Abstract
This correspondence concerns our publication: de Almeida AF, De Gaspari E. Pathog Dis. 2018 Feb 1;76(1) with results obtained using mouse model that analyzed the passage of IgG antibodies with high avidity through the placenta described for the first time for Neisseria meningitidis B. Below, we explain the importance of results obtained by calling attention to check vaccines for protection in this age group with a high number of cases, including São Paulo, Brazil. Thus, further studies on the use of maternal immunization and vaccines for protection against this disease are needed.
I would like to show the importance of translational research as in different diseases , especially in studies of vaccines to N.meningitidis as existing studies to Bordetella pertussis described in literature.
Key words
translational research, neisseria meningitis vaccines, maternal immunization
Meningococcal disease (MD) is present worldwide. Serogroup B is responsible for a large proportion of cases. Although people with less than 4 years of age are affected by meningococcal serogroup B, vaccine efficiency was not demonstrated in this age group. MD caused predominantly by Neisseria meningitidis serogroups A,B and C occurs predominantly in young children and remains a substantial cause of morbidity and mortality worldwide. In developed countries, the invasive meningococcal disease occurs primarily in infants aged less than 1 year, reaching a peak at around 6 months, with circulating maternal antibodies specific to bacterium decline [1].
As we know, the evaluation of new MD vaccines for over 40 years serum bactericidal (SBA) has been shown to be the most important serologic correlate to the effectiveness of vaccine protection. However, in infants, there was no evidence of a significant increase of SBA in response to vaccination after 2 or 3 vaccine doses to schemes with an epidemic Chilean strain, suggesting lack of protection regarding the vaccine evaluated in this age group [2].
Neisseria meningitidis serogroup B is the most common cause of meningococcal disease epidemic in developed countries. In an attempt to control the epidemic of meningococcal serogroup B in São Paulo, Brazil, during 1989 and 1990, Cuban-produced outer-membrane proteins of meningococcal serogroup B vaccine were aplied to approximately 2-4 million children aged 3 months-6 years [3]. However, the vaccine failed, as the vaccine previously tested in Chile [2].
While vaccines have been tremendously successful in reducing the incidence of serious infectious diseases, newborns remain particularly vulnerable in the first few months of life to life-threatening infections. There are a number of challenges to neonatal vaccination.Although the success of pediatric immunization for the prevention of infection in the early years of life is a testament of the capacity of adaptive immunity in infants to respond to vaccination, antibody responses at this age group differ from those of adults [4]. Recent studies have indicated that neonatal vaccination may be an effective strategy for protecting against early-life infections such as influenza, respiratory syncytial virus, and pertussis. Bacillus Calmette-Guerin (BCG), a live vaccine against tuberculosis, demonstrates that a single vaccine dose administered at birth can in principle provide lifelong protection [5,6].
Almost all vaccines work through induction of serum or mucosal antibodies, especially in young infants, where the lack of previous antigen exposure limits the effectiveness of T-cell responses [7]. Cell immunity is also required for protection against disseminated disease and recovery from measles and smallpox [8]. CD4+ T cells, especially follicular B-helper T cells (TFH), are instrumental in helping B cells to produce antigen-specific antibodies. Also, Th1- and cytotoxic T lymphocyte-mediated immunity is critical for protection against intracellular infections, as observed for BCG vaccine [8,9].
In general, antibody responses in infants are weaker and of shorter duration than those in adults. This is the case for meningococcal protein vaccines, where there is also evidence that potentially protective bactericidal antibody responses in infants are poorly cross-reactive among variants of the same antigen compared to responses of immunologically mature age [10,11].
In developed countries, invasive meningococcal disease occurs primarily in infants less than 1 year of age [12], reaching a peak at around 6 months as circulating maternal antibodies are specific for bacterium decline [13]. And the age range is high in children younger than 2 year , as can be seen in Brazil http://www.saude.sp.gov.br/resources/cve-centro-de-vigilancia-epidemiologica (Figure 1).
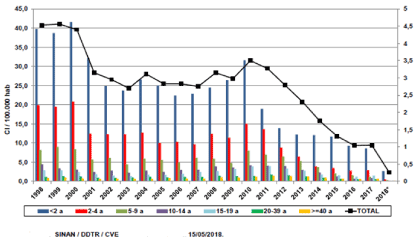
Figure 1. Meningococcal disease: incidence by age, state of Sao Paulo, 1998-2018
The rapid disease onset and the challenge of distinguishing it from other febrile illnesses in very young infants are particular problems and make prevention through prophylactic immunization the most attractive solution.Epidemiological evidence shows that although this disease most commonly occurs in infants, meningococcus is part of the nasopharyngeal microbiome and is mainly carried asymptomatically in young adults (12). This has implications for vaccination strategies that may be adopted to reduce infectious diseases [13].
