Abstract
The current study compared organization of the sensory receptors in duck and quail beak. Samples of beak were collected and processed for paraffin and semithin sectioning and SEM. The typical structure of the sensory receptors was detected by different histochemical stains. The topographical distribution of various types of mechanoreceptors was different within the two species. Higher proportions of sensory receptors were evaluated as Herbst and Grandry corpuscles in the bill tip organ, Herbst in the aboral surface of the lateral edge of the cranial part and in the middle site of the oral mucosa of the middle and caudal parts of duck beak. Prevalence of the sensory receptors in quail was also estimated; Merkel receptors in the tip, Ruffini corpuscles in the middle site of the oral mucosa of the cranial and caudal parts. Analysis of the predominant proportions of the sensory receptors in the different divisions of duck and quail beak outlined the functional map of the beak in both species. Duck beak was mostly responding to vibration stimuli particularly the cranial and the caudal part, while quail beak was more sensitive stretching, especially in the cranial portion. In duck beak, the functional map was organized as stretching sensors in the cranial part, pressure sensors in the cranial and the middle parts, velocity sensors in the cranial and caudal parts. While in quail beak, the functional map was organized as the vibration and stretching in the cranial part, pressure in the tip and the cranial portions.
Key words
beak, sensory receptors, Duck, Quail, SEM
Introduction
Different types of sensory endings are responsible for sensation in avian species; free nerve endings and dermal corpuscles including Herbst corpuscles, Grandry corpuscles, Ruffini and Merkel receptors. Three major parts comprised the basic structure of the dermal corpuscles; central mechanotransduction cells which surrounded by cells organize a lamellated envelope and outer capsular flattened cells. Herbst corpuscles homologous to mammalian Pacinian corpuscles respond to low frequency. They are distributed in beak, tongue, mucosa of the oropharynx [1-4], skin in association to the feather follicles [5], leg [6], and feet [7]. Herbst corpuscles composed of a central sensor axon which is ensheathed by sensory cells aligned in symmetrical rows along the central axon. Sensory cells are organized in lamellated layers and comprised the inner bulb. The axon and the inner bulb form the central core of the corpuscle. The central core is surrounded by mucin fluid-filled inner space or the peribulbar space which is ensheathed by an outer capsule. The capsule is formed of fibrocytes which interspersed between the loosely arranged collagen fibers. Herbst corpuscle is covered by a perineural lamellated capsule [1]. Herbst corpuscles sever as vibration and pressure-sensitive mechanoreceptors [8].
Grandry corpuscles are tactile mechanoreceptors. They are considered to be equivalent to the mammalian Meissner corpuscles. Grandry corpuscles are distributed in dermal tissue of the beak and tongue. They are characterized by an alternative organization of the sensory cells and the axon terminals. Grandry Sensory cells are in direct apposition with the discoid-shaped axon terminals. Both sensory cells and axon terminals are enclosed by satellite cells. Grandry corpuscles are encapsulated by collagen fibers and fibroblasts [1].
In mammals, Merkel cells are specialized intraepidermal cells for tactile sensation. In avian species, Markel cells organize subepithelial sensory units which are termed as Markel corpuscles. The distinguishable feature of Markel corpuscle is the alignment of Markel cells in parallel with the discoid nerve endings which are enclosed by lamellar cells [9].
Avian Ruffini corpuscles are modified nerve endings which function as stretching receptors. The axons give rise terminal extensions which enclosed by collagen capsule. Ruffini corpuscles in birds are described in joint capsule, beak and feathered skin [10].
Types of sensory corpuscles are varied among avian species. Herbst corpuscles are the most common type of avian mechanoreceptors. While Grandry corpuscles are limited to aquatic birds. Markel corpuscles are described in non-aquatic birds. Beak is a sensitive mechanosensory organ in avian species rich in sensory corpuscles [1]. The current study aims to investigated localization of different types of the sensory corpuscle in the beak of aquatic (duck) and non-aquatic bird (quail) using light and scanning electron microscope.
Material and methods
Sampling
The study was carried out using apparent healthy birds. Beak samples were taken from 1 week, 2 weeks and 6 weeks aged Quail birds (Coturnix coturnix japonicum) and 1 week, 1 month, 2 months aged duck (Cairina moschata). The birds were decapitated and the whole upper beak was carefully dissected and washed by saline before fixation.
Fixation of samples
Beak was fixed in 10% neutral buffered formalin for paraffin sections and a mixture of 20 mL of 2.5% glutaraldehyde and 80 mL 0.1 M Na-phosphate buffer (pH 7.2-7.4) for semithin sectioning and SEM [11].
Histological examinations
Formalin-fixed samples were extensively washed using 0.1 M Na-phosphate buffer (pH 7.2-7.4). Samples were dehydrated by ascending grades of alcohol (70, 80, 90, 100%). The dehydrated samples were cleared in methyl benzoate for 24 hours, impregnated, and embedded in paraffin wax for preparation of paraffin blocks. Serial sections (5-μm thickness) were cut. The sections were dewaxed and rehydrated in a descending series of ethanol (100, 95, and 70%) and DW. The sections were stained by different histochemical techniques. After staining, the sections were dehydrated again in an ascending series of ethanol (70, 80, 90, and 100%), cleared in xylene (2 minutes), and mounted with DPX.
Conventional histological staining
Paraffin sections were stained with Hematoxylin and Eosin stain [12], Crossman trichrome stain [13], Mallory trichrome [14], Heidenhain iron hematoxylin (Hx) [15], Methylene blue [11]. Stained sections were examined by Leitz Dialux 20 Microscope. Photos were taken using a Canon digital camera (Canon Powershot A95).
Preparations of resin embedding samples
Small specimens measured 2.0-3.0 mm from the beak were used in semi-thin sections. They were washed 4 times for 15 minutes in 0.1 M sodium phosphate buffer (pH 7.2) then were post-fixed in 1% osmic acid in 0.1 M Na-phosphate buffer at 4°C for 2 hours. The samples were again washed 3 times for 20 minutes in 0.1 M phosphate buffer (pH 7.2). Dehydration was performed through graded acetone (70, 80, 90, 100%), 10 minutes for each concentration. The dehydrated samples were immersed in a mixture of acetone/resin (1/1 for 1day, ½ for another day) and pure resin for three days. The resin was prepared by using 10 gm ERL, 6 gm DER, 26gm NSA and 0.3 gm DMAE and thoroughly mixed by a shaker. The specimens were embedded in the resin at 60 C° for 3 days. Polymerized samples were cut to semithin sections by using an ultramicrotome Ultracut E (Reichert-Leica, Germany) and stained with toluidine blue (Sodium tetraborate (borax) 1 gram, toluidine blue 1 gram, and Distilled water 100 ml.s) [11].
Scanning electron microscopy
Representative specimens from the beak were washed several times with normal saline and then fixed in a mixture of 2.5% paraformaldehyde and 5% glutaraldehyde in 0.1 M sodium phosphate buffer, pH 7.3, at 4°C for 24 h. Thereafter, they were washed 4 times for 5 min in the fixation buffer and postfixed in 1% osmic acid in 0.1 M sodium phosphate buffer for further 2 h at room temperature, followed by washing with 0.1 M sodium phosphate buffer for 15 min 4 times. The samples were dehydrated using increasing concentrations of alcohol: 50, 70, and 90% for 30 min at each concentration and 100% for 2 days (several changes) followed by isoamyl acetate for 2 days. The dehydrated samples were subjected to critical point drying with a Polaron apparatus. Finally, they were coated with gold using JEOL-1100 E ion sputtering device and observed with a JEOL scanning electron microscope (JSM 5500 LV) at 10 kV.
Comparative analysis of regional distribution of the sensory receptors in duck and quail beak
Regional counting of all types of sensory corpuscles in serial sections of duck and quail beaks was performed using Image J (1.41o). The counted areas were categorized according to the beak length to four parts; the beak tip, the anterior beak (cranial part), the middle beak and caudal part. In duck, the whole beak of the duck measured about 65.52 mm in length. The tip of the duck beak was represented by the bill tip organ which measured 12.01 mm. The anterior division of the duck beak measured 16.31 mm. The middle division measured 17.5 mm and the caudal division measured 19.70 mm of the whole length of the duck beak. Quail beak measured about 18.79 mm and was divided to the tip of the beak, cranial, middle and caudal parts. The tip of the beak measured 2.6 mm. The cranial portion measured 4.02 mm. The middle portion was measured 5 mm and the caudal portion measured 7.17 mm of the whole length of the quail beak. The each division of the beak is subsequently divided into lateral edges and middle sides.
