Background: Chronic low back pain is one of the most debilitating medical problems worldwide and difficult to treat. New methods are wanted. A cotton mat with 30% silica fibres produced from traditional Korean clay by nano-technology, fibrilium, has shown very good informal results in practice. We tested this mat for clinical effectiveness.
Methods and Findings: Randomized, placebo-controlled, blinded clinical trial in 50 chronic low back pain patients (median duration of illness 10 years; pain severity > 5 on a 10 point scale); 2 patients withdrew, 48 were analyzed. The study was fully blinded. Intervention was sleeping on a fibrilium mat on top of a normal matrace for 4 weeks or on a mat with only cotton fibres. Outcomes were functional scores (Oswestry Disability Score, Linton-Halldén Score, McGill Pain Questionnaire), sleep quality (Pittsburgh Sleep Quality Index), generic quality of life (SF 36), clinical ratings (pain, wellbeing), and physiological measures (skin conductance, heart rate, breathing frequency).
All 5 multivariate linear models with baseline scores as covariates showed significant differences between groups in all measures (p < .038 to p < .0.0002), with large effect sizes for the functional scores between d = 0.8 and d = 1.4. We observed no negative effects. The trial’s main limitation is its short observation period.
Conclusion: Sleeping on a fibrilium mat that contains 30% fibres made from silica containing clay using nano-technology has a strong therapeutic effect on chronic low back pain.
chronic low back pain placebo-controlled, randomized clinical trial, RCT, nano-technology, silica
Chronic low back pain is the most prevalent disorder worldwide regarding years lived with disability [1], and it is not easy to treat effectively, once it has set in. Recent guideline, especially in the light of the opioid crisis, discourage the usage of medication for chronic sufferers [2] and encourage active treatments such as exercise, which, however, only has a small to moderate effect size [3]. Prevention is possible by regular movement and exercise, but effects are not robustly proven, nor will they work for everyone [4]. Kinesio tapes are often used but have only small benefits [5]. Thus, the quest for a safe treatment that might also be used in a preventive way without requiring much effort or resources is still open.
It was in this light that we studied a new product that is alleged to treat and prevent pain syndromes by a fabric that contains up to 30% fibres produced from nano-particles, mostly special silicate structures, from a traditional Korean clay that is said to contain healing properties. This clay has been engineered into specific fibres using nanotechnology that can be woven into traditional cotton fabrics. In the case at hand a fabric, called fibrilium is woven into a cotton mat (30% fibrilium, 70% organic cotton) that is then used as mat to cover the conventional sleeping matrace to be slept on in the night. The hypothesis that this fabric reflects the body’s infrared and near-infrared radiation (4-14 nm wavelength) was supported by a recent laboratory The current research was set up to test the claim that the specific mat, containing 30% fibrilium fabric, is effective as a treatment for chronic pain patients. We set out to conduct three parallel blinded and placebo controlled randomized trials in patients with chronic low back pain, premenstrual syndrome and arthralgic pain, with 50 patients in each trial (150 patients each), conducted according to GCP. This is the first of this series of three trials which we report. It shows a very strong clinical effect in this strictly blinded and placebo-controlled trial over 4 weeks in chronic low back pain patients.
Patients
Patients were recruited from general practice via word of mouth and invitation by MT, a GP specialist for sports medicine. They had to suffer from chronic low back pain (i.e. pain duration at least 6 weeks or longer), with a pain level of at least 5 on a 10-point numerical rating scale and an age between 30 and 70 years. Patients of both genders were included equally. Since the recruitment center was in the central part of Austria with a homogeneous population, ethnic aspects were irrelevant for this study. Exclusion criterion was a previous surgical intervention to treat low back pain or any other surgical intervention on the spine. Patients were fully informed and signed an informed consent.
Interventions
The intervention was the usage of a fibrilium mat to sleep on for 4 weeks, or a placebo mat which did not contain the fibrilium fibres. Both mats were indistinguishable for patients and providers and were delivered in numbered packs to the GP who handed them out to the patients after consent was signed and the randomization result produced. The patient was supposed to put the mat on top of their normal sleeping matrace and cover it with a regular linen sheet.
