Purpose: Endometriosis is a chronic, estrogen-dependent, benign disease characterized by the presence of endometrial tissue outside the uterus. The benefit of nutritive complements on endometriosis-associated symptoms has been described. The purpose of this study was to evaluate in vivo the effect of probiotics on the development and pain due to endometriosis.
Method: We used a mouse model of endometriosis orally treated with one (P1: Saccharomyces Boulardii) or two probiotics (P2: Saccharomyces Boulardii + Lactobacillus Acidophilus), during 4 or 12 weeks. We followed the lesions growth by ultrasonography, the pain suffered by the mice with behavioral and qRT-PCR tests and their immune status by FACS and ELISA techniques.
Results: After 4 and 12 weeks of treatments, we observed that volume and size of the lesions were significantly lower. Clinically, heat sensitivity is decreased only by P1-treated at W4 while tactile sensitivity is higher in P2-treated mice. The levels of AOPP in the sera, reflect of the oxidation of proteins, is significantly reduced by all the treatments at W12. The serum levels of zonulin, a marker of the intestinal barrier permeability, and pro-inflammatory cytokines IL-6 and TNF-α were significantly reduced by the P1 and the P2 treatments.
Conclusion: Treatment with one or two probiotics, have different but both favorable effects on clinical, immune and physiologic parameters in endometriosis. Because of its better results on pain and a greater ease of handling, Saccharomyces boulardii seems to be more suited to be used as a new therapeutic strategy for endometriosis.
endometriosis, probiotics, inflammation, pain, saccharomyces boulardii, lactobacillus acidophilus
Endometriosis is a chronic, estrogen-dependent, benign disease characterized by the presence of endometrial tissue outside the uterus. The ectopic tissue is composed of the endometrial epithelium and stroma, and its growth is hormone-dependent and responds to the same cyclic variations as the eutopic endometrium. The prevalence of endometriosis varies from 6 to 10% in the general population of women of childbearing age and from 35 to 50% in women consulting for pelvic pain and / or infertility [1]. Its frequency makes it a real public health issue. The pathogenesis notably involves chronic inflammation, oxidative stress and fibrosis. The most accepted mechanism for the etiology of endometriosis is a dissemination of endometrial tissues from the uterus to the peritoneal cavity by retrograde menstrual flow. As the phenomenon of retrograde menstruations concerns a majority of cycling women, it suggests that individual susceptibilities are implicated. For example, intrinsic specific alterations of eutopic and/or ectopic endometrium gaining high survival capacity, invasive and proliferative capability [2], as well as defective immune clearance of ectopic cells have been described. Recently, it has been suggested that the ectopic implantation seen in endometriosis could be promoted by increased inflammation and priming of adaptive T regulatory cells, resulting in impaired cytotoxicity and enhanced immune suppression [3]. The pathophysiology of endometriosis is complex and still poorly understood [4]. Medical treatments based on hormonal therapies are neither always effective nor free of side effects [5]. The radical surgical treatment of endometriosis lesions has shown its effectiveness but presents a real risk of complications [6]. Patients with endometriosis are at high risk of several chronic diseases, such as autoimmune diseases, cancer, asthma/atopic diseases, cardiovascular diseases [7] and inflammatory bowel diseases [8].A better understanding of the mechanisms of endometriosis would pave the way for new medical treatments.
A recent review [9] described the benefit of additional intake of fatty acids, antioxidants and a combination of vitamins and minerals on endometriosis-associated symptoms. More studies are necessary to gain evidence on food products or nutrients efficacy to alleviate endometriosis syndromes, with an attention to their relative amounts [9]. The animal gut is a complex ecosystem of host cells, microbiota, and available nutrients, and the microbiota prevents several degenerative diseases in humans and animals via immunomodulation. Probiotics are microbial strains that are beneficial to health, and their potential has recently led to a significant increase in research interest in their use to modulate the gut microbiota [10]. The gut microbiota is involved in several immune, metabolic, and nutrients absorption functions that are crucial for the host homeostasis [11,12]. The gut microbiota contributes to innate and adaptive immunity [13,14]. Gut microbial metabolites play key roles in inflammatory signaling, interacting both directly and indirectly with host immune cells. They prevent colonization by opportunistic pathogens and participate to the maintenance of intestinal epithelial barrier [15].