Results
The current study investigated various types of sensory receptors in the beak of duck and quail using different histochemical techniques, SEM and comparing the regional distribution of the sensory receptors in duck and quail beak.
Avian Sensory corpuscles could be categorized depending on the general structure into two types. The first was formed of sensory nerve ending which established contact with the sensory cells and was surrounded by the perineural sheath. This type included Herbst, Grandry and Merkel corpuscles. The second type consisted of multiple axons ensheathed by capsule. This type was represented by Ruffini corpuscle.
Beak of duck had two surfaces; an internal oral surface and an external skin covering; the beak skin. The beak was supported by the bone tissue of the premaxilla (Figures 1A, D). In the beak tip, the connective core extended into the epithelium forming deep pits; bill tip organs, which were rich in sensory receptors including Herbst, Grandry and Ruffini (Figures 1B, C). In duck beak, Ruffini corpuscles were recognized in the stroma of the duck beak. They were located in the dermal tissue and also extended from the submucosa to the lamina propria (Figures 1F). Ruffini corpuscles contained both myelinated and unmyelinated axons surrounded by a distinguished capsule (Figures 1E, H, I). Ruffini corpuscles gave rise a nerve ending toward the epithelial covering (Figures 1G). Herbst corpuscles were identified in the dermal tissue, the lamina propria of the oral mucosa (superficial Herbst corpuscles) and submucosa (Deep Herbst corpuscles). The superficial Herbst corpuscles were larger than the deep Herbst corpuscles. The deep Herbst corpuscles located in the between the spicules of the premaxilla and could be enclosed by the periosteum (Figures 2A, C). Herbst corpuscles composed of symmetrically aligned sensory cells along the central axon which comprised the inner bulb. The inner space composed of concentric lamellar layers of fibroblasts-like cells and collagen fibers. Herbst corpuscle was surrounded by a capsule (Figures 2B, D-H). Grandry corpuscles were distributed in the lamina propria of the oral mucosa and the dermal tissue of the beak skin. They consisted of Grandry sensory cells which enclosed by a capsule. The nerve supply gave rise nerve terminals aligned with the sensory cells (Figures 3A-C). Unicellular Grandry corpuscle was composed of the sensory cell and surrounded by satellite cells (Figures 3C, E, F). The collagenous capsule of the unicellular Grandry corpuscle was identified by Crossman's trichrome (Figures 3F). The intracellular microfilaments of the Grandry sensory cells were detected by Heidenhain iron hematoxylin (Figures 3G). Two types of Merkel corpuscles were observed according to location. The superficial Merkel corpuscles were found in the dermal tissue and lamina propria of the oral mucosa (Figures 3J). While the deep Merkel corpuscles located in the submucosa at the integument side. Merkel corpuscles contained numerous Markel sensory cells which were surrounded by capsular cells (Figures 3D, H, I). Merkel cells in the dermal tissue (Figure 3K).
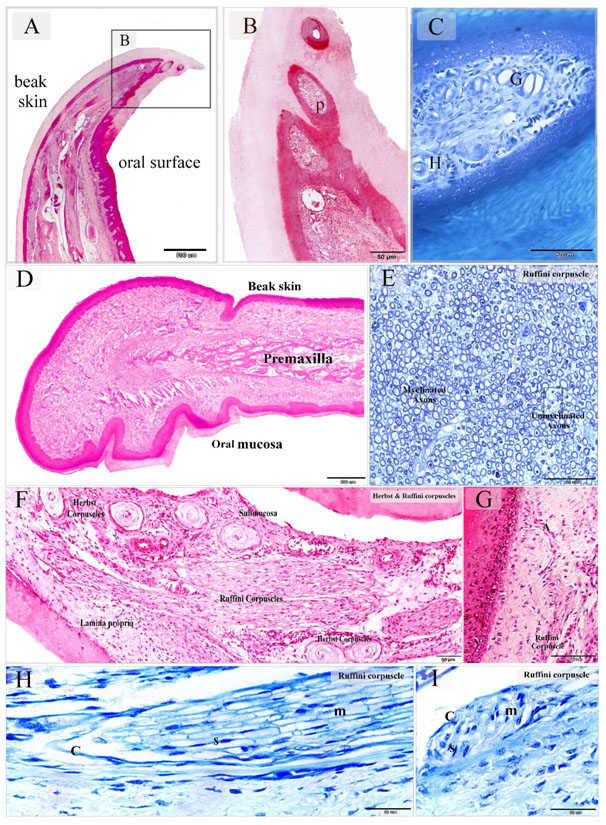
Figure 1. Sensory receptors in the bill Tip organ of duck beak and distribution of the Ruffini corpuscles in the dermal tissue and mucosa duck beak
Paraffin sections stained by H&E (A, B, D, F, G), methylene blue (C, H, I). Semithin section stained with toluidine blue (E). A-C: sagittal section of duck beak. Note pits (P) rich in sensory receptors. Herbst corpuscle (H), Grandry Corpuscle (G). D: showed the general histological features of duck beak. The beak had two surfaces; an oral mucosa and skin covering (beak skin). The bone tissue of the premaxilla supported the beak. E: Ruffini corpuscle composed of myelinated and unmyelinated axons. F: Sagittal section in duck beak. The oral mucosa and submucosa were rich in sensory corpuscles. Note Ruffini corpuscles extended between the lamina propria and submucosa. G: Ruffini corpuscle derived axon terminal (A) extended toward the epithelium. H: Sagittal section in duck beak showed Ruffini corpuscle. Note myelinated axons (m), Schwann cells (S), capsule (C). I: Cross section in duck beak showed Ruffini corpuscle. Note myelinated axons (m), Schwann cells (S), capsule (C).
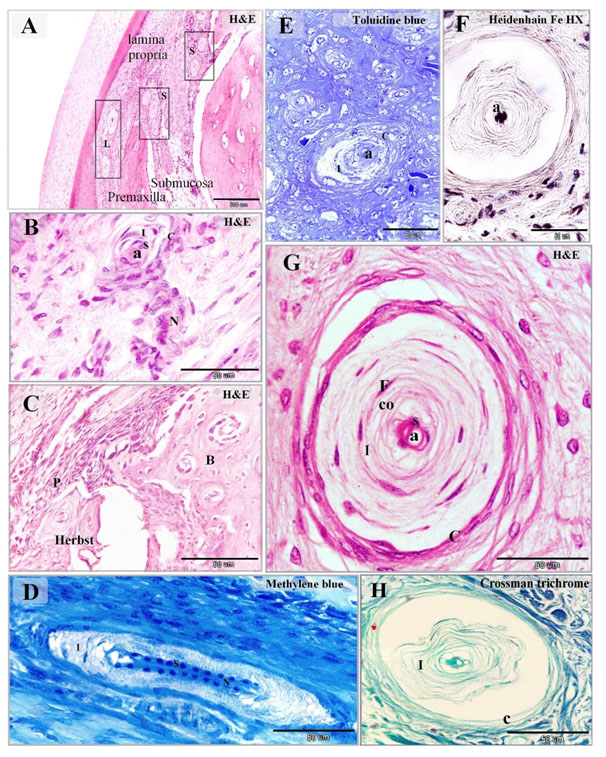
Figure 2. Distribution of Herbst corpuscles in the dermal tissue and mucosa of the duck beak
Paraffin (A-D, F-H) and semi-thin (E) sections stained with H&E (A-C, G), Toluidine Blue (E), Heidenhain iron hematoxylin (F), methylene blue (D), Crossman's trichrome (H). A: mucosa of duck beak contained large superficial Herbst (L) corpuscles situated in the lamina propria and smaller deep Herbst (S) corpuscles located in the submucosa between the spicules of the premaxilla. B: Herbst corpuscles as supplied by a nerve (N) which extended as the central axon (a) and surrounded by capsule (C). The sensory cells (S) surrounded the axon, inner space (I). C: Herbst corpuscles enclosed by the periosteum (P) of the premaxillary bone (B). D: the central axon stained by methylene blue. Note the symmetrical arrangement of the sensory cells (S) along the axon, inner space (I) and nerve (N). E, G: showed the detailed structure of the Herbst corpuscles. The sensory cells (S) aligned on both sides of the central axon (a). The inner space (I) composed of concentric lamellar layers of fibroblasts-like cells (F) and collagen fibers (co). Herbst corpuscle was surrounded by a capsule (C). F: the central axon (a) stained by Heidenhain iron hematoxylin. H: collagen fibers in the inner space (I) and capsule (C) stained green by Crossman's trichrome.