Randomisation and Blinding
Randomisation was conducted using the web-applicatoin www.randomizer.org. A person not otherwise involved in patient care accessed the randomization application and gave out the result to the physician, after the patient was enrolled in the study. According to this randomisation result one of the mats were handed out to the patient. As neither the treating GP nor the patient knew the content of the batches, and as the fabric itself cannot be distinguished from normal cotton fabric by simple means of touching and looking, the study was blinded in the sense that allocation to treatment and treatment itself were blinded, as well as the outcome measurements.
Outcome Measurement
Patients filled in a set of questionnaires at the initial visit and after inclusion into the study and after 4 weeks at a second visit to the clinic. At those occasions also heart rate, breathing and skin conductance level was measured as indicators of autonomic arousal. The physiological measurements took place after 5 minutes rest while seated for 10 minutes, using the Schuhfried Biofeedback 2000x-pert (Schuhfried, Vienna, Austria), radio moduls “Multi” and “Resp”. Skin conductance level (SCL) was measured by throwaway finger electrodes on the right indexfinger (sampling rate 2 kHz, measurement range 0 – 50 µSiemens; max error 0,65 µS; resolution 0,012 µS. Pulse rate was measured by period length of the blood volume pulse (RR-distances) and reflects heart rate, sampled at 500 Hz with a measurement range between 30 and 200 beats per minute. Respiration rate was measured with the modul “Resp” and an abdominal breathing belt. The measurement range is between 0,02 – 60 breaths per minute and is measured over an area of 20 cm with a resolution of 0,2 mm. The data were registered online and sent to the computer via a radio module, downsampled, analyzed and displayed graphically. The mean values, range and standard deviation for the measurement period per subject were recorded and used for further analysis. Apart from a clinical interview and the clinical measurements, patients filled in questionnaires: the SF 36 in its appropriate German version to document Quality of Life [7]; the McGill pain questionnaire in its short form [8-10]; the Pittsburgh Sleep Quality Index [11,12]; the Oswestry Low Back Pain Disability Score [13,14] and the Linton-Halldén Score [15]. All questionnaires were filled in by patients at their leisure. Since neither provider nor patients knew about their treatment allocation outcome measurement was fully blinded. As there was no experience with this intervention, all clinical outcomes (McGill pain questionnaire, Oswestry Low Back Pain Disability, Linton-Halldén and Pittsburgh Sleep Quality Index) and the physiological measures were treated as equally important. Quality of Life (SF 36) was considered secondary outcome.
Ethics
The study was conducted according to GCP guidelines and conforming to the declaration of Helsinki. Patients were fully informed and no invasive intervention was applied other than regular medical procedures and the application of a mat to sleep on. Patients were informed that the treatment might have a benefit, but that the clinical efficacy of the mat was unknown, which was the reason why it would have to be tested in a blind setting. The requirement of two GP visits within 4 to 5 weeks was normal procedure, and the application of physiological measurement was non-invasive. Considering the overall balance of benefit to harm patients had a reasonable chance to improve without any gross inconvenience, financial or other investment.
The study was submitted to and approved by the ethics committee in Carinthia with the EK-No: A35/16.
Statistics, Power, Data-Management
The estimation of patient numbers started from a pragmatic stance. Considering the empirical experience with the mat that single therapists and providers had we estimated that the effect would be sizeable and reasoned that with a pragmatically relevant effect size of d = 0.8 we would detect an effect with reasonable (i.e. 80%) chance if 25 patients were included in each arm. Any larger effect would be detectable with an even higher chance. As clinical trials must, from an ethical point of view, include as few patients as possible and as many as necessary the target of 25 patients per group seemed a reasonable choice.