For decades, the development of a vaccine has focused on protein antigens, subcapsular with outer membrane vesicles (OMV) for outbreaks and, more recently, used in multicomponent vaccines to offer better protection against antigenically different strains responsible for the disease during an epidemic. The inclusion of recombinant antigens is a strategy to overcome the immunogenicity of OMV and increase coverage. In the case of 4CMenB Bexsero, the vaccine consists of three recombinant antigens, identified for the first time by reverse vaccinology [14], formulated with detergent-extracted OMVs used in an outbreak-specific vaccine in New Zealand in 2004 [15].
Thus, the evidence for the efficacy of these vaccines against different strains and the contribution of specific protection antigens can only be provided by epidemiological analyses in large populations [15]. The recent inclusion of four components, meningococcus B (4CMenB) vaccine, Bexsero, in childhood immunization program in the UK has provided preliminary evidence that the vaccine is effective. Bexsero was approved in many countries around the world, which was subsequently included in various national or regional vaccination schedules. In September 2015, the United Kingdom included the vaccine into their national immunization program funded by the playground vaccines are approved based on serological criteria and not on evidence of direct protection, they offer the first opportunity to evaluate their impact against meningococcus [16].
Another circumstances vaccines recombinant antigen using the lipidated bivalent recombinant vaccine, FHbp rLP Trumenba® was approved by FDA for use at the age of 10-25 years, but it has yet to be approved in Europe. FHbp is expressed by the majority of isolates as an important virulence factor.
The antibodies produced against this antigen, therefore, potentially play a dual role in complementing activation, both being bactericide. Although FHbp is antigenically diverse, it can be broadly divided into two genetically distinct families, containing one representative from each [16-18].
Brazil has mandatory vaccination schedules, and since 2004, vaccination is the most effective way of preventing diseases. Brazil has evolved in recent years in this area, especially with the creation of the National Immunization Program (NIP), in 1973, which facilitated the access of the population to vaccines. The population has to be attentive to campaigns and the vaccination calendar, which corresponds to a set of priority vaccines for the country. They are all available free of charge in the public network. There are vaccination schedules to different populations: children, adolescents, adults, older adults and indigenous population.
Children, adolescents and adults need to attend health units in periods of campaign and take all the vaccines provided. Only with all vaccines, the population will be properly immunized, explains the coordinator of the National Immunization Program of the Ministry of Health. The campaigns follow these dates by the necessity of immunity from a group, so that all may be vaccinated at that moment.
Although there is no specific schedule, the female population has special attention, especially pregnant women. Women aged 12-49 years who have not received MMR (measles, mumps and rubella) in childhood should seek a health unit before pregnancy in order to prevent the transmission of rubella to the baby.
The double-adult and vaccine against hepatitis B should also be administered to newborns so they do not run the risk of suffering from diseases such as neonatal tetanus and hepatitis B. Pregnant women are also part of the target population of flu vaccine.
Technological advances in the production and introduction of new vaccines in the calendar of immunization campaigns make the work of research a priority in the health area. Advanced studies have contributed to the development of new products, since Brazil has technological mastery of the most modern vaccine generations.
In Brazil Oswaldo Cruz , Foundation, the main official vaccine and serum producers are: Ataulpho de Paiva, Fundation, Ezequiel Dias Fundation, Technology Institute of Parana, Vital Brazil Institute, Butantan Center of production and research.
The health system of Brazil is composed of two sub-sectors: public and private . The Basic Brazilian vaccination calendar is that defined by the National Immunization Program (NIP) and corresponds to the set of vaccines considered of priority interest to public health in the country. Currently, it consists of 12 recommended products to the population since birth until older age and distributed free of charge in vaccination stations of the public network [19].
The meningococcal vaccine B composed of recombinant proteins still generates doubts about its future use in the immunization schedule of the Ministry of Health of Brazil, because its priority and viability compared to others must be discussed. It requires the evaluation of criteria such as epidemiology, mortality, cost, target population, coverage expectation and economic planning.
