothelial cell density is inversely related to late endothelial failure [57], suggesting that preservation methods by minimizing initial endothelial loss may have a very significant contribution to a favorable long term outcome of keratoplasty.
Methods of corneal preservation
Since cornea is an avascular structure, selection criteria for corneal donors are less restrictive than for vascularized tissues. Nevertheless several infectious diseases have been transmitted through corneal grafts [58,59] underlying thus the importance of careful testing the donors according to the medical standards guidelines issued from the European Eye Bank Association [60] and the Eye Bank Association of America [61]. The same guidelines define also the methods the eye banks should use to assess the quality of donor corneas. The most important component of this quality control is the assessment of endothelial cell density and viability. This is performed with the use of specular or confocal light microscopy [62]. Regarding the age of the donor, although most eye banks in America prefer donors 65 years old or younger, this is recently changing given the results of the Cornea Donor Study which has shown that the five years success rate of corneal transplantation was not lower if the donors were 65-75 years old [63]. On the other hand, European eye banks tend to accept grafts form donors even older than 75 years. The minimum acceptable endothelial cell density also varies among eye banks. According to the 2010 European Eye Bank Association Report, 70% of the eye banks accept as minimum density 2000 cells / mm² while the rest have a minimum ranging from 2100 to 2500 cells / mm² [64]. In general, a cornea with an endothelial density of 2.200 cells / mm² should have a sufficient number of viable cells in order to retain its transparency for the next 25 years [65]. Nevertheless, the long term surviving of the graft is also dependent on several other factors like the allograft rejection and the recipient diagnosis [66].
The initial method of corneal preservation was the storage of the whole globe in the “moist chamber”, a moistened pot at 2-6 ºC as prescribed by Filatov in 1935 [32]. This type of storage was however limited by the availability of metabolic substrates and buildup of metabolic waste products in the aqueous humor. It was for this reason replaced by the storage of corneas excised from the eye along with a rim of sclera (a corneoscleral disk or button). The storage of grafts in serum was introduced in the 1960s and the serum was soon replaced by a synthetic solution whose composition mimicked that of aqueous humour. The revolutionary turn which followed in early 1970s, was the development of the MK-medium which extended the storage period in hypothermic conditions to 4 days. MK-medium is still in use in the developing countries due to its low cost and simplicity of production.
For the storage and preservation of corneoscleral disks three methods are today available: hypothermia, organ culture and cryopreservation. Of these, only cryopreservation permits the storage of occular tissue indefinitely. Indeed, cryopreserved corneas have been successfully transplanted on several occasions in the past [67-70]. More recently, it has been used in DALK [71] and as a tectonic graft for perforated corneas [72,73]. Cryopreservation, however, has been associated with variable and unpredictable rates of endothelial loss [74]. As a result, cryopreserved corneas are been currently used only occasionally in emergency situations when the aim is to save the eye [70]. On the other hand, optimization of cryopreservation protocols remains an open issue for cryobiologists [75], since cryopreservation could become an alternative in addressing the problem of global storage of donor corneas.
The hypothermic storage
Hypothermic storage is the most common method of corneal preservation in the USA and in most Asian countries [76]. It is based on the principle that cold reduces metabolic cellular demand. On the other hand, cooling has also deleterious effects on cells such as the suppression of active transport of ions across cell membranes leading to water influx and cellular edema and the disruption of calcium homeostasis and proton exchange leading to acidosis [77]. As a consequence, the storage time is limited to a maximum of 10-14 days. This time span has been claimed by the manufacturers of the newer storage solutions which have largely replaced the first hypothermic solution, the M-K medium (which permitted a maximum storage of only 4 days). These are: the modified M-K medium, the K-Sol, the Dexol, the Liquorol, the Optisol (GS and plus), the Chen medium, the Eusol-C (Al.Chi.mia, Padova, Italy), the Cornisol (Aurolab, Madurai, India) and the newest Life 40C (Numedis Inc. Minnesota, USA). It should be noted that despite the extended time limits offered by the manufacturers, most eye banks prefer to keep corneas no more than 7-10 days in hypothermic conditions due also to the fact that corneal epithelium is less well than endothelium preserved, and the extend of epithelial defects after transplantation increases also with storage time [78].