A bidirectional interaction between the gut and vaginal microenvironments is evidenced but not well studied. Some microorganisms colonize both the gastrointestinal and the reproductive tracts proving the interaction between these microbiomes. Ingestionof lactobacillusprobioticshas been shown to induce changes in the vaginal bacterial community, as well as an inflammatory response [16]. This provides evidence of interaction between the vaginal and gastrointestinal microbiomes, and supports the proposition that oral probiotics may reduce some vaginal infection and bacterial vaginosis [17,18]. Oral probiotics can moderate pro-inflammatory cytokine expression in the vagina, which can subsequently alter the inflammatory status in the gut by acting on bacteria and immune mediators including macrophages, lymphocytes and dendritic cells. Both the gut and the female genital tract microbiota produce fermentation by-products that can influence local immunological responses with systemic implications.
The purpose of this study was to evaluate in vivo the effect of oral probiotics on the development of endometriosis, but also on the pain caused by the lesions. The pain that occurs during endometriosis pathology may be nociceptive, inflammatory, neuropathic or a mix of these [19].The experience of pain is complex involving many mechanisms and interactions between the periphery and the central nervous system [20]. Recent work has shown alterations in both the peripheral and CNS of women with endometriosis-associated pain [20,21]. Oral administration of specificLactobacillusstrains can mediate analgesic functions in the gut, similar to the effects of morphine [22]. The microbiology of the intestinal tract could influence visceral perception and could be used for the treatment of abdominal pain occurring in endometriosis.
Therefore, we used a mouse model of endometriosis (syngeneic uterine horn peritoneal graft model). We assessed the effects of Saccharomyces Boulardii and Saccharomyces Boulardii/Lactobacillus Acidophilus per os on the evolution of endometriosis in mice. Studies have been previously conducted with these two probiotics to determine their effects on endometrial cells in vitro. However, their influence on intraperitoneal endometriotic lesions require a study in a whole organism, to evaluate the effect in vivo of probiotic ingestion on the intraperitoneal lesions’ growth, pain and immune reaction. Ultimately, the goal of this project is to be able to propose new therapeutic strategies for endometriosis using probiotics.
Mice model of endometriosis
The model of endometriosis performed for the study has already been described elsewhere [23,24]. This model is a syngeneic graft of uterine horns in immunocompetent mice to generate endometriosis like lesions. 6-week-old female Balb/C Jrj mice were purchased from the supplier Janvier Labs (Route du Genest, 53940 Le Genest-Saint-Isle). The mice are housed in a conventional animal facility (N ° C75-14-05 / DTPP 2014-208), with 12-hour automated day/night cycles, drink (tap water) and food (Teklad, Envigo, Gannat, France, # T.2016MI.12) ad libitum. This project is in strict compliance with the European guidelines for animal experimentation. The endometriotic mouse model by implantation (suturing) of syngeneic intraperitoneal horn is a model validated and published in our laboratory. Implantation of uterine horns intraperitoneally is performed under isoflurane anesthesia (3.5%, under oxygen 2.5 l / min) and post-operative analgesic (Buprecare, 10 µg / kg, sc). A laparotomy is performed, and a fragment is fixed on each side of the opening, on the internal wall of the peritoneum, by two stitches made with 5/0 absorbable surgical thread.
Probiotic treatments
The molecules and products used for the treatments are: Zinc-Bisglycinate Chelate (ALBION Laboratories, Clearfield, USA, # 03506), Gomme Acacia Seygal (Nexira Food, Rouen, France, #FibregumB), Trans-reseveratrol (Active Inside, Beychac and Caillau, France, # PR-0018), Curcumin (Wacker, Eddyville, IA, USA, # 60072151), Saccharomyces Boulardii (LHC Lesaffre Bio Springer, Maison Alfort, France, # VI1700368), Lactobacillus Acidophilus (Danisco US, Madison, WI, USA, #MSAMPHRUDOPH). The exact composition is a patented formula of Gynov Society (Bordeaux, France).
The vehicle without probiotics, P0, (31.89% Zinc-Bisglycinate, 32.52% Acacia-Seygal Gum, 14.24% Trans-reseveratrol, 21.35% Curcumin) and probiotic (s), P1 or P2, are administered per os 5 days a week in 200µl, during 4 (4W) or 12 weeks (12W). The groups of control or treated mice were the following: mice without implants without treatment (Sham), mice with implants and treated with the vehicle (group P0) or the vehicle + 1 probiotic (group P1: 18mg / kg Saccharomyces Boulardii), or the vehicle + 2 probiotics (group P2: 18mg / kg Saccharomyces Boulardii + 9mg / kg Lactobacillus Acidophilus).