The potential impact of maternal immunization as a public strategy to prevent disease in mothers and infants is well recognized. In addition, there are no vaccines currently approved or licensed specifically for use in pregnant women. Licensed vaccines that are recommended for non-pregnant adults may be administered to pregnant women based on the risk/benefit assessment. When the risk of exposure and disease from a vaccine preventable infection is high for mother and/or fetus, and an effective vaccine is available, the benefit of the vaccine protection is greater than any potential theoretical risk from the vaccine, which is in turn considered to be lower than the risk of acquiring the infection and disease that the vaccine can prevent. Licensed vaccines that have not been formally evaluated or approved for pregnant women are therefore recommended for administration during pregnancy by the WHO and the US Centers for Disease Control and Prevention (CDC), as well as local organizations in many countries .
Ensuring and evaluating the safety of vaccines administered to pregnant women is a key component of any maternal immunization program or recommendation. This is particularly true now that new vaccines that can benefit pregnant women and their infants are being developed, such as vaccines to protect against Guillain-Barré Syndrome and Respiratory syncytial virus. An important issue is the need for harmonization of standard definitions of key safety outcomes after maternal vaccination and systematic approach to the assessment of safety throughout the life cycle of a vaccine, but particularly after implementation so a large number of pregnant women are vaccinated. It is critical to consider the inherent risks associated with pregnancy itself and understanding the importance of vaccination and to clearly understand the background rate of these risks in specific populations. Furthermore, to evaluate the impact of maternal immunization as a public health strategy to reduce the burden of morbidity and mortality associated with infection, it is necessary to establish baseline rates of these outcomes to demonstrate the efficacy and benefit of vaccines for both mothers and infants. Finally, ethical and regulatory aspects surrounding the inclusion of pregnant women as research subjects also influence the development of vaccines for maternal immunization [9].
The importance of vaccination during pregnancy lies not only on the direct protection against diseases, but also by indirectly protecting small infants during the first few months of life [20]. Vaccination against flu and whooping cough is a priority within the comprehensive care strategy for pregnant women and small infants in Argentina, in the context of transitioning from child vaccination to family vaccination. In 2011, the flu vaccine was included in the National Immunization Schedule (NIS) as mandatory and free of charge, with the aim of decreasing complications and death due to influenza in the at-risk population in Argentina. The national vaccination coverage for pregnant women in the past 4 years (2011-2014) has been satisfactory; 88% coverage was obtained in the year that this program was introduced to the schedule. In the following years, coverage was maintained greater than 95% [20-22].
On February 2012, Argentina became the first country in Latin America to have a strategy of universal vaccination for pregnant women against whooping cough. This recommendation has been implemented throughout the country by vaccination from 20 weeks of pregnancy with the aim of reducing morbidity and mortality due to pertussis in infants younger.
It is important to consider that the goal of maternal immunization is to boost maternal levels of specific antibodies to provide newborns and young infants with sufficient IgG antibody concentrations at birth to protect them against infections occurring during a period of increased vulnerability, until they are able to adequately respond to their own active immunizations or infectious challenges as recently demonstrated by our group in an experimental model for maternal immunization against Neisseria meningitidis B [21,22].
Maternal immunization has potential to significantly improve maternal and child health worldwide by reducing maternal and infant morbidity and mortality associated with disease caused by pathogens that are particularly relevant in the perinatal period and in early life, and for which there are no alternative effective preventive strategies. Our primary concern is to share the Argentina experience in order to encourage other countries with similar social, economic, and cultural characteristics to determine the necessary actors to undertake the challenge of prioritizing vaccination during pregnancy, given the high impact that primary prevention has through vaccines in two vulnerable populations such as pregnant women and small infants.
Conclusion
The present vaccines for Neisseria meningitidis B use different antigens in various age groups; however, they do not protect children below two years of age and does not stimulate the immune system to trigger an effective protection. Our study with animal model has shown that protective IgG antibodies opens perspectives for the evaluation of vaccines with regard to the protection mechanism for the transfer of antibodies by maternal immunization. It is a fact that current vaccines do not induce an effective protection in the age below 2 years . Studies are needed to verify the future use of meningococcal vaccines in pregnant women in an attempt to reduce the incidence of the disease in this age group.
It is important to account attention to vaccines for Neisseria meningitidis in a general way ,especially for the being used and the new as Bexero and Trumemba to N.meningitidis B.
References
- Feavers IM, Maiden MCJ (2017) Recent Progress in the Prevention of Serogroup B Meningococcal Disease. Clin Vaccine Immunol 24. [Crossref]
- Tappero JW, Lagos R, Ballesteros AM, Plikaytis B, Williams D, et al. (1999) Immunogenicity of 2 serogroup B outer-membrane protein meningococcal vaccines: a randomized controlled trial in Chile. JAMA 28: 1520-1507.
