We focused on the importance of procedure strategy for the planning of treatment in a patient with calcified occlusive abdominal aorta with bilateral renal artery stenosis showing intermittent claudication. A 76-year-old man was admitted to our hospital with acute decompensated heart failure. He had a history of hypertension, dyslipidaemia, chronic kidney disease, smoking habit, heavy alcohol intake, and coronary artery bypass graft surgery 6 years ago. Examination revealed that the abdominal aorta was nearly completely occluded due to a large calcified nodule involving bilateral renal arteries. Because he suffered from bilateral intermittent claudication, we decided to undertake revascularization of the abdominal aorta and left renal artery by endovascular treatment (EVT) in a single session. We aimed to secure disappearance of the pressure gradient, but not full distension of the abdominal aorta. We undertook EVT with balloon-expandable stent in order to maintain superior mesenteric artery flow and to ensure firm dilation of the left renal artery. For the calcified abdominal aorta, we tried to construct bidirectional routes of abdominal aortic blood flow antegradely and retrogradely. Our technique is less invasive than bypass surgery and can be performed safely under local anaesthesia using percutaneous access.
endovascular treatment, calcified occlusive abdominal aorta, renal artery stenosis
Systemic atherosclerotic lesions are not rare in patients with multiple risk factors for atherosclerosis. In some cases, diffuse, heavily calcific lesions of the infrarenal abdominal aorta are treated with aortobifemoral bypass surgery, but not with balloon-expandable or self-expandable stents, because of the risk of incomplete apposition or aortic rupture. Recently, aortic stent grafts have become feasible in endovascular treatment (EVT) of these patients. However, in a patient with juxtarenal aortic occlusion disease, simple EVT with stent grafts is not applicable [1,2]. We report on a rare case of calcified occlusive abdominal aorta with bilateral renal artery stenosis with symptomatic intermittent claudication. We focused on the importance of procedure strategy for the planning of treatment in this case.
A 76-year-old man had been admitted to another hospital with acute decompensated heart failure (ADHF) but discharged himself before improvement of his symptoms. However, 2 weeks after his discharge, dyspnoea on exertion became worse, and leg edema appeared. He visited our hospital and was admitted as an emergency. He had a history of hypertension, dyslipidaemia, chronic kidney disease, and coronary artery bypass graft (Left internal thoracic artery- left anterior descending artery [LITA-LAD], saphenous vein graft [SVG]-#4PL, SVG-#4PD) surgery 6 years ago. He had a longstanding smoking habit (20/day for 50 years) and indulged in heavy alcohol intake.
After his admission, during decongestion, he showed flush pulmonary edema with bacterial infection. Heart failure had recovered after intensive care therapy; however, detailed examination revealed atherosclerotic lesions of bilateral renal arteries and the abdominal aorta. The abdominal aorta was nearly completely occluded due to a large calcified nodule involving bilateral renal arteries (Figure 1). The right renal artery was occluded, and the left renal artery had severe stenosis consisting of calcified deposits. In particular, the left renal artery needed urgent treatment to maintain his hemodynamic. Additionally, he suffered from Rutherford category class 3 (his ankle-brachial index was 0.62/0.52) bilateral intermittent claudication; therefore, the abdominal aorta also needed treatment. Considering his impaired cardiac function (left ventricular ejection fraction 38%), open surgery carried a grave risk of perioperative cardiovascular events, and we decided to attempt revascularization of the abdominal aorta and left renal artery by EVT, concurrently.
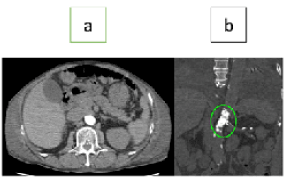
Figure 1. Abdominal plain computed tomography (calcified lesion of abdominal aorta) (a) Axial section, (b) Coronal section
We set the guiding sheath antegradely (Destination 6Fr 45 cm, Terumo, Tokyo, Japan) via the left brachial artery and retrogradely (Ansel 7Fr 55 cm, COOK Japan, Tokyo, Japan) via the right common femoral artery, and imaging catheter (pigtail 4Fr 110 cm, Terumo) via the right radial artery. The guidewire was crossed to the left renal artery antegradely (Cruise 235 cm, Asahi Intecc, Aichi, Japan), and to the abdominal aorta retrogradely (VASSALLO G40, Cardinal Health Japan, Tokyo, Japan) through the calcified lesion using a micro-catheter (Corsair Armet 90 cm, Asahi Intecc). First, we performed vessel preparation for both the aorta and the left renal artery (SHIDEN HP 3×40 mm, Kaneka Medix, Osaka, Japan) and made cracks in the calcified lesions (cutting balloon 5×20 mm, Boston Scientific Japan, Tokyo, Japan). Second, we delivered a balloon-expandable stent (Expess LD 7×57 cm, Boston Scientific Japan) to the left renal artery without dilatation using the support of a guide extension (Guidezilla PV 6Fr LONG, Boston Scientific Japan). Third, we performed size upped dilatation for the abdominal aorta (Ultraverse 10×40 mm, Bard Medical, Covington, USA). Then, we deployed a self-expandable stent (S.M.A.R.T. control 14×60 mm, Cardinal Health Japan). After that, we deployed a renal stent for the left renal artery using the kissing balloon technique. Finally, we checked that the pressure gradients of the left renal artery and the abdominal aorta had been nullified. Thus, we succeeded in revascularization of both the left renal artery and the abdominal aorta in a single session (Figure 2). One year after EVT, the patient had no recurrence of heart failure and his ankle-brachial index was 1.14/1.10.