Four or 12 weeks after implantation, animals were sacrificed by cervical dislocation. Blood, both endometriotic lesions and spleen were retrieved for further analysis.
At the beginning of the experiment the number of mice per group were: Sham, n = 8 – P0, n = 11 - P1, n = 15 - P2, n = 15. For the first sacrifice at W4 we used: Sham, n = 5 – P0, n = 5 - P1, n = 10 - P2, n = 10. For the second sacrifice at W12 we used: Sham, n = 3 – P0, n = 6 - P1, n = 5 - P2, n = 5.
Ultrasonography monitoring
The evolution of the volume of lesions is monitored by ultrasonography under general anesthesia to confirm the viability of the implant and to measure its size as previously described [25]. The high-resolution ultrasound (40mHz) allows to follow the evolution of the size of the uterine horn implants in a precise, non-invasive way and to quickly assess the progression of endometriosis. This procedure was performed at PIV (Small Animal Imaging, Cochin Institute, Paris, France) at W1, W3, W8, W11 after the implantation of the horn’s fragments.
Immunohistology
The validation of the endometriosis model is confirmed by histologic evaluation of the endometriotic lesion. The presence of glandular and stromal cells validated the endometriosis model. Endometriotic implants were surgically removed at sacrifice and weighed, then half of it was being fixed with 10% formaldehyde and set in paraffin. The samples are sliced into sections of 5µm thick and stained with H&E. This method was performed at the Histim (Platform of immunohistlogy, Cochin Institute, Paris, France).
In vivo hot hyperesthesia
Hot hyperesthesia was evaluated in the model of endometriotic mice every week and averaged over 2 weeks, during the 4- or 12-weeks probiotic treatment period. Each mouse is placed on a metal surface [26] (cold/hot plate from BIOSEB) maintained at a constant temperature, set at + 50 ° C ± 0.2 ° C. The mice were used to being manipulated by the experimenters during the week preceding the endometriosis induction surgeries. Before each test session, the mice are left for 10 min in the environment of the experiment structure to be acclimated before being placed individually on the hot metal surface of the plate inside a plexiglass enclosure. A maximum of 1 min exposure of the mice to the hot plate was decided in order not to degrade their nerve sensitivity. The response latency, which is the time until the investigator observes a positive nocifensive behavior, is recorded. Nocifensive behaviors include forepaw withdrawal or licking, hind paw withdrawal or licking, stamping, leaning posture and jumping. This reaction is compared to the one of the sham group mice and considered positive when the delay for the mouse to raises its foot or frantically licks its paw to cool it is significantly different.
In vivo tactile hypoesthesia
The abdominal tactile sensitivity is evaluated every two weeks on endometriotic mice by a manual Von Frey test. Punctate mechanical allodynia and hyperalgesia can be assessed by the application of von Frey filaments of varying forces. A synthetic monofilament is applied perpendicularly to the tested surface, delivering a constant pre-determined force (typically 0.2–13.7 mN for mice) for 2–5 s. A response is considered positive if the animal exhibits any nocifensive behaviors, including brisk paw withdrawal, licking, or shaking of the paw, either during application of the stimulus or immediately after the filament is removed. While the plantar surface of the hind paw is the most commonly used area for testing, other areas of the body, including the abdomen can also be used [27]. We used the “ascending stimulus” method to determine mechanical sensitivity using manual Von Frey.
The mean of the monofilaments sizes that induce a nocifensive reaction for a group of mice is compared to the one of the sham group mice and considered positive when there is a significant difference.
qRT-PCR
After surgical resection, half of the lesions were immediately frozen in liquid nitrogen. Total RNA was extracted from mouse tissue (endometriosis-like lesions) using the Trizol reagent (Invitrogen, Carlsbad, USA), according to the manufacturer's instructions.
