Thyroid hormone (TH) plays a profoundly important role in fetal neurological maturation. The differentiation and maturation of the brain is chronologically programmed by TH [1]. The changes in TH levels in fetuses may have serious and long-term neurodevelopmental consequences. In humans, early diagnosis and treatment are essential to ensure the normal Central Nervous System (CNS) development and prevent the sequelae of congenital hypothyroidism; cretinism is the most serious form. Currently, screening for congenital hypothyroidism is initiated at 2 to 3 days after birth by measuring TSH levels in the neonatal heel blood and starting therapy in the postnatal period. This neonatal screening strategy may be late for securing a normal brain development that starts at first trimester of pregnancy [2,3]. A significant number of school age children diagnosed hypothyroidism in utero had mild to moderate leaning attentional problems, despite early diagnosis and treatment postnatally [4,5]. A fetal functional marker in maternal serum or urine would provide a convenient method for screening congenital hypothyroidism in utero, rather than postnatally.
Sulfoconjugation is the major pathway for thyroid hormone metabolism in mammalian fetuses
In sheep study, we have demonstrated that the high production rate (µg/kg/d) of T4 sulfate (T4S) in fetuses reflects the dominate role of the sulfation pathway in TH metabolism. It also predicts that 3,3’-diiodothyronine sulfate (T2S) is a major thyroid hormone metabolite in the fetus [6]. The high gradient between fetal and maternal serum concentrations of iodothyronine sulfates raises the possibility that there may be significant fetal to maternal transfer of sulfated TH metabolites (iodothyronine sulfoconjugates). When the ovine fetus was infused with pharmacological amounts of T3 or T3S, significant fetal to maternal transfer of T2S and T3S occurred [6-8]. In humans, we found high levels of radioimmunoassayable T2S in maternal serum [9] and urine [10].
W-Compound, a T2S-Crossreactive Material, as a Potential Marker for Fetal Thyroid Function:
In humans, we found high levels of radioimmunoassayable T2S in maternal serum [9,11]; levels increased with the progression of pregnancy and peaked before parturition. At delivery, a 20-fold increase in serum “T2S” was found compared to non-pregnant women and “T2S” levels returned to non-pregnant values in 7 to 10 days (Figure 1,2). On a closer examination, the radioimmunoassayable “T2S” did not cochromatography with synthetic T2S by HPLC [9]. Over 40 known synthetic thyroid hormone analogs were examined and none was found to be identical to the T2S-like material in pregnant women’s serum [11]. Thus, the name W-Compound was given. It is postulated that W-Compound is a side-chain modification of T2S, which cross-reacts with T2S antibody but is slightly more hydrophobic than T2S. Consistent with being an analogue of iodothyronine, we recently found high level of iodine content in highly purified W-Compound preparation analyzed by a Triple Quadrupole ICP-MS (Inductively Coupled Plasma Mass Spectrum) (Xi BX, Synold T, Wu SY. unpublished results).
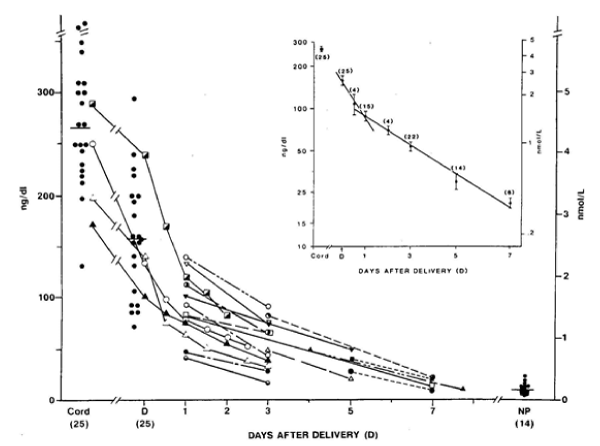
Figure 1: Concentrations of T2S and W-compound in cord serum of newborns and W-compound levels in maternal serum samples at the time of deliver (D). The connected lines represent serial measurements in the same patients (n = 18). T2S concentrations also were measured in 14 nonpregnant women (NP) for comparison. The decrease in serum W-compound concentrations after parturition is depicted in the semilog plot in the inset. The closed circles in vertical bars represent the mean (±SEM) and (n) represent the total number of samples studied at each time period in a total of 35 patients.
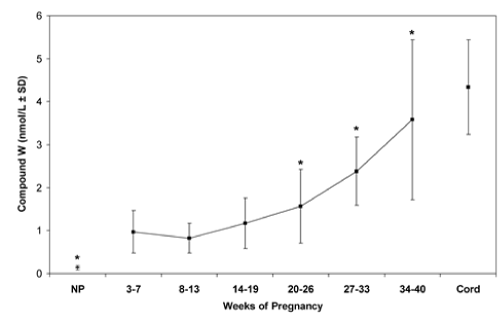
Figure 2: Normal values of T2S-crossreactive material (W-Compound) in serum from pregnant women, nonpregnant women, and newborns. Vertical bars are mean ± 1 SD. * p < 0.05 cf. 3-7 weeks pregnancy.
In normal pregnancies, both maternal and fetal W-Compound levels increased progressively with a significant direct correlation (p<0.001, in both mothers and fetuses) [12] (Figure 2). In addition, in 436 paired cord and maternal sera obtained from women at delivery, a highly significant correlation was found between the concentrations of Compound W in newborn cord and maternal serum (p<0.01) [12]. A significant positive correlation was observed in serum levels between fetal W-Compound and fetal T4 (p<0.003) and between maternal and fetal W-Compound (p<0.0001) [12] whereas no correlation was observed between maternal serum W-Compound and maternal serum T4 in euthyroid or hyperthyroid women. Thus, these data strongly suggest the fetal origin of W-Compound.