The technique of hypothermic storage is rather simple and inexpensive. The corneas are stored in vials and refrigerated at 2-6 0C. The vials may allow inspection of corneal endothelium with specular microscopy. During the storage period the corneas remain thin and they are readily available for surgical use. All the hypothermic storage media consist of a tissue culture medium supplemented with deturgescent agents like dextran and chondroitin sulfate to prevent corneal swelling. Chondroitin sulfate is considered the most crucial ingredient since it presumably plays an important role in the intracellular redox system as an antioxidant and as a membrane and growth factor stabilizer. It was the addition of chondroitin sulfate to tissue culture media which lead from the M-K medium to the development of the newer solutions. Other additives include antibiotics, energy sources, antioxidants, membrane stabilizers and growth factors. All storage solutions are commercially available and ready for use [79].
From all the above storage media, Optisol GS (from Chiron Ophthalmics Irvin, CA, until 1997 and after that form Bausch & Lomb Inc., Rochester, NY, USA) remains the most popular. It was introduced in 1991 as a hybrid of K-Sol, Dexol and CSM (culture storage medium). It contains: 2.5% chondroitin sulfate, 1% dextran, Fe, 14 vitamins, amino acids, cell metabolites, antioxidants and precursors of adenosine triphosphate [80]. Studies have repeatedly shown a high percentage of clear grafts and a low percentage of endothelial loss after corneal transplantation [81,82]. Moreover, other commercially available hypothermic storage media (Cornisol, Life 40C and Eusol-C) have shown no superiority against Optisol-GS in comparative studies [83-85].
Since the donor’s eye is usually contaminated, decontamination of the corneal graft is an essential part of the storage process. Antibiotics (mainly gentamycin) are for this reason added in all storage solutions. However, antibiotics are more effective when the bacteria are more metabolically active which certainly does not happen under hypothermic conditions. Nevertheless, preoperative warming of the donor corneas enhances the decontaminating effect of the antibiotics which have been stored in the tissue during the hypothermic storage period [78]. On the other hand, hypothermic storage solutions do not routinely contain antifungal agents, even though most postkeratoplasty endophthalmitis and keratitis cases are of fungal origin. Recent studies suggest that the addition of the antifungal agent amphotericin B in Optisol GS significantly improves the activity against the contamination with candida species albeit with the expense of increased toxicity against the corneal endothelial cells [86].
The main advantages of hypothermic storage are the simplicity of the technique and the low cost due to minimal equipment requirements and minimal handling. The storage solutions are readily available and easy to transport to procurement sites making possible the recovery of corneas from donors even in remote areas. As a consequence, the availability of corneas is dramatically increased while the eye bank processing can be performed without sophisticated infrastructure and with minimal training of the personnel.
The storage in organ culture medium
Summerlin and colleagues [87] were the first to report the preservation of corneas in organ culture medium at 370 C for 4 weeks. This was shortly followed by the successful transplantation of organ cultured corneas by Doughman and colleagues [88]. This preservation method, although pioneered by American ophthalmologists, predominates now days in Europe and in Australia. Of the 62 eye banks included in the 2010 European Eye Bank Association Directory, 47 used organ culture, 9 used hypothermic storage and 6 used both methods [63]. Organ culture allows a significantly longer period of preservation which is typically 4 weeks [53] although successful transplantations with the use of corneas preserved even for 7 weeks have also been reported [89]. The extended storage period comes however at a cost of more complicated technique in comparison to hypothermia. The corneas are stored in an incubator at 30-370C. The storage solution consists of tissue culture medium (most commonly Eagle’s essential medium), supplemented with 2-10% fetal or newborn calf serum, antibiotics (mostly penicillin and streptomycin) and antifungal agents (amphotericin B) [78]. The presence of calf serum has raised concerns about possible transmission of Creutzfeldt-Jacobs prion protein during the periods of disease outbreak and alternative animal product-free solutions have been successfully tested [90-92]. Nevertheless, the common practice of including calf serum in the organ culture medium has not been substantially changed. The majority of the eye banks change the culture medium every 1-2 weeks while the rest keep the same solution for the whole storage period. Since dehydration macromolecules are ingested from corneal cells at these storage temperatures, they are not added in the solution. Therefore, the cornea swells to about twice its normal thickness. The corneal swelling has to be reversed before its use for transplantation. The corneal grafts are placed for this reason in a solution containing dextran. The same solution is used for the transport of the cornea. The extend of deswelling depends on the dextran concentration which varies between 4-8% in the different banks and the time varies also from less to one up to seven days. All the above minor differences in handling techniques are nevertheless associated with similar results in terms of graft outcomes [78]. The possible toxic effect of dextran due to unexpected penetration into the graft tissue along with the difficulty to prepare the solution has triggered recently the search for an alternative macromolecule [93]. Despite the initial encouraging results, the use of dextran solution remains the standard method for the reversal of corneal swelling prior to transplantation. The inspection of corneal endothelium under specular microscopy is not feasible with organ culture. Therefore, phase contrast or bright field light microscopy is necessary. To visualize the endothelial cells, swelling of the intercellular space with a hypotonic solution is required and it should be performed in aseptic conditions [94]. The swelling is very transient, lasting only a few minutes and is dependent on the storage time and on the particular composition of the storage medium [95]. The whole process is technically demanding and requires experienced observers for the interpretation of the images. Regarding microbiological safety, organ culture appears superior to hypothermic storage since the antibiotics and antifungal agents included in the storage solution are far more effective at 30-370C. In addition, contaminated tissue will be easily recognized since the micro-organisms will grow rapidly in this temperature. Screening through the examination of a sample of the medium normally takes place after 7 days in culture and just before the transplantation. So, the risk of transplanting contaminated tissue is minimized since up to 3.5% of the corneas in organic culture have been reported to be discarded due to contamination [96]. These corneas would probably have been distributed for transplantation if they had been stored under hypothermic conditions. The incidence of endophthalmits has been reported as low as 0.1% after storage in organ culture medium [78].
The main advantage of organ culture is the length of storage period. This 4-week period permits the implementation of microbiological testing for safety, as well as a more thorough evaluation of the corneal endothelium also after vital staining such as with tryphan blue for the more accurate recognition of necrotic cells. Given the great variability regarding endothelial loss among stored corneas, organ culture is considered a ``stress test`` for the recognition of those graft with irreversibly affected vitality [97]. This way, the most suitable corneas for each procedure can be selected. Moreover, the capacity of corneal endothelium for self-repair is maintained only with organ culture [98]. On the other hand, maintenance in organ culture medium is definitely a more complicated preservation method with a higher cost compared to the hypothermic storage (Table 2). Nevertheless, despite their differences, the few studies which have compared the two methods in terms of clinical outcome and of post-operative decline in endothelial cell density, have demonstrated similar results showing this way no definite superiority of one method against the other [99,100].
Table 2. Hypothermic storage versus storage in organ culture medium
Hypothermic Storage (2-60C) |
Storage in Organ Culture Medium (30-370C) |
Most common storage method in USA and in Asia |
Most common storage method in Europe and in Australia |
Storage period: 7-10 days |
Storage period: 4-6 weeks |
Offers thin grafts ready to use |
Pre-operative deswelling of the graft is necessary |
Minimal bacterial growth in low temperatures.
Antibiotics added provide coverage after intra-operative warming.
Antifungal agents are not added in the solution |
At 30-37°C the graft contamination becomes more obvious and the antibiotics and the antimycotics are more effective.