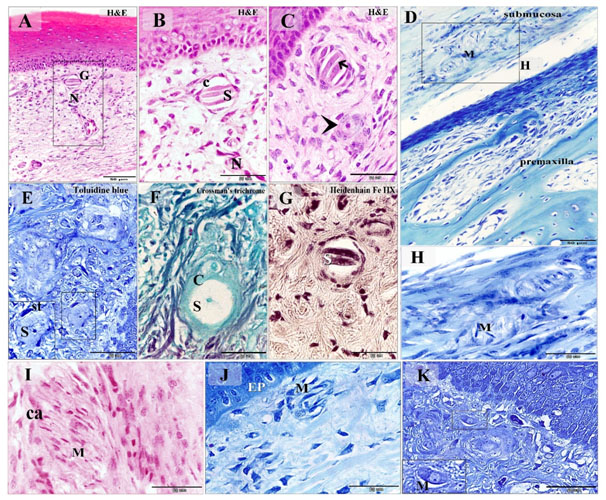
Figure 3. Distribution of Grandry, Merkel corpuscles and Merkel cells in the dermal tissue and mucosa of the duck beak
Paraffin (A-D, F-J) and semi-thin (E, K) sections stained with H&E (A-C, I), Toluidine blue (E, K), Crossman's trichrome (F), Heidenhain iron hematoxylin (G), Methylene blue (D, H, J). A: Grandry corpuscle (G) located in the lamina propria note the nerve (N) supply. B: Higher magnification of (A) note Grandry sensory cells (S) enclosed by capsule (C). C: nerve terminal (arrow) between the sensory cells. The arrowhead refers to unicellular Grandry corpuscle. D: Merkel corpuscles (M) located in the submucosa adjacent to the premaxilla. E: unicellular Grandry corpuscle composed of the sensory cell (S) which surrounded by satellite cells (st). F: The collagenous capsule (C) of the unicellular Grandry corpuscle stained by Crossman's trichrome, note, sensory cell (S), capsule (C). G: the intracellular filaments of the Grandry sensory cells (S) stained positive by Heidenhain iron hematoxylin. H: Higher magnification of the squared area in (D) showed Merkel corpuscles (M). I: Merkel corpuscles (M) contained numerous Markel sensory cells. Note capsular cells (ca). J: Subepithelial Merkel corpuscle (M). Note epithelium (EP). K: Subepithelial Merkel cells (M)
By SEM, aggregation of the sensory corpuscles located in the lamina propria at the lateral edges of the duck beak (Figures 4A, B). Herbst corpuscles had a central axon which was ensheathed by the sensory cells (Figures 4C, E, F). The inner space contained fibroblast-like cells. Herbst corpuscles were surrounded by a capsule (Figures 4C, D). Herbst corpuscle was supplied by nerve fiber (Figures 4D). Ruffini corpuscles might locate in groups in the submucosa. They axons organized in a strap-like arrangement (Figures 4G, H). Grandry corpuscles had Grandry sensory cells which were elongated in shape. They surrounded by satellite and capsular cells. Digitations of both satellite cells and Grandry cells could be observed (Figure 5A-C). The nerve fiber supplying the Grandry corpuscles gave rise a discoid-shaped nerve terminal (Figures 5D). Merkel corpuscles were large, had elongated sensory cells which separated by nerve terminals (Figures 5E, F).
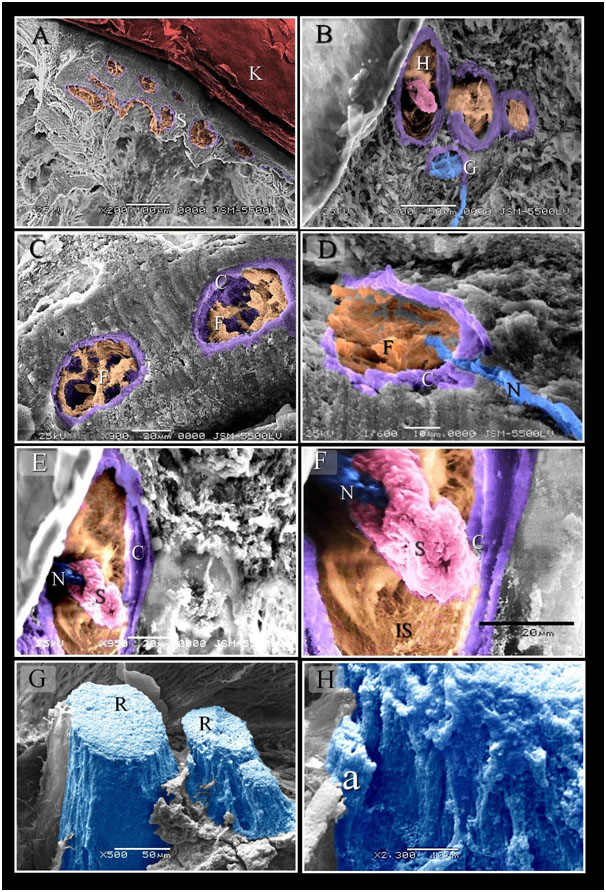
Figure 4. Scanned Electron micrographs illustrated the structure of Herbst and Ruffini corpuscles
Colored scanned electron micrographs of the dermal tissue and mucosa of the duck beak. A: General view of the beak of duck. Aggregation of the sensory corpuscles (S) in the lamina propria. Note keratin (K). B: aggregation of the sensory corpuscles in the dermal tissue note Herbst (H) and Gandy (G) corpuscles. C, D: Fibroblasts like cells (F) in inner space of Herbst corpuscles, note Capsule (C). Nerve (N). E, F: the central nerve terminal (N) surrounded by the sensory cells (S). note inner space (IS), capsule (C). G, H: a group of Ruffini corpuscles (R) was located in the submucosa of the beak. Note Axon (a).
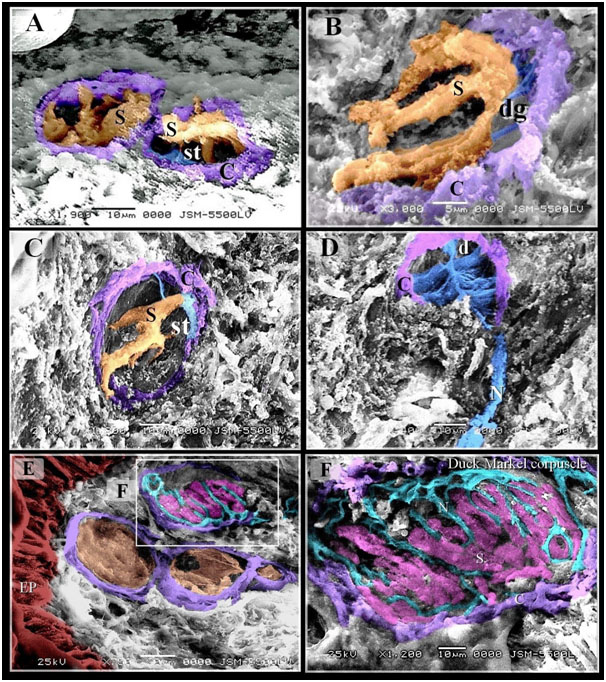
Figure 5. Scanned Electron micrographs illustrated the structure of Gandy corpuscles and Merkel corpuscles
Colored scanned electron micrographs of the dermal tissue and mucosa of the duck beak. A, B, C: Grandry sensory cells had elongated shape. Note capsule (C). D: Nerve (N) supply the Gandy corpuscles in which nerve terminals were expanded and formed discs (d), digitations (dg; blue colored) of satellite cells (st) and Grandry cells. E: Merkel corpuscle (the squared area) located under the epithelium (EP). F: Nerve terminals (N) between Merkel sensory cells (S).
The general structure of the quail beak was similar to duck beak expect the tip of the beak had no bill tip organ (Figures 6A, 7A). The oral mucosa in the beak tip was rich in Merkel corpuscles (Figures 6B). Sub-epithelial Merkel cells were arranged along the lamina propria of the oral mucosa in quail beak (Figures 6C). Quail beak had a mucosal and integumentary covering and supported by premaxillary bone. The submucosa was rich in cavernous tissue (Figures 6D, E, and 7A). Most Herbst corpuscles were located in the mucosa of quail beak (Figures 9F-H). Herbst corpuscles of quail were different from duck that had fine collagen lamellae. Some Herbst corpuscles had two axons (Figures 6I). Quail beak was dominated by Ruffini corpuscles. They represented the continuation of the sensory nerve fibers (Figures 7B, C). Melanocytes were common around Ruffini corpuscles (Figures 7D).