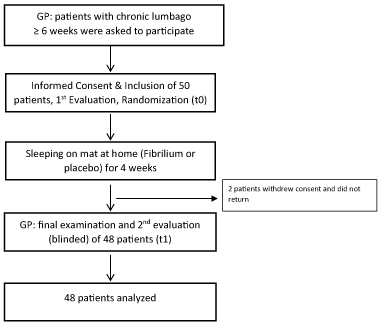
Data management and statistics was handled blindly as well. Two patients did not return and were considered having withdrawn their consent, because they were unavailable and did not answer multiple phone calls. Apart from these two patients, all clinical data were complete and no missing data treatment was necessary. It was decided to not interpolate data of these two patients and analyze the remaining full data set, as no information about the withdrawal of these patients could be obtained and this was the first study of its kind. Some physiological data were not available due to instrumentation failure or movement artefacts. Since there was no systematic relationship with treatment missing data were not interpolated and physiological data were evaluated as given.
Since the protocol did not stipulate any detailed analyses an analysis plan was developed checking for data integrity and distribution but before running any exploratory analyses. According to this plan five separate linear models with baseline scores as covariates were to be calculated: one for the three main pain and disability indeces (McGill, Oswestry, Linton-Halldén), one for the scales of the SF36, one for the Pittsburgh Sleep Quality Index, one for two single numerical rating scales of well-being and pain from the clinical interview, and one for the physiological data. In case of multivariate significance of the model univariate models were explored to study the source of the effect and graphical inspection of residuals were used to check for the adequacy of the model. Only after all analyses were finished and a first report logged were the codes broken. All analyses were calculated with Statistica Version 13.
Between Jan 2017 and Dec 2017 altogether 50 patients were entrolled in the trial. The trial flow is depicted in Figure 1. The baseline characteristics of the patients are given in Table 1. As can be seen from Table 1, randomization worked well and the groups were well balanced, confidence intervals all overlapping. All outcome data except SCL were approximately normally distributed and fulfilled the preconditions for linear modelling. SCL data were therefore log-transformed.
Table 1. Sociodemographic Variables and Outcome Variables (means, standard deviations & 95% confidence intervals; frequencies, percent) at Baseline
Variable |
Fibrilium (n=22) |
Placebo (n=26) |
Total (n=48) |
Age |
46,8 (9,0) |
44,3 (8,4) |
45,4 (8,7; 42,89-47,95) |
Weight (kg) |
75,4 (11,9) |
71,9 (14,7) |
73,5 (13,5; 69,6-77,4) |
Height (cm) |
173,2 (7,6) |
172,8 (8,7) |
172,9 (8,1; 170,6-175,3) |
Duration of Back Pain (years) |
10,1 (8,0) |
9,0 (9,9) |
9,5 (9,0; 6,9-12,2) |