The risk of transplanting contaminated tissue is reduced |
The more easily transported hypothermic medium to procurement sites facilitates getting more donor corneas from remote areas. |
More thorough evaluation of the stored corneas permits selecting the most suitable cornea for each procedure |
Simplicity in equipment, no need for sophisticated staff training, lower cost |
Technical complexity, need for qualified staff, higher cost |
In conclusion, corneal transplantation or keratoplasty remains the only available and effective therapy of corneal blindness worldwide. Trauma and genetic or degenerative ocular disorders have currently replaced infectious diseases as the main indications of keratoplasty in the western world countries. In the developing world, though, eye infections remain the main reason to perform keratoplasties. The method has also evolved over time from the initial full thickness transplantation (penetrating keratoplasty) to the selective replacement of corneal endothelium and Descemet's membrane only (lamellar or endothelial keratoplasty). A turning point in the history of keratoplasty was the development of eye banks. Eye banking allows the storage of donor corneal grafts by preserving the integrity of corneal endothelium, a critical factor for a successful long-term outcome of keratoplasty. A major limitation of this exciting therapeutic modality is, however, the shortage of human donor corneas. For this reason, the appropriate storage and the preservation of the quality of every single available corneal graft are of paramount importance. Since corneal endothelial cells have a critical role in the maintenance of corneal transparency, it is not surprising that the main goal of corneal preservation through eye banking is to minimize the unavoidable time-dependent decline in endothelial cell density. Hypothermic storage and storage in organ culture medium are the two currently in use preservation techniques. Hypothermic storage limits the graft preservation to a maximum of 10-14 days. It is a simple method of low-cost using solutions readily available and permitting the recovery of corneas from donors even in remote areas. The alternative storage technique in organ culture medium is more costly and technically more complex. It is however, associated with a reduced risk of graft contamination and offers a substantially longer storage period of 4-6 weeks. Despite their differences, it has not been shown any superiority of one method against the other and their use remains for each eye bank a matter of preference and of resource availability. Nevertheless, only a new method combining the advantages of both, by further expanding the time limits of storage and preserving at the same time the integrity of corneal endothelium at a reasonable cost, could effectively help the clinicians to overcome the problem of graft shortage. Research efforts in this field are urgently needed.
We thank Dr. Filip Filev for his valuable medical advice and Mrs. Sibylle Altenahr for her technical information and counselling. Please note that this paper is based on an expanded version of the introduction of the medical thesis of Ismini Koulouri.
- Lamm V, Hara H, Mammen A, Dhaliwal D, Cooper DK, et al. (2014) Corneal blindness and xenotransplantation. Xenotransplantation 21: 99-114.
- Oliva MS, Schottman T, Gulati M (2012) Turning the tide of corneal blindness. Indian J Ophthalmol 60: 423-427.
- Whitcher HP, Srinivasan M, Upadhyay MP (2001) Corneal blindness: a global perspective. Bull World Health Organ 79: 214-221
- Thomas PA, Geraldine P (2007) Infectious keratitis. Curr Opin Infect Dis 20: 129-141.
- Arentsen JJ, Morgan B, Green WR (1976) Changing indications for keratoplasty. Am J Ophthalmol 81: 313-318.
- Pahor D, Gracner B, Falez M, Gracner T (2007) Changing indications for penetrating keratoplasty over a 20-years period, 1985-2004. Klin Monbl Augenheilkd 224: 110-114.
- Wang J, Hasenfus A, Schirra F, Bohle RM, Seitz B, et al. (2013) Changing indications for penetrating keratoplasty in Homburg / Saar from 2001 to 2010-histopathology of 1,200 corneal buttons. Graefes Arch Clin Exp Ophthalmol 251: 797-802.
- Damji KF, Rootman J, White VA, Dubord PJ, Richards JS (1990) Changing indications for penetrating keratoplasty in Vancouver, 1978-1987. Can J Ophthalmol 25: 243-48.
- Kang PC, Klintworth GK, Kim T, Carlson AN, Adelman R, et al. (2005) Trends in the indications for penetrating keratoplasty, 1980–2001. Cornea 24: 801-803.
- Le R, Yucel N, Khattak S, Yucel YH, Prud’homme GJ, et al. (2016) Current indications and surgical approaches to corneal transplants at the University of Toronto: A clinical-pathological study. Can J Ophthalmol 52: 74-79.
- Dekaris I (2013) Current trends in corneal transplantation. Medical Sciences 39: 35-46.
- Rahman I, Carley F, Hillarby C, Brahma A, Tullo AB, et al. (2009) Penetrating keratoplasty: indications, outcomes and complications. Eye 23: 1288-1294.
- Feizi S, Zare M (2011) Current approaches for management of postpenetrating keratoplasty astigmatism. J Ophthalmol 708736.