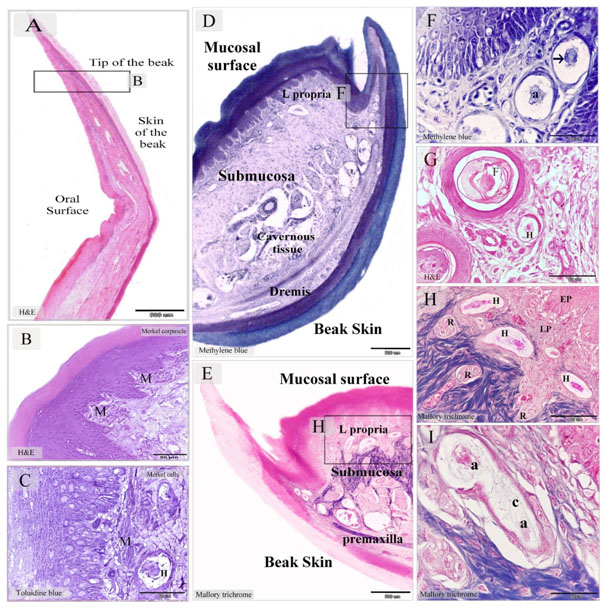
Figure 6. Merkel receptors in the tip of Quail beak and Herbst corpuscles in the dermal tissue and mucosa of Quail beak
Paraffin sections stained by H&E (A, B, G), Mallory trichrome (E, H, I), methylene blue (D, F). Semithin section stained with toluidine blue (C). A: sagittal section in the tip of beak in quail bird. B: numerous Markel corpuscles (M) in the lamina propria C: Merkel cells distributed along the lamina propria. Note Herbst corpuscle (H). D, E: showed the general histological features of Quail beak; mucosal surface and beak skin, submucosa, the cavernous tissue (cavernous tissue). Note the submucosa (submucosa) between the oral mucosa and the premaxilla. The lamina propria (L.propria) of the mucosa. The dermal tissue (Dermis) of the beak skin. F: higher magnification of the squared area in (D). Aggregation of Herbst corpuscle in the dermal tissue of the lateral papillae of the beak. Note Axon (a), the arrow refers to the sensory cell. G: Herbst corpuscles (H) closed to the feather follicle (F) in the caudal part of the beak. H: Higher magnification of the squared area in (E). Herbst (H) and Ruffini corpuscles (R) in the lamina propria (LP), epithelium (EP). I: Herbst corpuscle with two axons (a). Note the fine collagen fibers (C) in the inner space stained by Mallory trichrome.
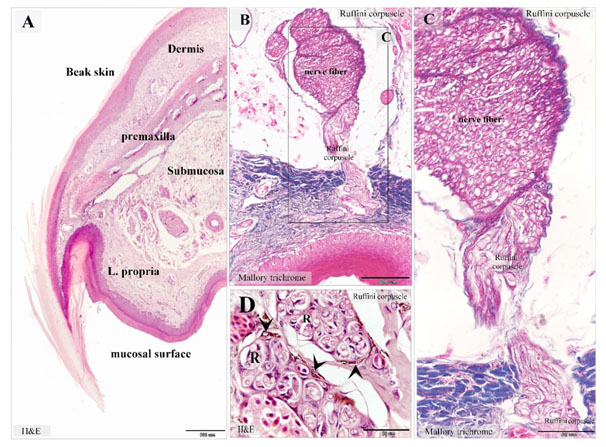
Figure 7. Ruffini corpuscles in Quail beak
Paraffin sections stained with H&E (A), Mallory trichrome (B-D). A: showed the general histological features of Quail beak. The beak had two surfaces; an oral mucosa and skin covering (beak skin). The bone tissue of the premaxilla supported the beak. Note the submucosa (submucosa) between the oral mucosa and the premaxilla. The lamina propria (L. propria) of the mucosa. The dermal tissue (Dermis) of the beak skin. B, C: Ruffini corpuscles in the lamina propria extended from the nerve fiber of the submucosa. D: dermal tissue rich in Ruffini corpuscles (R). Melanocytes (arrowheads) were common around Ruffini corpuscles.
By SEM, aggregations of the sensory receptors were common the mucosal surface (Figures 8A). The general structure of Herbst corpuscle was similar to those of the duck but the inner space was narrow (Figures 8B-C). Merkel corpuscles had discoid-shaped sensory cells which separated by axon terminals (Figures 9A-C). Ruffini corpuscles extended in the submucosa and contained myelinated and unmyelinated nerve fibers of quail beak (Figures 9D-F).
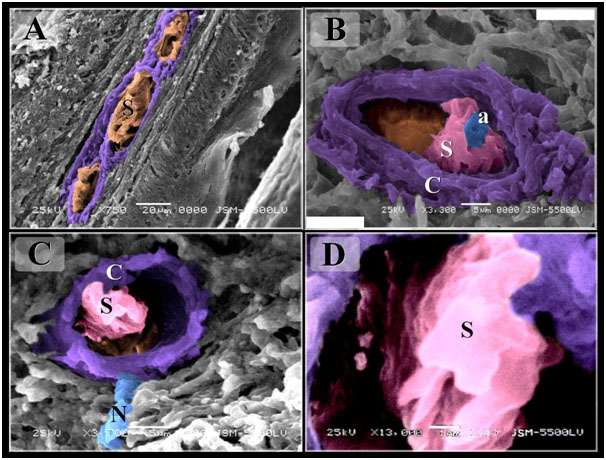
Figure 8. Scanned Electron micrographs illustrated the structure of Herbst corpuscles in Quail
Colored scanned electron micrographs of the mucosa of the Quail beak A: Aggregation of sensory receptors (S). B-D: Herbst corpuscles. Note Sensory cells (s), axon (a), capsule (C), nerve (N).
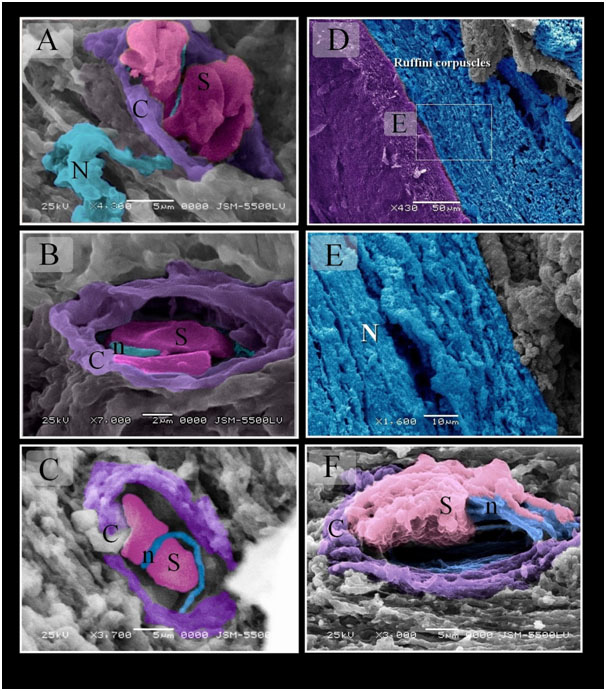
Figure 9. Scanned Electron micrographs illustrated the structure of Merkel and Ruffini corpuscles in Quail
Colored scanned electron micrographs of the mucosa of the Quail beak. A, B, C: Merkel corpuscle. Nerve terminals (n) closely apposed sensory cells (S). Note capsule (C). Nerve (N). D, E: Ruffini corpuscles extended in the submucosa of quail beak. Note myelinated nerve (N). F: Ruffini corpuscle enclosed by capsule (C). Note unmyelinated nerve (n), Schwann cells (S).