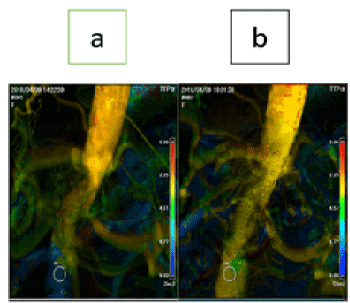
Figure 2. Parametric imaging (time to peak) – Angiogram (a) Pre endovascular treatment, (b) Post endovascular treatment
Written informed consent for patient information and images to be published was provided by the patient.
Although occlusion of the right renal artery had previously been detected, calcified partial occlusion of the abdominal aorta and severe stenosis of the left renal artery were observed during the admission for ADHF. These findings were uncommon clinical entities and were identified as an advanced stage of a severe chronic obliterative atherosclerotic process in the aorta [3,4]. Revascularization of renal artery stenosis in patients with overt heart failure is recommended [5]. In patients with infrarenal aortic artery occlusion without renal artery lesions, aortic stent grafts are available [6]. In this case, revascularization of the left renal artery was essential because of right renal artery occlusion. In view of the significant perioperative morbidity and mortality of surgical treatment (owing to the low ejection fraction and chronic kidney disease) [7,8] and intermittent claudication, we adopted EVT in a single session. In the subset of patients in whom the stenosis is more diffuse and not focal in origin, the usefulness of traditional techniques may be limited. Although stent graft was available for revascularization of the renal artery, we undertook EVT with a balloon-expandable stent to ensure preservation of superior mesenteric artery flow and firm dilation of left renal artery. For the treatment of calcified abdominal aorta, over-extension of the affected part of the aorta was avoided to prevent aortic rupture. We also attempted to construct bidirectional routes of the abdominal aortic blood flow retrogradely to avoid causing deformity of the renal artery stent. Our aim was to abolish the pressure gradient, but not to achieve full distension of the abdominal aorta. This technique is less invasive than bypass surgery and can be performed safely under local anesthesia using percutaneous access.
In this case, acute heart failure and the past medical history of coronary artery bypass graft surgery were a clue to the presence of systemic atherosclerotic disease, especially calcified occlusion of the abdominal aorta with bilateral renal artery stenosis. Progression of chronic renal disease and hypertension, possibly due to renal artery involvement (renovascular hypertension), may have provoked the acute onset of heart failure. The presence of intermittent claudication may also be a manifestation of the progression of the calcified occlusive lesion of the abdominal aorta. Since ankle-brachial index was maintained within normal limits one year after EVT in this case, the procedural strategy used for the treatment may be correct. However, further follow-up examination is needed in this patient, who had complicated lesions. In patients with multiple risk factors for atherosclerosis, the measurement of ankle-brachial index is important in order to detect lesions early, and hence to enable effective and timely treatment.
In a patient with almost completely occluded calcified abdominal aorta involving bilateral renal arteries and suffering from bilateral intermittent claudication, we undertook EVT with balloon-expandable stent in order to preserve superior mesenteric artery flow and firm dilation of the left renal artery. We attempted bidirectional access routes to the abdominal aorta antegradely and retrogradely. Our technique is less invasive than bypass surgery and can be performed safely under local anaesthesia using percutaneous access.
None
None
- Onal B, Ilgit ET, Yücel C (1998) Primary stenting for complex atherosclerotic plaques in aortic and iliac stenoses. Cardiovasc Intervent Radiol 21: 386-392.
- Karkos CD, D'Souza SP, Hughes R (2000) Primary stenting for chronic total occlusion of the infrarenal aorta. J Endovasc Ther 7: 340-344.
- Holfeld J, Gottardi R, Zimpfer D (2008) Treatment of symptomatic coral reef aorta by endovascular stent-graft placement. Ann Thorac Surg 85: 1817-1819.
- Uberoi R, Tsetis D (2007) Standards for the endovascular management of aortic occlusive disease. Cardiovasc Interv Radiol 30: 814-819.
- Prince M, Tafur JD, White CJ (2019) When and how should we revascularize patients with atherosclerotic renal artery stenosis? JACC Cardiovasc Interv 12: 505-517.
- Chehab B, Vamanan K, Gupta K (2012) Percutaneous treatment of severe diffuse stenosis in heavily calcified infrarenal abdominal aorta using iliac extender endoprosthesis: a case series. Catheter Cardiovasc Interv 79: 439-443.
- Eliason JL, Clouse WD (2007) Current management of infrarenal abdominal aortic aneurysms. Surg Clin North Am 87: 1017-1033.
- Schaefer PJ, Mueller-Huelsbeck S, Lukas R (2008) Low-profile primary stent placement for the treatment of focal calcified ulcerated stenosis in the infrarenal aorta. J Vasc Interv Radiol 19: 182-188.