- Purcell JJ, Jr, Krachmer JH, Doughman DJ, Bourne WM (1982) Expulsive hemorrhage in penetrating keratoplasty. Ophthalmology 89: 41-43.
- Henein C, Nanavaty MA (2017) Systematic review comparing penetrating keratoplasty and deep anterior lamellar keratoplasty for management of keratoconus. Cont Lens Anterior Eye 40: 3-14.
- Patel AK, Scorcia V, Kadyan A, Lapenna L, Ponzin D, et al. (2012) Microkeratome-assisted superficial anterior lamellar keratoplasty for anterior stromal corneal opacities after penetrating keratoplasty. Cornea 31: 101-105.
- Shousha MA, Yoo SH, Kymionis GD, Ide T, Feuer W, et al. (2011) Long-term results of femtosecond laser-assisted sutureless anterior lamellar keratoplasty. Ophthalmology 118: 315-323.
- Karimian F, Feizi S (2010) Deep anterior lamellar keratoplasty: Indications, surgical techniques and complications. Middle East Afr J Ophthalmol 17: 28-37.
- Yeung SN, Lichtinger A, Kim P, Amiran MD, Rootman DS (2012) Retrospective contralateral study comparing deep anterior lamellar keratoplasty with penetrating keratoplasty: a patient’s perspective. Can J Ophthalmol 47: 360-364.
- Mosca L, Fasciani R, Mosca L, Guccione L, Legrottaglie EF, et al. (2011) Graft rejection after femtosecond laser--assisted deep anterior lamellar keratoplasty: report of 3 cases. Cornea 30: 912-6.
- Sharma N, Kandar AK, Singh Titival J (2013) Stromal rejection after big bubble deep anterior lamellar keratoplasty: case series and review of the literature. Eye Contact Lens 39: 194-198.
- Price MO, Price FW Jr (2006) Descemet’s stripping with endothelial keratoplasty. Comparative outcomes with microkeratome-dissected and manually dissected donor tissue. Ophthalmology 113: 1936-1942.
- Covert DJ, Koenig SB (2007) Descemet stripping and automated endothelial keratoplasty (DSAEK) in eyes with failed penetrating keratoplasty. Cornea 26: 692-696.
- Price MO, Price FW Jr (2007) Descemet stripping with endothelial keratoplasty for treatment of iridocorneal endothelial syndrome. Cornea 26: 493-497.
- Maier P, Reinhard T, Cursiefen C (2013) Descemet Stripping Endothelial keratoplasty-Rapid recovery of visual acuity. Dtsch Arztebl Int 110: 365-371.
- Shulman J, Kropinak M, Ritterband DC, Perry HD, Seedor JA, et al. (2009) Failed Descemet-stripping automated endothelial keratoplasty grafts: a clinicopathologic analysis. Am J Ophthalmol 148: 752-759.
- Terry MA, Straiko MD, Veldman PB, Talajic JC, VanZyl C, et al. (2015). Standardized DMEK Technique: Reducing Complications Using Prestripped Tissue, Novel Glass Injector, and Sulfur Hexafluoride (SF6) Gas. Cornea 34: 845-852.
- McCauley MB, Price MO, Feirchild KM, Price DA, Price FW Jr, et al. (2011) Prospective study of visual outcomes and endothelial survival with Descemet membrane automated endothelial keratoplasty. Cornea 30: 315-319.
- Ang M, Wilkins MR, Mehta JS, Tan D (2016). Descemet membrane endothelial keratoplasty. Br J Ophthalmol 100: 15-21.
- Busin M, Madi S, Santorum P, Scorcia V, Beltz J, et al. (2013) Ultrathin descemet's stripping automated endothelial keratoplasty with the microkeratome double-pass technique: two-year outcomes. Ophthalmology 120: 1186-1194.
- Doughman DJ (1998) Corneal tissue preservation, chapter 34. In: Leibowitz HM, Waring GO. Corneal Disorders: Clinical Diagnosis and Management. 2nd ed. Philadelphia, PA: WB Saunders Co 871-872.
- Filatov VP (1937) Transplantation of the cornea from preserved cadavers’ eyes. Lancet 229: 1395-7.
- Chu W (2000) The past 25 years in eye banking. Cornea 19: 754-765.