Regional distribution of the sensory receptors in duck beak was summarized in Fig. 10. In the bill tip organ, Herbst corpuscles were about 44%, Grandry corpuscles were 43.5%, Ruffini corpuscles were 12.5%. There were no Merkel receptors in the bill tip organ. In the cranial part, Herbst receptors were 20.30% in the aboral surface of the lateral edge, 7.51% in the oral surface of the lateral edge, 15.78% in the aboral surface of the middle aspect and 16.54% in the oral surface of the middle aspect. Grandry corpuscles were 6.76% in the aboral surface of the lateral edge, 1.5% in the oral surface of the lateral edge, 0.75% in the aboral surface of the middle aspect and 3.82% in the oral surface of the middle aspect. Ruffini corpuscles were 2.25% in the aboral surface of the lateral edge, 3% in the oral surface of the lateral edge, 6.76% in the aboral surface of the middle aspect and 9.02% in the oral surface of the middle aspect. Merkel receptors were 0.2% in the aboral surface of the lateral edge, 0.3% in the oral surface of the lateral edge, 4.1% in the aboral surface of the middle aspect and 1.5% in the oral surface of the middle aspect. In the middle part, Herbst receptors 15% in the aboral surface of the lateral edge, 5.5% in the oral surface of the lateral edge, 25% in the aboral surface of the middle aspect and 18.75% in the oral surface of the middle aspect. Grandry corpuscles were 3.72% % in the aboral surface of the lateral edge, 0.16% in the oral surface of the lateral edge, 0.25% in the aboral surface of the middle aspect and 3.5 % in the oral surface of the middle aspect. Ruffini corpuscles were 1.1% in the aboral surface of the lateral edge, 0.2% in the oral surface of the lateral edge, 0.1% in the aboral surface of the middle aspect and 1.72% in the oral surface of the middle aspect. Merkel receptors were 25% in the oral surface of the middle aspect. The aboral, oral surfaces of the lateral edge and the aboral surface of the middle aspect lack Merkel receptors. In the caudal part, Herbst corpuscles were 9.27% in the aboral surface of the lateral edge, 18.55% in the aboral surface of the middle aspect and 54.63% in the oral surface of the middle aspect. The oral surface of the lateral edge was devoid of Herbst sensory units. Grandry receptors were absent in the aboral surface of the lateral edge and were about 9.27 % in the oral surface of the lateral edge, 4.12% in the aboral and the oral surfaces of the middle aspect. The caudal part of duck’s beak was devoid of Ruffini and Merkel receptors.
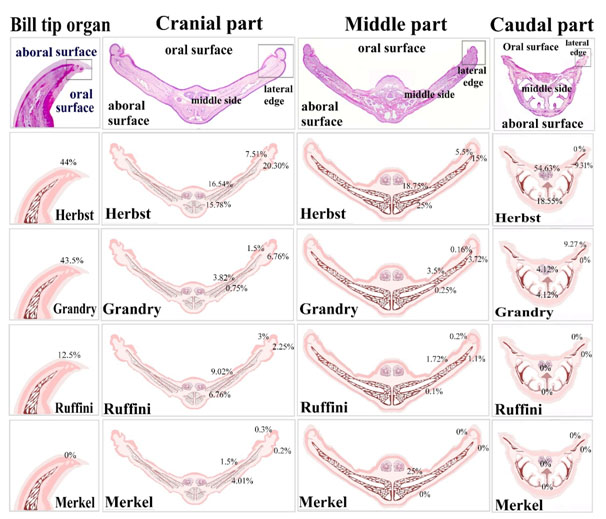
Figure 10. Illustration summarize the distribution of the sensory receptors in the duck beak
In the bill tip organ, Herbst corpuscles were about 44%, Grandry corpuscles were 43.5%, Ruffini corpuscles were 12.5%. There were no Merkel receptors. In the cranial part, Herbst receptors were 20.3% in the aboral surface of the lateral edge, 7.51% in the oral surface of the lateral edge, 15.78% in the aboral surface of the middle aspect, and 16.54% in the oral surface of the middle aspect. Grandry corpuscles were 6.76% in the aboral surface of the lateral edge, 1.5% in the oral surface of the lateral edge, 0.75% in the aboral surface of the middle aspect, 3.82% in the oral surface of the middle aspect. Ruffini corpuscles were 2.25% in the aboral surface of the lateral edge, 3% in the oral surface of the lateral edge, 6.76% in the aboral surface of the middle aspect, 9.02% in the oral surface of the middle aspect. Merkel receptors were 0.2% in the aboral surface of the lateral edge, 0.3% in the oral surface of the lateral edge, 4.1% in the aboral surface of the middle aspect, 1.5% in the oral surface of the middle aspect. In the middle part, Herbst receptors 15% in the aboral surface of the lateral edge, 5.5% in the oral surface of the lateral edge, 25% in the aboral surface of the middle aspect, 18.75% in the oral surface of the middle aspect. Grandry corpuscles were 3.72% % in the aboral surface of the lateral edge, 0.16% in the oral surface of the lateral edge, 0.25% in the aboral surface of the middle aspect, 3.5 % in the oral surface of the middle aspect. Ruffini corpuscles were 1.1% in the aboral surface of the lateral edge, 0.2% in the oral surface of the lateral edge, 0.1% in the aboral surface of the middle aspect, 1.72% in the oral surface of the middle aspect. Merkel receptors were 25% in the oral surface of the middle aspect. The aboral, oral surfaces of the lateral edge and the aboral surface of the middle aspect lack Merkel receptors. In the caudal part, Herbst corpuscles were 9.31% in the aboral surface of the lateral edge, 18.55% in the aboral surface of the middle aspect, 54.63% in the oral surface of the middle aspect. The oral surface of the lateral edge was devoid of Herbst sensory units. Grandry receptors were absent in the aboral surface of the lateral edge and were about 9.27 % in the oral surface of the lateral edge, 4.12% in the aboral and the oral surfaces of the middle aspect. The caudal part of duck’s beak was devoid of Ruffini and Merkel receptors.
Regional distribution of the sensory receptors in quail beak was summarized in Figure 11. In the tip of the beak, Herbst corpuscles were about 20%, Ruffini corpuscles were 15% and Merkel receptors were 65%. In the cranial part, Herbst corpuscles were about 9.78% in the aboral surface of the lateral edge, 7.34% in the oral surface of the lateral edge, 3.65% in the aboral surface of the middle aspect and 2.43% in the oral surface of the middle aspect. Ruffini corpuscles were 3.65% in the aboral surface of the lateral edge, 6.09% in the oral surface of the lateral edge, 12.19% in the aboral surface of the middle aspect and 41.09 % in the oral surface of the middle aspect. There was no Merkel receptor in the aboral, oral surfaces of the lateral edge and in the aboral surface of the middle aspect. Merkel receptors were 14.85 % in the oral surface of the middle aspect. In the middle part, there was no sensory receptor. In the caudal part, the aboral and the oral surface of the lateral edge and the aboral surface of the middle aspect had no sensory receptors. Herbst corpuscles were 33.33% and Ruffini receptors were 66.66% in the oral surface of the middle aspect.
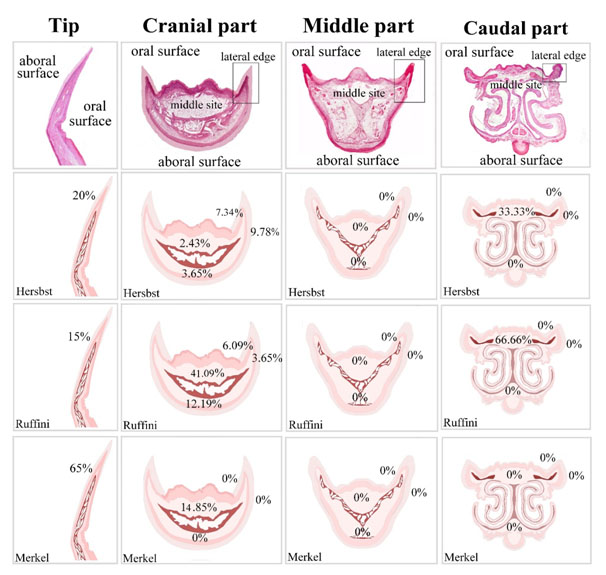
Figure 11. Illustration summarize the distribution of the sensory receptors in the quail beak
In the tip of the beak, Herbst corpuscles were about 20%, Ruffini corpuscles were 15% and Merkel receptors were 65%. In the cranial part, Herbst corpuscles were about 9.78% in the aboral surface of the lateral edge, 7.34% in the oral surface of the lateral edge, 3.65% in the aboral surface of the middle aspect, 2.43% in the oral surface of the middle aspect. Ruffini corpuscles were 3.65% in the aboral surface of the lateral edge, 6.09% in the oral surface of the lateral edge, 12.19% in the aboral surface of the middle aspect, 41.09 % in the oral surface of the middle aspect. There was no Merkel receptor in the aboral surface of the middle aspect, in the aboral and oral surfaces of the lateral edge. Merkel receptors were 14.85 % in the oral surface of the middle aspect. In the middle part, there was no sensory receptor. In the caudal part, the aboral and the oral surface of the lateral edge and the aboral surface of the middle aspect had no sensory receptors. Herbst corpuscles were 33.33% and Ruffini receptors were 66.66% in the oral surface of the middle aspect.