The amplified genes are: col1 (collagen 1) [28], Ptgs2/cox2 (cyclooxygenase 2), nrf (Nerve growth factor), igf1 (Insulin Growth Factor 1), Pecam1/cd31. The cDNA was synthesized using the Maxima H Minus cDNA Synthesis Master Mix kit (Thermo Fisher Scientific). The qPCR was performed using the SensiFAST SYBR No-ROX kit (Bioline). The transcription levels of the selected genes were quantified in a LightCycler480® (Roche, San Francisco, CA) thanks to calibration samples (serial dilution) and normalized to two reference genes (Actinb and Tbp) in the LightCycler480 software. The primer sequences used for qPCR are as follows (Ptgs2 = Cox2, Pecam1 = Cd31):
mm-Col1a1_F |
CCGAACCCCAAGGAAAAGA |
mm-Col1a1_R |
CTGTTGCCTTCGCCTCTGA |
mPtgs2-F |
TGAGCAACTATTCCAAACCAGC |
mPtgs2-R |
GCACGTAGTCTTCGATCACTATC |
mPecam1-F |
CTGCCAGTCCGAAAATGGAAC |
mPecam1-R |
CTTCATCCACCGGGGCTATC |
mTbp-F |
AGAACAATCCAGACTAGCAGCA |
mTbp-R |
GGGAACTTCACATCACAGCTC |
m-bActin-F |
ACCACCATGTACCCAGGCATT |
m-bActin-R |
CCACACAGAGTACTTGCGCTCA |
m-Ngf-F |
CCAGTGAAATTAGGCTCCCTG |
m-Ngf-R |
CCTTGGCAAAACCTTTATTGGG |
m-Igf1-F |
CTGGACCAGAGACCCTTTGC |
m-Igf1-R |
GGACGGGGACTTCTGAGTCTT |
Flow cytometry
Spleen cell suspensions from each mouse were incubated for 20 min with 10 µg/mL anti-CD16/CD32 antibody (clone 93, eBiosciences) for Fc receptor saturation and then stained with the appropriate labeled antibody at 4°C for 30 min in the dark in PBS with 2% FBS. The FACS Fortessa II flow cytometer (BD Biosciences) was used to perform flow cytometry according to standard techniques. For spleen characterization of immune cells, two cocktails of monoclonal antibodies were used: Panel A for T and B cells characterization: B220-APC, CD69-PE, CD8-BV605, CD40-PerCP/Cy5.5, MHCII-FITC, CD4-BV510. Panel B for macrophage phenotyping and M1/M2 characterization: CD206-AF647, CD86-BV510, Ly6C PE-Cy770, CD11b-APC-Cy7, F4/80-BV711, purchased from BioLegend, Ozyme (Montigny-le-Bretonneux, France). M1 macrophages were defined as B220−F4/80+CD11b+Ly6CHighCD206- and M2 macrophages as B220−F4/80+CD11b+Ly6cLowCD206+. Data were analyzed with FlowJo software (Tree Star, Ashland, OR).
Statistical study of the different results
The comparative statistical analysis of the different groups of mice was carried out by two-way ANOVA if the measured values followed a normal distribution, Kruskal-Wallis test otherwise. with multiple comparisons Bonferroni and Dunn’s tests respectively.
Endometriosis-like lesions evolution and related symptoms in mice with probiotic treatments
The vehicle without probiotics, P0, (31.89% Zinc-Bisglycinate, 32.52% Acacia-Seygal Gum, 14.24% Trans-reseveratrol, 21.35% Curcumin) and probiotic (s), P1 or P2, are administered per os 5 days a week in 200µl for 4 (4W) or 12 weeks (12W). The groups following: mice without implants without treatment (Sham), mice with implants and treated with the vehicle (group P0) or the vehicle + 1 probiotic (group P1: Saccharomyces Boulardii), or the vehicle + 2 probiotics (group P2: Saccharomyces Boulardii + Lactobacillus Acidophilus).
The implanted fragments of horn have been weighed before surgery (W0) and at the sacrifices (W4 and W12). The weights and volumes of the lesions were homogeneous at implantation (P0, n = 10 - P1, n = 20 - P2, n = 20). There is a significant growth of the implants with no difference between P0, P1 and P2 treatment groups concerning the lesions weight at W4 and W12. Nevertheless, the ultrasound imaging analysis of the implants showed that the lesions sizes are significantly decreased by the 2 treatments at W3 and even more at W11 (p<0.0001 for P0 42.03±1.28 vs P1 31.57±1.46 and P2 33.55±0.94 at W3 - P0 49.36±2.86 vs P1 31.82±2.51 and P2 17.41±1.90 at W12). P2 treatment is more efficient than P1 on the evolution of the volume of the lesions.