- Payne JW (1980) New directions in eye banking. Trans Am Ophthalmol Soc 78: 983-1026.
- Wilson SE, Bourne WM (1989) Corneal preservation. Surv Ophthalmol 33: 4.
- McCarey BE, Kaufman HE (1974) Improved corneal storage. Invest Ophthalmol 13: 165-173.
- Boyd B (2010) Chapter 22. The Cornea. Part VI Eye Banks. In: Boyd B. Modern Ophthalmology: The Highlights. Jaypee Highlights Medical Publishers, Inc.; 3rd revised edition 318.
- http://www.eeba.eu/article/The%2BEEBA/c/1
- Gain P, Jullienne R, He Z, Aldossary M, Acquart S, et al. (2016). Global survey of corneal transplantation and eye banking. JAMA Ophthlamol 134: 167-173.
- Nishida T (2005) Cornea. In: Krachmer J, Mannis M, Holland E, editors. Cornea: Fundamentals, Diagnosis and Management. Vol 1. Philadelphia, PA: Elsevier-Mosby 3-26.
- Polisetti N, Joyce NC (2013). The culture of limbal stromal cells and corneal endothelial cells. Methods Moll Biol 1014: 131-139.
- Bahn CF, Falls HF, Varley GA, Meyer RF, Edelhauser HF, et al. (1984) Classification of corneal endothelial disorders based on neural crest origin. Ophtalmology 91: 558-563.
- Bahn CF, Glassman RM, MacCallum DK, Lillie JH, Meyer RF, et al. (1986) Postnatal development of corneal endothelium. Invest Ophthalmol Vis Sci 27: 44-51.
- Nucci P, Brancato R, Mets MB, Shevell SK (1990). Normal endothelial cell density range in childhood. Arch Ophtalmol 108: 247-248.
- Yee RW, Matsuda M, Schultz RO, Edelhauser HF (1985) Changes in the normal corneal endothelial cellular pattern as a function of age. Curr Eye Res 4: 671-678.
- Joyce NC (2003) Proliferative capacity of the corneal endothelium. Pro Retin Eye Res 22: 359-389
- Johnson DH, Bourne WM, Campbell RJ (1982). The ultrastructure of Descemet's membrane. II. Aphakic bullous keratopathy. Arch Ophthalmol 100: 1948-1951.
- Meek K, Knupp C (2015) Corneal structure and transparency. Prog Retin Eye Res 49: 1-16.
- Bourne WM (2003) Biology of the corneal endothelium in health and disease. Eye 17: 912-918.
- Mergler S, Pleyer U (2007) The human corneal endothelium: New insights into electrophysiology and ion channels. Prog Retin Eye Res 26: 359-378.
- Yee RW, Matsuda M, Schultz RO, Edelhauser HF (2011) Human corneal endothelial cell expansion for corneal endothelium transplantation: An overview. Transplantation 91: 811-819.
- Bourne WM, Nelson LR, Maguire LJ, Baratz KH, Hodge DO, et al. (2001) Comparison of Chen medium and Optisol-GS for human corneal preservation at 4°C. Results of transplantation. Cornea 20: 683-686.
- Pels E, Schuchard Y (1983) Organ-culture preservation of human corneas. Doc Ophtalmol 56: 147-153.
- Albon J, Tullo AB, Aktar S, Boulton ME (2000) Apoptosis in the endothelium of human corneas for transplantation. Invest Opthalmol Vis Sci 41: 2887-2893.
- Bourne WM, Hodge DO, Nelson LR (1994) Corneal endothelium five years after transplantation. Am J Ophthalmol 118: 185-196.
- Ing JJ, Ing HH, Nelson LR, Hodge DO, Bourne WM, et al. (1998) Ten-year postoperative results of penetrating keratoplasty. Ophthalmology 105: 1855-1865.
- Nishimura JK, Hodge DO, Bourne WM (1999) Initial endothelial cell density and chronic endothelial loss rate in corneal transplants with late endothelial failure. Ophthalmology 106: 1962-1965.
- Eastlund T (1995) Infectious disease transmission through tissue transplantation: Reducing the risk through donor selection. Cell Tranplant 4: 455-477J.