The total proportions of the sensory receptors were estimated in different regions of duck and quail beak (Figures 12). In duck beak; Herbst corpuscle was 2.68% in the tip, 30.65% in the cranial part, 9.19% in the middle part and 26.81% in the caudal part. Grandry corpuscle was 2.68% in the tip, 6.51% in the cranial part, 0.38% in the middle part and 6.51% in the caudal part. Ruffini corpuscle was 0.76% in the tip, 10.72% in the cranial part, 0.38% in the middle part and was absent in the caudal part. Merkel receptors were absent in the tip and caudal parts and were 3.6% in the cranial and middle parts of duck beak. In quail beak; Herbst corpuscle was 3.8% in the tip, 14.28% in the cranial part, 0.95% in the caudal part and was absent in the middle part. Ruffini corpuscles were 2.89% in the tip, 51.42% in the cranial part, 1.9% in the caudal part and was absent in the middle part. Merkel receptors were 12.38% in the tip and the cranial part and were absent in the middle and the caudal part of quail beak.
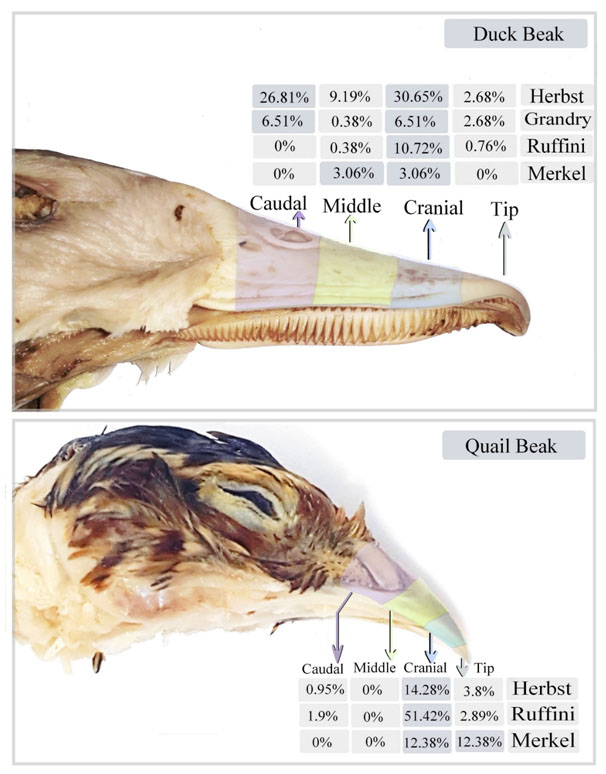
Figure 12. Illustration summarizes the proportions of the sensory receptors in the different divisions of duck and quail beak.
The total proportions of the sensory receptors were estimated in different regions of duck and quail beak. In duck beak; Herbst corpuscle was 2.68% in the tip, 30.65% in the cranial part, 9.19% in the middle part, 26.81% in the caudal part. Grandry corpuscle was 2.68% in the tip, 6.51% in the cranial part, 0.38% in the middle part, 6.51% in the caudal part Ruffini corpuscle was 0.76% in the tip, 10.72% in the cranial part, 0.38% in the middle part and was absent in the caudal part. Merkel receptors were absent in the tip and caudal parts and were 3.6% in the cranial and middle parts of duck beak. In quail beak; Herbst corpuscle was 3.8% in the tip, 14.28% in the cranial part, 0.95% in the caudal part and was absent in the middle part. Ruffini corpuscles were 2.89% in the tip, 51.42% in the cranial part, 1.90% in the caudal part and was absent in the middle part. Merkel receptors were 12.38% in the tip and the cranial part and were absent in the middle and the caudal part of quail beak.
Discussion
The beak considered a high-sensitive organ in avian species. Studying a comparative organization of variable types of sensory receptors in aquatic and non-aquatic birds provide a significant information to the functional contribution. The current study concerned to analyze the distribution of different types of the sensory units in duck and quail beak and discussed their biological significance in each species.
The current study investigated different types of the sensory receptors in duck and quail beaks. The beak was divided into four regions; the tip, the cranial, the middle and the caudal part. Bill tip organ located at the pointed end of the duck beak and have multiple pits which were rich in mechanosensitive nerve endings. Tip of the quail beak lack the pits. Herbst, Grandry and Ruffini corpuscles located in the bill tip organ of duck while the beak tip of quail had Merkel, Herbst and Ruffini receptors. Herbst, Grandry and Merkel sensory endings participate in tactile perception in the bill tip organ [16]. Tactile receptors of the bill organ act as ion detectors. Tactile mechanoreceptors of eagles are highly sensitive to recognize the undesirable food and metal particles; particularly iron. Bill tip of raptors act as a refining device which picks off foreign materials such as scales, feathers, hairs of the prey, it also dissecting the flesh from the prey carcass, selection of tiny bites of food for feeding young chicks [17]. As one of the features of the behavioral adaptation in the insect-eating birds; the probe-foraging birds, the pits in of the bill tip organ have aggregations of the Herbst corpuscles which assist in recognition of the buried or submerged prey [18].
In the current study, Herbst, Grandry, Ruffini and Merkel receptors resembled the sensory nerve endings the duck beak while Herbst, Ruffini, and Merkel represented the significant mechanoreceptors of quail beak. Different sensory receptors were commonly described in the Upper digestive tract in avian species. Herbst corpuscles or pressure sensors are prevalent in the bill skin (Rhamphotheca) and oropharynx of the ostrich and emu [2]. Herbst corpuscles and Grandry corpuscles were described in the tongue and bill of the mallard [19]. Pressure sensors of mammals; Pacini corpuscles, have been detected in aponeuroses and tendons of skeletal muscles, ligaments, joint capsule, periosteum and beneath the interosseous membrane, in the epineurium, in the adventitia of the blood vessels, at the arteriovenous anastomoses, fibrous capsules of parenchymatous organs such as pancreas, in the pleura, mesentery and colon, penis, glabrous skin such as fingerpad and footpad [20]. Although Grandry corpuscle, velocity sensors, has been specified for aquatic birds including duck [21] and geese [22], they have been also mentioned in the bill tip organ of the non-aquatic bird such as chicken [23]. Grandry corpuscles are not specific to the ratites [2]. Mammalian velocity sensors or Meissner corpuscles are found in highly sensitive integument such as nipple, lips, external genitalia, fingertips, and eyelids [24]. Markel’s cells in birds are either intra-epidermal or dermal cells. Intra-epidermal Merkel cells are described in hard palate of fowl [25]. Dermal cells may be single or in the group which encapsulated by perineural sheath. They are also located in beak, tongue, toes, feathered skin [21]. Merkel cell receptors are identified in the bill and on the tongue of nonaquatic birds (Necker 2000). Merkel cells are identified in different species such as fish, reptiles, and mammals. They are located in the basal layer of epidermis of the hairy skin, glabrous or hairless skin of plantar and palmar surfaces, and some mucosal epithelia. while in birds they are found in the dermis [26]. Ruffini endings are common in mammals but avian Ruffini corpuscles are described in the quail bills, geese bill and joint capsules [10,27]. Ruffini's corpuscles are located in the dermis subcutaneous tissue and joint capsule [28].
In the current study, the sensory receptors shared a distinct structure. The Axon terminals were closely related to the Schwann or sensory cells which were enveloped by the perineural sheath. Duck beak was rich in Herbst, Grandry, Merkel and Ruffini corpuscles while quail beak had Herbst, Merkel, and Ruffini corpuscles. Similar structure of Grandry and Herbst corpuscles is mentioned in the palatine mucosa, gingival mucosa and beak skin of the duck [21].
The structure of Herbst corpuscles of the beak was species-specific. Herbst corpuscles of quail resembling those of duck but minor differences were observed by light microscope and SEM. In paraffin sections, the inner space was wide and lamellae layers were thick in duck. Quail Herbst corpuscles had a narrow inner space which had fine lamellae. In Scanned samples, the lamellar structure of the inner space was well-organized in duck while quail Herbst corpuscles had less-distinct lamellar fibrils. Two different types of Herbst corpuscles, regarding location, dimensions, and structure, are described in duck, chicken, and quail. The widely distributed type is identified in the chicken, quail, and duck except for duck beak and tongue. This type appears elongated with different dimensions which measured about 25 x 12 11m to 300 x 110 11m. The inner bulb is large contained about 50 sensory cells, which organize numerous rows. While the lamellar structure of the inner space is less distinct. The second type is restricted to duck beak and tongue. This type has an ovoid shape with constant dimensions, their average is 160 x 100 11m; extreme values 130 x 80 11m and 190 x 120 11m. The internal bulb has about 20 sensor cells aligned in two identical rows. The internal space has an apparent lamellar structure [29].