Relationship with doctor (1: sufficient; 3: very good) |
2,1 (0,7) |
2,2 (0,8) |
2,16 (0,7; 1,9-2,4) |
Expectation (0: no improvement; 10: free of pain |
5,7 (1,9) |
5,8 (1,9) |
5,8 (1,9; 5,2-6,3) |
Gender: female |
15 (57,7%) |
12 (54,5%) |
27 (56 %) |
Education: |
basic |
9 (34,6 %) |
13 (59%) |
22 (45,8%) |
A-Level |
6 (23%) |
2 (9%) |
8 (16,7%) |
College |
3 (11,5%) |
3 (13,6%) |
6 (12,5%) |
University |
8 (30,7%) |
4 (18,2%) |
12 (25%) |
Smoker: |
no |
21 (80,8%) |
14 (66,7%) |
35 (74,5%) |
Yes: 1-10
Cigarettes per day |
3 (11,5%) |
3 (14,3%) |
6 (12,8%) |
Yes: 11-20 per day |
2 (7,7%) |
4 (19%) |
6 (12,8%) |
Alcohol: |
|
|
|
never |
4 (15,4%) |
3 (13,6%) |
7 (14,6%) |
Rarely |
7 (27%) |
9 (41%) |
10 (33,3%) |
Sometimes |
14 (53,8%) |
9 (41%) |
23 (47,9%) |
Often |
1 (3,8%) |
1 (4,5%) |
2 (4,2%) |
Coffe: |
never |
3 (11,5%) |
2 (9,1%) |
5 (10,4%) |
Rarely |
4 (15,4%) |
0 (0%) |
4 (8,3%) |
Sometimes |
0 (0%) |
4 (18,2%) |
4 (8,3%) |
Exercise: |
never |
0 (0%) |
1 (4,5%) |
1 (2,1%) |
Rarely |
3 (11,5%) |
6 (27,2%) |
9 (18,7%) |
Sometimes |
17 (65,4%) |
9 (40,9%) |
26 (54,2%) |
Often |
6 (23,1%) |
4 (18,2%) |
10 (20,8%) |
Daily |
0 (0%) |
2 (9%) |
2 (4,2%) |
Outcome Measures: Self-Report |
Pain Severity |
5,9 (1,3) |
5,5 (1,6) |
5,7 (1,4; 5,2-6,1) |
General Feeling |
5,9 (1,9) |
5,5 (1,9) |
5,7 (1,9; 5,1-62) |
SF36 Physical Functioning |
75,4 (16,8) |
79,0 (19,3) |
77,4 (18,1; 72,1-82,7) |
SF36 Role Physical |
57,9 (35,7) |
55,8 (36,3) |
56,8 (35,6; 46,4-67,1) |
SF36 Role Emotional |
68,2 (40,5) |
75,6 (40,6) |
72,2 (40,3; 60,5-83,9) |
SF36 Energy |
46,8 (17,6) |
41,0 (17,5) |
43,6 (17,6; 38,5-48,8) |
SF36 Emotional Wellbeing |
62,5 (17,9) |
59,8 (17,5) |
61,1 (17,5; 56,0-66,2) |
SF36 Social Functioning |
68,7 (23,1) |
65,4 (21,6) |
66,9 (22,1; 60,5-73,3) |
SF36 Pain |
44,0 (13,3) |
48, 1 (15,4) |
46,2 (14,4; 42,0-50,4) |
SF36 General Health |
63,6 (19,3) |
59,8 (19,0) |
61,6 (19,0; 56,0-67,1) |
Pittsburgh Sleep Quality Index |
8,2 (3,0) |
9,5 (4,2) |
8,9 (3,7; 7,8-10,0) |
McGill Total Pain Score |
13,9 (6,9) |
13,0 (8,0) |
13,4 (7,4; 11,3-15,6) |
Oswestry Disability Index |
21,5 (13,2) |
21,8 (12,7) |
21,7 (12,8; 18,0-25,4) |
Linton-Hallgrén Score |
78,7 (24,9) |
85,7 (21,9) |
82,5 (23,3; 75,7-89,3) |
Outcome Measures: Physiology* |
Pulse |
71,4 (9,5) |
70,8 (10,9) |
71,1 (10,2; 68,1-74,1) |
Skin Conductance Level |
3,7 (5,3) |
3,5 (7,7) |
3,6 (6,7; 1,7-5,6) |
Breathing Frequency |
16,0 (3,4) |
16,1 (3,9) |
16,1 (3,7; 15,0-17,2) |
*One missing data set for physiology data at baseline in Fibrilium group due to instrumentation failure
Linear Models
All linear models were significant. Results of the statistical analyses are given in Table 2. Results of the outcome measures are presented in Table 3.