- Armitage WJ, Tullo AB, Ironside JW (2009) Risk of Creutzfeldt-Jakob disease transmission by ocular surgery and tissue transplantation. Eye (Lond) 23: 1926-1930.
- EEBA (2016) Minimum Medical Standards. Revision 2. Operative from 1.1.2016. http://eeba.eu/downloads/EEBA%20Minimum%20Medical%20StandardsRev02-2016.pdf.
- EBAA (2011) Medical Standards. Published by: EBAA 1015 18th Street, NW, Suite 1010, Washington, DC 20036, USA October 2011. http://restoresight.org/wp-content/uploads/2011/11/Medical-Standards-October-2011.pdf
- Price M, Price F (2012) Monitoring and maintaining endothelial cell health. Expert advice on diagnostic tests and their clinical relevance. Ophthalmology Management 16: 40-44.
- Writing Committee for the Cornea Donor Study Research Group, Mannis MJ, Holland EJ, Gal RL, Dontchev M, et al. (2013). The effect of donor age on penetrating keratoplasty for endothelial disease: graft survival after 10 years in the Cornea Donor Study. Ophtalmology 120: 2419-2427.
- Claerhout I, Maas H, Pels E (2010) European Eye Bank Association Directory Report, 18th ed.2010. www.europeraneyebanks.org.
- Armitage WJ, Dick AD, Bourne WM (2003) Predicting endothelial cells loss and long-term corneal graft survival. Invest Ophthalmol Vis Sci 44: 3326-3331.
- Patel SV (2011) Graft survival after penetrating keratoplasty. Am J Ophthalmol 151: 397-398.
- Ehlers N, Sperling S, Olsen T (1982) Post-operative thickness and endothelial cell density in cultivated, cryopreserved human corneal grafts. Acta Ophthalmol (Copenh) 60: 935-944.
- Kaufmann HE, Escapini H, Capella JA, Robbins JE, Kaplan M, et al. (1966) Living preserved corneal tissue for penetrating keratoplasty. Arch Ophthalmol 76: 471-476.
- McCarey BE, Kaufman HE (1974) Improved corneal storage. Invest Ophtalmol Vis Sci 13: 165-173.
- Brunette I, Le François M, Tremblay MC, Guertin MC (2001) Corneal transplant tolerance of cryopreservation. Cornea 20: 590-596.
- Chen W, Lin Y, Zhang X, Wang L, Liu M, et al. (2010) Comparison of fresh corneal tissue versus glycerin-cryopreserved corneal tissue in deep anterior lamellar keratoplasty. Invest Ophthalmol Vis Sci 51: 775-781.
- Jang JH, Chang SD (2011) Tectonic deep anterior lamellar keratoplasty in impending corneal perforation using cryopreserved cornea. Korean J Ophthalmol 25: 132-135.
- Shi W, Liu M, Gao H, Li S, Wang T, et al. (2009) Penetrating keratoplasty with small-diameter and glycerin-cryopreserved grafts for eccentric corneal perforations. Cornea 28: 631-637.
- Halberstadt M, Böhnke M, Athmann S, Hagenah M (2003) Cryopreservation of human donor corneas with dextran. Invest Opthalmol Vis Sci 44: 5110-5115.
- Marquez-Curtis LA, McGann LE, Elliott JAW (2017) Expansion and cryopreservation of porcine and human corneal endothelial cells. Cryobiology 77: 1-13.
- Powers RM, Linden JV (2016) Tissue Banking. In: Rossi’s Principles of Transfusion Medicine. Simon TL, McCullough J, Snyder EL, Solheim BG, Strauss RG (Editors). Wiley Blackwell 474
- Armitage WJ (2011) Preservation of human cornea. Transfus Med Hemother 38: 143-147.
- Armitage WJ (2008) Developments in corneal preservation. In: Cornea and external eye diseases. Reinhard T, Larkin F. (editors). Springer-Verlag Berlin Heidelberg 103-104.
- Pels E, Beele E, Claerhout I (2008) Eye bank issues: II. Preservation techniques: warm versus cold storage. Int Ophthalmol 28: 155-163.