Two types of Herbst corpuscles were observed in duck beak. The superficial Herbst corpuscles in the lamina propria were small and the large deep Herbst corpuscles located in the submucosa. Herbst corpuscles of variant size are documented in other species; large Herbst and Mini-Herbst corpuscles. Large Herbst has an obvious capsular space. Mini-Herbst corpuscles are described in the papillae of the bill organ and the lamina propria of the tongue of the parrot. Mini-Herbst has an affinity for metachromatic dyes. Thus, Mini-Herbst corpuscle has functional specialization regarding proteoglycan components [30]. Herbst corpuscles are described closed to premaxillary bones, mostly at the anterior apex of the premaxillae [25]. Aggregations of Herbst corpuscles in the interosseous membrane in legs of some birds are known as strands or Herbstscher strang [21]. Localization of Herbst corpuscles in proximity to the premaxilla may reveal raising the sensitivity of Herbst corpuscles [31].
Mechanoreceptors are categorized based on the rate of stimulation into rapid and slow adapting response. Slow adapting type is classified into two subtypes. Slow adapting I receptors represent a random variable distribution. Unlike, the normal distribution (slow adapting subtype II receptors) exhibits the symmetrical frequency distribution [10]. Most mechanoreceptors have a rapid adapting response [32].
Types and functional implications different of mechanoreceptors have been described. Herbst corpuscles are rapidly adapting mechanoreceptors. They respond to vibration rather than high frequency [33]. Herbst corpuscles also respond to the acceleration components of vibratory stimuli [34]. Thus, they serve in the perception of pressure and vibration from the surrounding environment [35]. Orientation (rostrocaudal) of Herbst corpuscles in the organs renders them to receive the maximal effective stimulation [19]. Herbst corpuscles, is similar to other lamellated corpuscle, detect rapid mechanical deformation. Herbst corpuscles are implicated in committing mechanoelectrical transductions. Sensory reception occurs via assimilation of the mechanical stimuli by the lamellar structure of the Herbst corpuscle which in turn exerts neural signaling to the central axon terminal. The structural components of the Herbst corpuscle serve as a mechanical filter system. The capsule acts as the first filter while the second is the adaptive response of the nerve fiber [21,25]. The lamellar structure of the inner core is responsible for detect high-frequency vibrations up to 2000 Hz [36,37]. However, different vibration limits are described for Herbst corpuscles. Vibration ranges of Herbst corpuscles reach 40-1500Hz, the maximal response is detected at 300-600Hz [25].
Grandry corpuscles are believed to be rapidly adapting velocity sensors [32,33,38] in respect to the morphological criteria [10]. Merkel cell receptors are thought to act as rapid and slow adapting receptors [10]. Merkel cells formed complex sensory corpuscles which serve in picking worms. Extensive Markel corpuscles in tongue of birds which have special feeding behavior in seed husking [32]. Merkel corpuscles in tongue and beak of the land birds are described as slow adapting pressure-sensitive nerve endings [37]. They are also amplitude-sensitive receptors [32]. Although vibrotactile Herbst corpuscles in bird wings are described in flight control, the Merkel receptors are observed in wings of the flying mammals [39]. Merkel cell-associated hairs are likely responsible for flight control in bat wings [40]. Ruffini corpuscles are considered as Slow adapting II receptor for stretching [33] and are intensely sensitive to pressure [41]. Ruffini corpuscles involved in mechanoelectrical transduction via establishment contact between the axon terminals and collagen bundles which are likely the point where the sensory signals generate [10,36].
The sensory receptors satisfy the functional demand of the organs. Functional specializations of mechanoreceptors have been detected depending on their location. Herbst corpuscles localized in organs of the upper gastrointestinal tract have a potential role in feeding behavior of different species of birds. They are involved in identification and manipulation of food. They are contributed in capture and handle the favorable food in beak of parrots and waterfowl [17]. Herbst and Grandry corpuscles in implication to detection of the prey via vibrotactile sensation [16,37]. Vibration sensitivity of Herbst corpuscles capable of monitoring the intensity of waves during insect burrowing [29]. Herbst corpuscles in the oropharynx are implicated in assisting in establishing an appropriate positioning of the tongue and laryngeal mound for cleaning the choana (internal nares) after swallowing [42]. Corpuscles in oropharynx are contributed in selection and transferring food [42]. Herbst corpuscles in the wings of pigeon act as vibration sensors which are involved in adjustment of the air flow to facilitate flying and control the flight behavior [43]. Both Herbst and Merkel receptors, which located in feathered skin, are implicated in detection of feather posture and serve in bird flight [27]. Wing skin has abundant mechanoreceptors, which exist around the feathers follicles. Mechanoreceptors seem to regulate flight. They monitor airspeed and detect a stall and turbulence [44]. Corpuscles situated in the legs probably act as an alarm for protection from danger [45]. Herbstscher strang in legs are implicated in detection of vibrations exerted in ground and earthquakes [21], in gallinaceous birds, responsible for the habitual feeding behavior such as scratch off the food.
In the current study, the topographical distribution of various types of mechanoreceptors was different within the two species. The type of the higher proportion among the sensory receptors in bill tip organ of duck beak was Herbst and Grandry corpuscles. The highest proportion of the sensory receptors was Herbst in the aboral surface of the lateral edge of the cranial part of duck beak, while Herbst corpuscles were the dominated type in the middle site of the mucosa of the middle and caudal part of duck beak. Merkel receptors were the most common type in the tip of the quail beak. Ruffini corpuscles prevailed the middle site of the oral mucosa of the cranial and caudal part of quail beak. Domination of particular type of sensory receptors in the duck and quail beak acquire them specific regional sensitivity. Duck beak seems to be designed mostly for Vibro-reception. The aboral surface of the lateral edge of the cranial part considered the most vibration sensing region, while the middle aspect of the oral mucosa of the middle and caudal portion had less vibration sensing capacity. The tip of quail beak mostly received pressure sensation. The majority of vibration sensors located in quail were located in the middle portion of the oral mucosa. The middle portion of the oral mucosa of the cranial and the caudal regions were the most sensitive region for stretching.
Species differences in the type and the distribution of the sensory units probably regard to nature of food. Quail beak adopts to eat harden food such as seeds, grains, vegetables, insects. Ducks also tend to feed on seeds, grains, vegetables, insects but usually favor to moisten their food. Organization of Herbst corpuscles in the beak is related to type of diet of birds [16]. Similar results are documented in different avian species. Herbst corpuscles comprise a major component of the highly sensing region; bill tip organ of geese [22], duck [21,46] and chicken [23]. The distribution of Herbst corpuscles exhibits regional differences in the oropharynx of different species of ratites particularly ostrich and emu which indicate variations of the tactile sensitivity between the two species [42]. The distribution of Herbst corpuscles is studied in the beak of fowl. The most anterior corpuscles are situated at the distance from the dertrum to the anterior edge of the external nares. These anterior corpuscles are at first dorsal, in the region of the culmen (aboral surface), but increasing numbers occur posteriorly and latero-ventrally. A few corpuscles extend posteriorly along the tomia (lateral edges) and some are present in the region of the exterior nares and the operculum. The second concentration of corpuscles occurs between the posterior half of the external nares and the sub-adjacent tomia [25]. Grandry corpuscles are restricted to the upper digestive tract of the aquatic bird. They locate in the dermal tissue of the duck and geese in the bill-tip organ [21] and beak, palate, and tongue of the mallard [19].
Analysis of the predominant proportions of the sensory receptors in the different divisions of duck and quail beak outlined the functional map of the beak in both species. Duck beak was mostly responding to vibration and pressure stimuli, while quail beak was more sensitive stretching and pressure. Stretching sensors occurred mainly in the cranial part of the duck beak. Velocity sensors mostly occurred in the cranial and caudal parts in duck. The cranial and the middle parts were the most sensitive areas for pressure sensation in duck. Pressure sensation almost occurred via Herbst and Ruffini receptors in the cranial and caudal parts in duck. In quail, the functional map was organized as the vibration and stretching in the cranial part, the pressure in the tip and the cranial portions. In duck, vibration sensors may distinguish movement of aquatic and land prey such as fish, Frogs, salamanders and amphibians, aquatic and land insects, Small crustaceans. Snails, and mollusks. Velocity sensors may detect Speed of water prey such as fish, water worms, insects. Pressures sensors seem to assist in dredging the ground searching for foods and identify texture of food either hard or moisten food and selection of favorite food such as Seeds, grain, Grass, leaves, and weeds. Stretching receptors may serve in the manipulation of the prey during movement. In quail, vibration receptors detect movement of the prey such as earthworm during digging the ground and locomotion of Insects. Pressures may serve as Peeling coated seeds as rice, barley, wheat, seeds of sunflowers. sensors may assist stretching in food manipulation. A comparative study explores the distribution of Herbst corpuscle in oropharynx of ostrich and Emu. Large number of Herbst corpuscle in the rostral or the cranial mandible compared with the caudal mandible [42].