- de Moraes JC, Perkins BA, Camargo MC, Hidalgo NT, Barbosa HA, et al. (1992) Protective efficacy of a serogroup B meningococcal vaccine in Sao Paulo, Brazil. Lancet 340: 1074-1078. [Crossref]
- Fortner KB, Nieuwoudt C, Reeder CF, Swamy GK (2018) Infections in Pregnancy and the Role of Vaccines. Obstet Gynecol Clin North Am 45: 369-388. [Crossref]
- Basha S, Surendran N, Pichichero M (2014) Immune responses in neonates. Expert Rev Clin Immunol 10: 1171-1184. [Crossref]
- Keller-Stanislawski B, Englund JA, Kang G, Mangtani P, Neuzil K, Nohynek H, et al. (2014) Safety of immunization during pregnancy: a review of the evidence of selected inactivated and live attenuated vaccines. Vaccine 12: 7057-7064.
- Plotkin SA (2008) Vaccines: correlates of vaccine-induced immunity. Clin Infect Dis 47: 401-409. [Crossref]
- Slifka MK (2004) Immunological memory to viral infection. Curr Opin Immunol 16: 443-450. [Crossref]
- Keller-Stanislawski B, Englund JA, Kang G, Mangtani P (2014) Safety of immunization during pregnancy: a review of the evidence of selected inactivated and live attenuated vaccines. Vaccine 12: 7057-7064.
- Wood N, Siegrist CA (2011) Neonatal immunization: where do we stand? Curr Opin Infect Dis 24: 190-195. [Crossref]
- Morris MC, Surendran N (2016) Neonatal Vaccination: Challenges and Intervention Strategies. Neonatology 109: 161-169.
- Harrison LH, Pelton SI, Wilder-Smith A, Holst J, Safadi MA, et al. (2011) The Global Meningococcal Initiative: recommendations for reducing the global burden of meningococcal disease. Vaccine 29: 3363-3371. [Crossref]
- Borrow R, Alarcon P, Carlos J, Caugant DA, Christensen H, et al. (2017) The Global Meningococcal Initiative: global epidemiology, the impact of vaccines on meningococcal disease and the importance of herd protection. Expert Rev Vaccines 16: 313-328. [Crossref]
- Pizza M, Scarlato V, Masignani V, Giuliani MM, Arico B, et al. (2000) Identification of vaccine candidates against serogroup B meningococcus by whole-genome sequencing. Science 287: 1816-1820.
- Oster P, Lennon D, O'Hallahan J, Mulholland K, Reid S, Martin D, et al. (2005) MeNZB: a safe and highly immunogenic tailor-made vaccine against the New Zealand Neisseria meningitidis serogroup B disease epidemic strain. Vaccine 23: 2191-2196.
- Ladhani SN, Campbell H, Parikh SR, Saliba V, Borrow R, et al. (2015) The introduction of the meningococcal B (MenB) vaccine (Bexsero) into the national infant immunisation programme-new challenges for public health. J Infect 71: 611- 614.
- Vesikari T, Esposito S, Prymula R, Ypma E, Kohl I, et al. (2013) EU Meningococcal B Infant Vaccine Study group (2013). Immunogenicity and safety of an investigational multicomponent, recombinant, meningococcal serogroup B vaccine (4CMenB) administered concomitantly with routine infant and child vaccinations: results of two randomised trials. Lancet 9: 825-835.
- Gossger N, Snape MD, Yu LM, Finn A, Bona G, et al. (2012) European MenB Vaccine Study Group Immunogenicity and tolerability of recombinant serogroup B meningococcal vaccine administered with or without routine infant vaccinations according to different immunization schedules: a randomized controlled trial. JAMA 8: 573-882.
- Calendario basico de vacinação (PNI). https://sbim.org.br/calendarios-de-vacinacao.
- Vizzotti C, Neyro S, Katz N, Juarez MV, Perez Carrega ME, et al. (2015) Maternal immunization in Argentina: A storyline from the prospective of a middle-income country. Vaccine 25: 6413-6419.
- de Almeida AF, De Gaspari E (2018) Dioctadecyldimethylammonium bromide (DODAB-BF) as a new adjuvant for maternal-fetal immunization in mice against Neisseria meningitidis: evaluation of humoral response. Pathog Dis 1:76.
- Rubio DM, Schoenbaum EE, Lee LS, Schteingart DE, Marantz PR, et al. (2010) Defining translational research: implications for training. Acad Med 85: 470-475.