- Szafilk J, Liberek I, Brix M (2000) Corneal storage methods. Transplantation Proceedings 32: 1424-1425.
- Lindstrom RL, Kaufman HE, Skelnik DL, Laing RA, Lass JH, et al. (1992) Optisol corneal storage medium. Am J Opthalmol 114: 345-356.
- Wagoner MD, Gonnah el-S (2005) Corneal graft survival after prolonged storage in Optisol - GS. Cornea 24: 976-979.
- Soni NG, Hoover CK, Da Silva H, Jeng BH (2015) Preservation of the corneal epithelium in different corneal storage media. Cornea 34: 1400-1403.
- Basak S, Prajna NV (2016) A prospective, in vitro, randomized study to compare two media for donor corneal storage. Cornea 35: 1151-1155.
- Kanavi MR, Ali Javadi MA, Chamani T, Fahim P, Javadi F, et al. (2015) Comparing quantitative and qualitative indices of the donated corneas maintained in Optisol-GS with those kept in Eusol-C. Cell Tissue Bank 16: 243-247.
- Layer N, Cevallos V, Maxwell AJ, Hoover C, Keenan JD, et al. (2014) Efficacy and safety of antifungal additives in Optisol-GS corneal storage medium. JAMA Ophthalmol 132: 832-837.
- Summerlin WT, Miller GE, Harris JE, Good RA (1973) The organ-cultured cornea: an in vitro study. Invest Ophthalmol Vis Sci 12: 176-180.
- Doughman DJ, Harris JE, Schmitt MK (1976) Penetrating keratoplasty using 370C organ cultured cornea. Trans Sect Ophthalmol Am Acad Ophthalmol Otolaryngol 81: 778-793.
- Ehlers H, Ehlers N, Hjortdal JO (1999) Corneal transplantation with donor tissue kept in organ culture for 7 weeks. Acta Ophthalmol Scand 77: 277-278.
- Bednarz J, Doubilei P, Wollnik P, Engelmann K (2001) Effect of three different media on serum free culture of donor corneas and isolated human corneal endothelial cells. Br J Ophthalmol 85: 1416-1420.
- Hempel B, Bednarz J, Engelmann K (2001) Use of a serum-free medium for long-term storage of human corneas. Influence on endothelial cell density and corneal metabolism. Graefes Arch Clin Exp Ophtalmol 239: 801-805.
- Smith VA, Johnson T (2010) Evaluation of an animal product-free variant of MegaCellTMMEM as a storage medium for corneas destined for transplantation. Ophtalmic Res 43: 33-42.
- Smith V, Johnson T (2012) Identification and evaluation of a thinning agent compatible with MegaCell DCS, an animal product-free corneal storage medium. Graefes Arch Clin Exp Ophthalmol 250: 1777-1786.
- Sperling S (1986) Evaluation of the endothelium of human donor corneas by induced dilatation of intercellular spaces and trypan blue. Graefe’s Arch Clin Exp Ophthalmol 224: 428-434.
- Thuret G, Manissolle C, Herrag S, Deb N, Cambos-Guyotat L, et al. (2004) Controlled study of the influence of storage medium type on endothelial assessment during corneal organ culture. Br J Ophthalmol 88: 579-581.
- Pels L (1997) Organ culture: the method of choice for preservation media. Br J Ophthalmol 81: 523-525.
- Böhnke M (1991) The Hamburg system of corneal preservation. Klin Mbl Augenheil 198: 562-571.
- Nejepinska J, Juklova K, Jirsova K (2010) Organ culture, but not hypothermic storage, facilitates the repair of the corneal endothelium following mechanical damage. Acta Ophthalmol 88: 413-419.
- Rijneveld WJ, Beekhuis WH, Van Rij G, Rinkel-van Driel B, Pels E, et al. (1982) Clinical comparison of grafts stored in Mc Carey-Kaufman medium at 480C and in corneal organ culture at 310C. Arch Ophthalmol 110: 203-205.
- Rijneveld WJ, Remeijer L, van Rij G, Beekhuis H, Pels E, et al. (2008) Prospective clinical evaluation of McCarey-Kaufman and organ culture cornea preservation media: 14-year follow-up. Cornea 27: 996-1000.