Table 2. Results of the Linear Models of Outcome Measures – Multivariate Statistics for Models and Univariate Estimates for Variables; testing was multivariate for each model first, except for sleep quality index, which was only univariate
|
Models/ Variables |
Wilk’s Lamda for Multivariate Model/
Adjusted R2 for Univariate Analysis |
F |
P
|
|
Model 1 – Pain Scores |
0.65 |
F4/41 = 7.39 |
0.0005 |
|
McGill Total Score |
0.46 |
F4/43 = 11.08 |
0.000003 |
|
Oswestry Disability Index |
0.48 |
12.05 |
0.000001 |
|
Linton-Hallgren Score |
0.46 |
11.22 |
0.000001 |
|
Model 2 – Sleep: Pittsburgh Sleep Quality Index |
0.43 |
F2/32 = 18.3 |
0.000002 |
|
Model 3 – Quality of Life/SF36 |
0.51 |
F8/31 = 3.8 |
0.003 |
|
SF36 Physical Function |
0.32 |
F9/38 = 3.52 |
0.003 |
|
SF36 Role Limitation Physical |
0.45 |
5.35 |
0.0001 |
|
SF36 Role Limitation Emotional |
0.31 |
3.36 |
0.004 |
|
SF36 Energy |
0.31 |
3.32 |
0.004 |
|
SF36 Emotional Wellbeing |
0.43 |
4.89 |
0.0002 |
|
SF36 Social Functioning |
0.54 |
7.29 |
0.000005 |
|
SF36 Pain |
0.58 |
8.39 |
0.000001 |
|
SF36 General Health |
0.54 |
7.17 |
0.000005 |
|
Model 4 – Self Ratings |
0.57 |
F2/43 =16.4 |
0.000005 |
|
Pain Severity |
0.58 |
F3/44 =22.9 |
<0.00001 |
|
General Feeling |
0.37 |
10.44 |
0.00003 |
|
Model 5 – Physiology Data |
0.79 |
F3/36= 3.1 |
0.038 |
|
Breathing |
0.55 |
F3/38 =17.8 |
<0.00001 |
|
Skin Conductance Level |
0.03 |
1.4 |
0.2 |
|
Pulse |
0.41 |
10.5 |
0.00004 |
Table 3. Outcome Measures (weighted means*, 95% Confidence Intervals) after Treatment
Variable |
Fibrilium |
Placebo |
Pain Measures |
McGill Total Score |
8,46 (3,26-9,66) |
16,18 (12,94-19,42) |
Oswestry Disability Index |
15,23 (7,86-22,60) |
28,91 (22,63-35,18) |
Linton-Hallgren Score |
56,23 (41,50-70-96) |
83,54 (73,85-93,24) |
Pittsburgh Quality of Sleep Index |
4,58 (2,88-6,23) |
9,41 (8,22-10,60) |
Quality of Life |
SF36 Physical Function |
79,61 (69,32-89,91) |
67,04 (56,95-77,14) |
SF36 Role Limitation Physical |
75,0 (60,44-89,56) |
45,45 (30,69-60,22) |
SF36 Role Limitation Emotional |
85,90 (73,17-98,63) |
60,61 (45,73-75,48) |
SF36 Energy |
60,77 (52,21-69,33) |
42,27 (36,98-47,56) |
SF36 Emotional Wellbeing |
71,54 (62,82-80,26) |
51,64 (44,85-58,43) |
SF36 Social Functioning |
79,33 (69,34-89,32) |
56,82 (50,25-63,38) |
SF36 Pain |
69,52 (58,85-80,19) |
38,75 (31,41-46,09) |
SF36 General Health |
73,46 (64,62-82,31) |
54,54 (48,02-61,97) |
Self-Ratings |
Pain Severity |
3,15 (2,08-4,23) |
6,23 (5,49-6,97) |
General Feeling |
7,61 (6,85-8,38) |
5,23 (4,30-6,15) |
Physiological Variables |
Breathing |
15,44 (14,49 – 16,39) |
17,09 (16,05-18,14) |
Skin Conductance Level (transformed)
(untransformed raw mean) |
0,09 (-0,48-0,66)
3,79 (1,63-5,94) |
0,66 (0,03-1,29)
2,57 (0,93-4,20) |
Pulse |
68,68 (65,95-71,40) |
72,91 (69,91-75,91) |
*for skin conductance also untransformed, unweighted raw means are given.
Outcome was uncorrelated with expectation of treatment or trust in physician measured at baseline (data not shown).