References
- Saxod R (2013) Mechanoreceptors: Development, Structure, and Function, in P.H.T.S.R.V.a.Z. Jirina, Editor, Springer Science & Business Media. Germany, pp: 3-8.
- Crole MRL, du Plessis, Soley JT (2015) Morphological features of Herbst corpuscles in the oropharynx of the ostrich (Struthio camelus) and emu (Dromaius novaehollandiae). Anat Rec (Hoboken) 298: 783-96. [Crossref]
- Skieresz-Szewczyk K, Jackowiak H (2016) Morphofunctional study of the tongue in the domestic duck (Anas platyrhynchos f. domestica, Anatidae): LM and SEM study. Zoomorphology 135: 255-268. [Crossref]
- Chouchkov C (2012) Cutaneous Receptors, Springer Science & Business Media, pp: 30.
- Cunningham SJ, Alley MR, Castro I (2011) Facial bristle feather histology and morphology in New Zealand birds: implications for function. J Morphol 272: 118-128. [Crossref]
- Zelená J, Halata Z, Szeder V, Grim M (1997) Crural Herbst corpuscles in chicken and quail: numbers and structure. Anat Embryol (Berl) 196: 323-333. [Crossref]
- Burns RB, Wight PA (1970) The distribution of Herbst corpuscles in the foot of the domestic fowl (Gallus domesticus). Res Vet Sci 11: 585-587. [Crossref]
- Stettenheim P (2013) Avian Biology, in the integument of birds, Farner DS and James, (Eds), Elsevier: Netherlands: 16.
- Toyoshima K, Shimam2021 Copyright OAT. All rights reserv Corpuscles in the Tongue of the Finch, Lonchura-Striata. Cell Tissue Res 264: 427-436.
- Necker R (1999) Sturkie's Avian Physiology, Whittow (Ed), Academic Press, USA.
- Bancroft JD, Layton C, Suvarna SK (2013) Bancroft's Theory and Practice of Histological Techniques. Churchill Livingstone, London, 7th (Edn), United Kingdom.
- Harris HF (1898) A new method of ripening hematoxylin. In: Mikroskopische Technik. Romeis (Ed.), Oldenburg, München, Germany.
- Crossman GA (1937) A modification of Mallory's connective tissue stain with a discussion of principles involved. Anal Rec 69: 33-38.
- Mallory FB (1936) The aniline blue collagen stain Stain Technol 11: 101-110.
- Heidenhai NM (1896) Noch einmal uber die Darstellung der centralkorper durch Eisenhamatoxylin nebst einigen allgemeinen Bemer Kungen uber die Hamatoxylin fabren. Zeitchrift fur wissenchaftliche Mikroskopie und fur Mukroskopich technik 13: 180.
- Campbell B, Lack E (2013) A Dictionary of Birds. London, A&C Black, United Kingdom, pp: 604.
- Nadjafzadeh M (2011) Feeding Ecology of and Lead Exposure in a Top Predator: The White-tailed Eagle (Haliaeetus Albicilla), Berlin, Logos Verlag Berlin GmbH, Germany, pp: 108.
- Cunningham SJ (2013) The anatomy of the bill tip of kiwi and associated somatosensory regions of the brain: comparisons with shorebirds. PLoS One 8: e80036. [Crossref]
- Berkhoudt H (1980) The Morphology and Distribution of Cutaneous Mechanoreceptors (Herbst and Grandry Corpuscles) in Bill and Tongue of the Mallard (Anas-Platyrhynchos L). Netherlands Journal of Zoology 30: 1-34. [Crossref]
- Zelená J (1994) Nerves and Mechanoreceptors: The Role of Innervation in the Development and Maintenance of Mammalian Mechanoreceptors, Springer Science & Business Media, Germany, pp: 147.
- Watanabe I, Usukura J, Yamada E (1985) Electron microscope study of the Grandry and Herbst corpuscles in the palatine mucosa, gingival mucosa and beak skin of the duck. Arch Histol Jpn 48: 89-108. [Crossref]
- Gottschaldt KM, Lausmann S (1974) The peripheral morphological basis of tactile sensibility in the beak of geese. Cell Tissue Res 153: 477-96. [Crossref]
- Gentle MJ, Breward J (1986) The bill tip organ of the chicken (Gallus gallus var. domesticus). J Anat 145: 79-85. [Crossref]
- Waldman SD (2016) Pain Review E-Book, Elsevier Health Sciences, USA, pp: 112.
- Malinovsky L (2013) Biology of the Integument: 2 Vertebrates, Bereiter-Hahn J, Matoltsy AG, Richards KS (Eds) Springer Science & Business Media, USA, pp: 534-549.
- Halata Z, Grim M, Bauman KI (2003) Friedrich Sigmund Merkel and his "Merkel cell", morphology, development, and physiology: review and new results. Anat Rec A Discov Mol Cell Evol Biol 271: 225-39. [Crossref]
- Beason RC (2012) Through a Bird’s Eye-Exploring Avian Sensory Perception. J Ornithol 153: S23-S48.
- Hisa Y (2016) Neuroanatomy and Neurophysiology of the Larynx, Springer, Germany, pp: 8.
- Saxod R (2013) Mechanoreceptors: Development, Structure, and Function. Jirina (Eds) Springer Science & Business Media, Germany, pp: 3-8.
- During MV, Andres KH (2012) The Primary Afferent Neuron: A Survey of Recent Morpho-Functional Aspects. Zenker W, Neuhuber WL (Eds), Springer Science & Business Media, Germany, pp: 10-12.
- Wight PAL, Siller WG, Mackenzie GM (1970) The distribution of herbst corpuscles in the beak of the domestic fowl. J Br Poult Sci 11: 165-170.
- Weijs WA (2012) Biomechanics of Feeding in Vertebrates. Bels VL, Chardon M, Vandewalle P (Eds), Springer Science & Business Media, Germany, pp: 265.
- Thewissen JGM, Nummela S. Sensory Evolution on the Threshold: Adaptations in Secondarily Aquatic Vertebrates.
- Gottschaldt KM (1985) Structure and function of avian somatosensory receptors, in Form and Function in Birds, King AS, McLelland J (Eds) London, Academic Press, United Kingdom, pp: 375-461.
- Zweers GA, Gerritsen AFC (1997) Transitions from pecking to probing mechanisms in waders. Netherlands Journal of Zoology 47: 161-208. [Crossref]
- Gottschaldt KM (1982) Thermosensitivity and its possible fine-structural basis in mechanoreceptors in the beak skin of geese. J Comp Neurol 205: 219-245. [Crossref]
- Walsh S, Milner A (2011) Living Dinosaurs: The Evolutionary History of Modern Birds. Dyke G, Kaiser G (Eds), John Wiley & Sons, USA, pp: 292.
- Iggo A (2011) Somatosensory and Visceral Receptor Mechanisms. Iggo A, Ilyinsky OB (Eds), Elsevier, USA.
- Yin J, Wang H, Racey P, Zhang S (2009) Distribution and ultrastructure of Merkel cell of the fishing bat (Myotis ricketti). Sci China C Life Sci 52: 802-806. [Crossref]
- Sterbing-D'Angelo S, Chadha M, Chiu C, Falk B, Xian W, et al. (2011) Bat wing sensors support flight control. Proc Natl Acad Sci U S A 108: 11291-11296. [Crossref]
- Carter R (2014) The Brain Book, London. Dorling Kindersley Ltd, United Kingdom, pp: 102.
- Crole MR, Soley JT (2014) Comparative distribution and arrangement of Herbst corpuscles in the oropharynx of the ostrich (Struthio camelus) and emu (Dromaius novaehollandiae). Anat Rec (Hoboken) 297: 1338-1348. [Crossref]
- Horster W (1990) Histological and Electrophysiological Investigations on the Vibration-Sensitive Receptors (Herbst Corpuscles) in the Wing of the Pigeon (Columba-Livia). J Comp Physiol A Neuroethol Sens Neural Behav Physiol 166: 663-673.
- Brown RE, Fedde MR (1993) Airflow sensors in the avian wing. J Exp Biol 179: 13-30.
- Shen JX, Xu ZM (1994) Response Characteristics of Herbst Corpuscles in the Interosseous Region of the Pigeons Hind-Limb. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 175: 667-674.
- Berkhoudt H (1975) The Epidermal Structure of the Bill Tip Organ in Ducks. Netherlands Journal of Zoology 26: 561-566.