Furthermore, animals submitted to the probiotic treatment had smaller and less active implants (no fresh blood, no angiogenesis and few glands), whereas control P0 group showed persistent, larger and more active lesions through macroscopic (Fig. 1C) evaluation.
Pain and sensitivity tests induced by murine endometriosis lesions
The von Frey tactile sensitivity test is a time efficient and sensitive method which can be used together with other established tests to evaluate pain outcome in the mouse. Most pathological pain conditions in patients and rodent pain models result in marked alterations in mechano-sensation and the gold standard way to measure this is by use of von Frey fibers. These graded monofilaments are used to gauge the level of stimulus-evoked sensitivity present in the affected dermal region. Higher is the size of the filament lower is the sensitivity.
At W7, and until the end of the experiments, the abdominal tactile sensitivity (Figure 2A) of endometriotic mice starts to be significantly decreased under the effect of P2 (W7: P0 vs P2, p=0.0080; W12: P0 vs P2; p=0.0039) but not with P1 treatment, when compared to P0. At W3 and W7 the sensitivity to heat (Figure 2B) is significantly lower in mice treated with P1 compared to P0 alone (W3: P0 vs P, p=0.05; W7: P0 vs P1; p=0.0066) but the difference is no longer observable at W11.
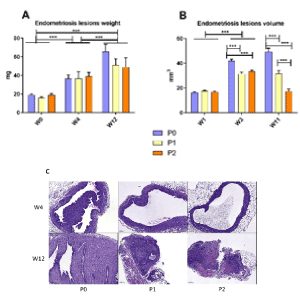
Figure 1. Effects of probiotic P1 and P2 treatments on endometriotic lesion development in mice (A) Weight of the endometriosis lesions on W4 and W12 (B) Volume of the endometriotic lesions on W3 and W11 obtained with measurements on ultrasonographic images of peritoneal implants in mice (C) Coloration with H&E of lesions at W4 and W12. Data are expressed as the mean ± SEM. The scale represents 200µm. Statistical analysis of the different groups of mice was carried out by one-way ANOVA followed by a Bonferroni test. The W3-W12 comparison by ANOVA followed by a Bonferroni test. NS, non-significant; ∗P ≤ 0.05; ∗∗P ≤ 0.01; ∗∗∗P ≤ 0.001. Scale bar, 200 μm
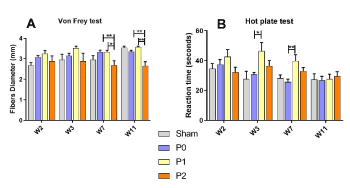
Figure 2. In vivo effects of P0, P1 and P2 treatments on sensitivity and pain evaluated through mice abdominal sensitivity and hot hyperalgesia. Mice received P0, P1 or P2 administered per os 5 days a week in 200µl all along the experiment. (A) Nociception was evaluated using iterative von Frey tests. Measurements were realized every week and averaged over 2 weeks, during the 4- or 12-weeks probiotic treatment period. (Sham, n = 3 - M, n = 6 - P1, n = 5 - P2, n = 5) (B) Hot hyperalgesia was evaluated using a hot plate set at + 50°C ± 0.2°C. (Sham, n = 3 - M, n = 6 - P1, n = 5 - P2, n = 5). Data are expressed as the means ± SEM. Statistical analysis of the different groups of mice was carried out by one-way ANOVA followed by a Bonferroni test. The W3-W12 comparison by ANOVA followed by a Bonferroni test. NS, non-significant; ∗P ≤ 0.05; ∗∗P ≤ 0.01; ∗∗∗P ≤ 0.001
Gene expression change in the endometriosis-like lesions with probiotic treatments
In order to evaluate characteristics of the endometriosis-like lesions, gene expression for markers related to growth (Igf1), to pain (Ngf)(29), to angiogenesis (Pecam1/Cd31), to inflammation (Ptgs2/Cox2) and to fibrosis (ColI) were evaluated by RT-qPCR at week 4 and week 12. At 4 weeks (Figure 3A), there were no significant differences between P0 and P1 or P2 for these markers. The mRNA level for ColI was lower in P1 than in P2 (p=0.0187). At 12 weeks (Fig 3B), there were no significant differences for Igf1, Ptgs2/Cox2 and ColI between the groups. There was a significant reduction of Ngf in P2 compared to P1. For Pecam1/Cd31, there is a significant effect within the 3 groups (significant Kruskal Wallis test) with a tendency for a reduced level with P1 (p=0.11) and P2 (p=0.06) compared to P0, indicating a potential beneficial effect of the probiotic treatment to limit angiogenesis within the endometriosis like lesions.