This randomised, placebo controlled, blinded clinical trial showed that sleeping on a mat containing 30% fibrilium fibres, a fibre made from nano-particles of a special Korean silica soil, alleviates chronic low back pain. The result is unequivocal, as all outcome domains – pain scores, quality of life, sleep, self-ratings and physiological data – showed significant changes. The result is reliable, because the study was double-blind and bias provides no viable explanation. Effect sizes are large with d = 1.3 for the McGill Total score, d = 1.4 for the Pittsburgh Sleep Quality Index, d = 0.8 for the Oswestry Disability Index, and d = 1.0 for the Linton-Halldén Score. To our knowledge it is the first clinical study demonstrating the effect of this fibrilium mat in a clinical population.
The result is clinically relevant, as the patients had a chronic condition having suffered from chronic low back pain since 10 years and their current pain level was more than 5 on a 10 point rating scale. The randomisation resulted in nicely comparable groups, and hence undocumented variables such as other concomitant treatments are not a likely explanation for the differential improvements. Note that the placebo group either remained at their baseline level or was worse at post-treatment. Thus, unlike in other pain trials, we could not observe a placebo-effect of any relevant size and hence our contrast was strong. This is probably due to the fact that there is no general information available that textures or fibres may have an influence on pain and hence patients had likely no specific expectations regarding the treatment. This is bolstered by a lack of correlation between initial expectation and outcome. Hence, our comparison had a strong contrast and psychological effects are not a likely explanation.
This raises the question regarding possible mechanisms. There is very little research on the physiological effects of this fibre. One laboratory study shows that it reflects infrared and near-infrared radiation. One very obvious explanation would thus be that the mat provides the body with extra warmth during sleep and thus contributes to a relaxation or regulation of physiological processes during rest. Another potential explanation is a subtle feedback mechanism triggered by this fibre. It is well known that human and other organisms radiate ultra-weak pulses of light, so called biophotons [15-17]. It is unclear, whether they are simply reflections of metabolic processes, indicating free-radical accumulation [19], or whether there is more to it. At any rate, the reflection of infrared radiation might trigger an as yet unknown feedback process. Another potential explanation might be the enhancement of infrared radiation during sleep through this mat. Infrared radiation is used by all organisms to structure water in the interstitial tissue of cells, in the so called matrix [20]. This might support various physiological functions, from transport of nutrients to faster removal of waste products, which is one of the tasks of the interstitial tissue, among others [21-23]. The mat might contribute to accelerating this process during sleep and thus contribute to faster regeneration with the result of a reduction in pain.
Although the knowledge about this silica containing soil is ancient, it is only a recent advance through nanotechnology that the soil and the silicates contained therein can be engineered into fibres of various thickness. This might open up the venue for interesting kinds or applications, in fibres for clothing and other appliances. Hence our finding has an important bearing on future advances.
Our study has a few limitations that should be noted. Because there was a lack of knowledge regarding the potential effects the protocol stipulated a broad array of outcome measures. As it happened all of them were quite sensitive to change and showed strong effects apart from skin conductance. Thus, even if a very conservative procedure for multiple testing were to be applied the overall result would remain significant. We tried to mitigate the problem of multiple testing by using multivariate linear models that account for correlations between outcome measures in similar domains. All of them were quite unequivocal, with exception of the physiological data which just reached significance. The fact that the physiological data, at least breathing and pulse, supported the self-report findings is encouraging, except that skin conductance level is a notoriously difficult variable as it is influenced by many situational aspects and its distribution is far from ideal for linear modelling. The log-transformation could only partially remedy this, and hence this lack of sensitivity is likely due to the distributional features of this variable.
The study was small and unicentric. However, since the effect was so strong, a larger sample of patients would only have led to overpowering the study and not helped in increasing the reliability. Thus, what is needed are independent replications to avoid type 2 errors.
Apart from that we have produced the first set of reliable data documenting the clinical effect of a fibrilium mat in chronic pain patients against a placebo mat in a double-blind randomised study. We conclude that it is clinically effective in alleviating pain in chronic low back pain patients.
This study was funded by the International Society for Regenerative Research, Bad Vigaun, Austria, a public charity.
- Global Burden of Disease Study 2013 Collaborators (2015) Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990-2013;2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 386: 743-800.