To further explore the possible origin of W-Compound, the serum concentrations of sulfated iodothyronines from cord arterial and venous samples were compared. There were no significant differences between the mean T3S, T4S, or reverse-T3S (rT3S) concentrations of arterial and venous serum samples. However, the venous T2S-equivalent concentration was higher than arterial in seven of the paired samples and lower in two. The mean “corrected” W concentration in nine pairs of cord serum was found to be significantly higher in venous samples than in arterial samples [11]. In addition, the mean of the maternal serum concentrations of T2S-reactive material was significantly lower than that of the paired cord serum concentrations. The rapid disappearance of W from maternal serum immediately after delivery supports this hypothesis [9] (Figure 1). A similar disappearance slope of serum W was also found in newborn infants [13]. These findings support the postulation that W is produced in the placenta with iodothyronine precursor of fetal origin.
Sulfoconjugation is a major metabolic pathway for thyroid hormone in developing mammals. The significant rise of sulfated iodothyronines in mammalian fetal compartments raises the possibilities that significant fetal to maternal transfer of the TH sulfo-conjugates may occur in the late gestation as the fetal hypothalamic-pituitary-thyroid system become more mature. This transfer may be a novel mechanism to maintain low T3 states or regulate serum 3,3’-T2, a thermogenic hormone [14], that is important for normal tissue maturity. The possibility that the transferred iodothyronine sulfate, especially 3,3’-T2S and its metabolite may serve as a marker of fetal thyroid function needs to be further explored. To facilitate the further study on W-Compound as a fetal thyroid function marker, we have recently developed a non-isotopic method [15].
- Bernal J (2015) Thyroid hormones in brain development and function, NCBI Bookshelf, www.endotext.org. Updated September 2, 2015.
- Stiles J, Jernigan TL (2010) The basics of brain development. Neuropsychol Rev 20: 327-348. [Crossref]
- Clairman H, Skocic J, Lischinsky JE, Rovet J (2015) Do children with congenital hypothyroidism exhibit abnormal cortical morphology. Pediat Res 78: 286-297. [Crossref]
- Rovet JF (1999) Long-term neuropsychological sequelae of early-treated congenital hypothyroidism: Effects in adolescence. Acta Paediatrica Suppl 88: 88-95. [Crossref]
- Leger J, Ecosse E, Roussey M, Lanoe JL, Larroque B (2011) French Hypothyroidism Study Group. Subtle health impairment and socioeducational attainment in young adult patients with congenital hypothyroidism diagnosed by neonatal screening: a longitudinal population-based cohort study. J Clin Endocrinol Metab 96: 1771-1782. [Crossref]
- Wu SY, Green WL, Huang WS, Hays MT, Chopra IJ (2005) Alternate pathways of thyroid hormone metabolism. Thyroid 15: 945-960. [Crossref]
- Wu SY, Polk D, Fisher DA, Huang WS, Reviczky AL, et al. (1995) Identification of 3,3’-diiodothyronine sulfate (3,3’-T2S) as a fetal thyroid hormone derivative in maternal urine in sheep. Am J Physiol 268: E33-E39. [Crossref]
2021 Copyright OAT. All rights reserv
- Wu SY, Polk DH, Huang WS, Fisher DA (1999) Fetal-to-maternal transfer of 3,3’5-triiodothyronine sulfate and its metabolite in sheep. Am J Physiol 277: E915-E919. [Crossref]
- Wu SY, Polk DH, Chen WL, Fisher DA, Huang WS, et al. (1994) A 3,3’-diiodothyronine sulfate crossreactive compound in pregnant women serum. J Clin Endocrinol Metab 78: 1505-1509. [Crossref]
- Wu SY, Fisher DA, Huang WS, Beck-Peccoz P, Emerson CH, et al. (1998) Urinary compound W in pregnant women is a potential marker for fetal thyroid function. Am J Obst-Gynecol 178: 886-891. [Crossref]
- Wu SY, Huang WS, Ho E, Wu ESC, Fisher DA (2007) A 3,3’-diiodothyronine sulfate cross-reactive substance,compound W, in serum from pregnant women – a potential marker for fetal thyroid function, Pediatr Res 61: 307-312.
- Cortelazzi D, Morpurgo PS, Zamperini P, Fisher DA, Beck-Peccoz P, et al. (1999) Maternal compound W serial measurements for the management of fetal hypothyroidsm. Eur J Endocrinol 141: 570-578. [Crossref]
- Chen D, Yu H, Bao J, Xue W, Xing Y, et al. (2012) 3, 3’-Diiodothyronine sulfate cross-reactive material (Compound W) in human newborns. Pediatr Res 72: 521-524. [Crossref]
- Lanni A, Moreno M, Cioffi M, Goglia F (1992) Effect of 3,3’-diiodothyronine and 3,5-diiodothyronine on rat liver oxidative capacity, Mol Cell Endocrinol 86: 143-148. [Crossref]
- Huang B, Yu H, Bao J, Zhang M, Green WL, et al. (2018) A homogeneous time-resolved fluorescence immunoassay method for the measurement of Compound W. Biomark Insights 13: 1177271918757484 . [Crossref]