- Qaseem A, Wilt TJ, McLean RM, Forciea M (2017) Noninvasive treatments for acute, subacute, and chronic low back pain: A clinical practice guideline from the american college of physicians. Ann Intern Med 166: 514-530.
- Chou R, Deyo R, Friedly J, Skelly A, Hashimoto R, et al. (2017) Nonpharmacologic therapies for low back pain: A systematic review for an american college of physicians clinical practice guideline. Ann Intern Med 166: 493-505.
- Steffens D, Maher CG, Pereira LS, Stevens ML, Oliveira VC, et al. (2016) Prevention of Low Back Pain: A Systematic Review and Meta-analysis. JAMA Intern Med 176: 199-208. [Crossref]
- Ilic B, Nikolic A, Ilic D (2017) Efficiency of kinesio taping in prevention and rehabilitation of sport injuries. Sportlogia13: 53-65.
- Tonelli MR (2013) Optical characterisation of probes of "cafissi" in the spectral range of micrometer. 4: 1-14.
- Bullinger M, Kirchberger I (1998) Der SF-36 Fragebogen zum Gesundheitszustand (SF-36). Handbuch für die deutschsprachige Fragebogenversion. Göttingen: Hogrefe.
- Dworkin RH, Turk DC, Farrar JT, Haythornthwaite JA, Jensen MP, et al. (2005) Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain 113: 9-19. [Crossref]
- Kiss I, Müller H, Abel M (1987) The McGill Pain Questionnaire--German version. A study on cancer pain. Pain 29: 195-207. [Crossref]
- Melzack R (1983) Pain Measurement and Assessment. New York: Raven Press.
- Backhaus J, Junghanns K, Broocks A, Riemann D, Hohagen F (2002) Test-retest reliability and validity of the Pittsburgh Sleep Quality Index in primary insomnia. J Psychosom Res 53: 737-740.
- Buysse D, Reynolds C, Monk T, Berman S, Kupfer D (1989) The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res 28: 193-213.
- Fairbank J, Davies J, Couper J (1980) The Oswestry low back pain disability questionnare. Physiotherapy 66: 271-273.
- Mannion AF, Junge A, Fairbank JC, Dvorak J, Grob D (2006) Development of a German version of the Oswestry Disability Index. Part 1: cross-cultural adaptation, reliability, and validity. Eur Spine J 15: 55-65.
- Linton SJ, Halldén K (1998) Can we screen for problematic back pain? A screening questionnaire for predicting outcome in acute and subacute back pain. Clin J Pain 14: 209-215. [Crossref]
- Hammerschlag R, Levin M, McCraty R, Bat N, Ives JA, et al. (2015) Biofield physiology: A framework for an emerging discipline. Glob Adv Health Med 4: 35-41.
- Ives JA, van Wijk EPA, Bat N, Crawford C, Walter A, et al. (2014) Ultraweak Photon Emission as a Non-Invasive Health Assessment: A Systematic Review. PLoS ONE 9: e87401.
- Popp FA, Biophotons - Background, experimental reuslts, theoretical approach and applications. Frontier Perspectives 11: 16-28.
- van Wijk R, van Wijk EPA, Wiegant FAC, Ives J (2008) Free radicals and low-level photon emission in human pathogenesis: State of the art. Indian Journal of Experimental Biology 46: 273-309.
- Pollack GH (2013) The Fourth Phase of Water: Beyond Solid, Liquid, and Vapour. Seattle: Ebner & Sons.
- Bordoni B, Marelli F (2017) Emotions in motion: myofascial interoception. Complementary Medicine Research 24: 110-113.
- Langevin HM, Keely P, Mao J, Hodge LM, Schleip R, et al. (2016) Connecting (t)issues: How research in fascia biology can impact integrative oncology. Cancer Research 76: 6159-6162.
- Tozzi P (2015) A unifying neuro-fasciagenic model of somatic dysfunction - underlying mechanisms and treatment - part I. J Bodyw Mov Ther 19: 310-326.