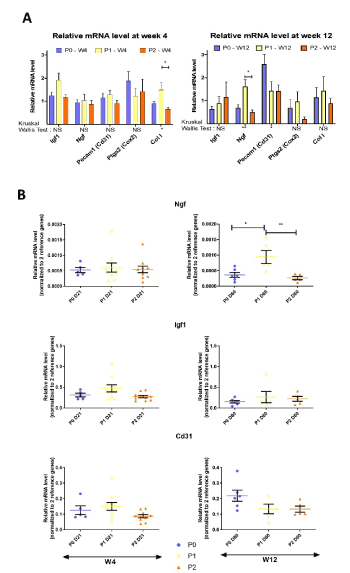
Figure 3. (A) Relative mRNA level of Igf1, Ngf, Pecam1, Ptgs2 and ColI by RT-qPCR in endometriosis-like lesions removed at W4 and W12 (B) from each endometriotic mouse (W4, n=5 for P0, n=10 for P1 and P2; W12, n=6 for P0, n=5 for P1 and P2). Data are expressed as the mean ± SEM of relative mRNA level normalized to 2 reference genes for each sample. Kruskal Wallis tests were used for the 3 groups comparison and when significant, Dunn's multiple comparisons tests were done. NS, non-significant; ∗P ≤ 0.05; ∗∗P ≤ 0.01
Probiotic treatments affect the splenic immune cells’ phenotype and activation in endometriotic mice
As endometriosis is characterized by an alteration of the systemic immune response [2], we investigated the impact of probiotic treatments on splenic immune cells frequency and activation. The frequency of CD4+ and CD8+ T-cells remained unchanged (Figure 4) in the different groups at W4 and W12. Interestingly, mice receiving the vehicle P0 and the P2 treatment exhibited a significant reduction of CD8+ T cell activation compared to the sham group at W4, and the difference is extended to the probiotic treatment P1 at W12.
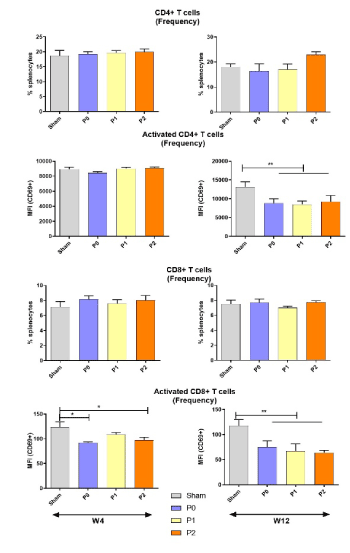
Figure 4. Probiotic treatments influence on T lymphocytes. Flow cytometric characterization of splenic CD4+ T and CD8+ T cells activated or not, at week 4 or week 12. Data represent the absolute count for T CD4+/ T CD8+ and percentage of activated cells (Expression of CD69) with SEM from independent samples. The ANOVA test with Bonferroni correction was used to detect significant differences between the groups. NS: Non-significant; *p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.001
Neither the treatments nor the vehicle affected the frequency of B cells (Figure 4). Activation of B cells were significantly reduced by P2 probiotics at W4 and by all the treatments at W12. At W4, the frequency of splenic NK cells was significantly reduced while their activation was increased in mice receiving the probiotic treatments compared to the Sham. At W12, a tendency to an increased activation of the NK cells by the probiotic treatments is observed, though not significantly.
We analyzed the impact of the different treatments on the macrophage’s polarization into the pro-inflammatory M1 or the anti-inflammatory/profibrotic M2 profile. The frequency of the splenic macrophages remained globally unchanged. At W4, the probiotic treatment P2 significantly increased the ratio M1/M2 and this tendency was maintained and extended to the P1 treatment at W12. (Figure 5).
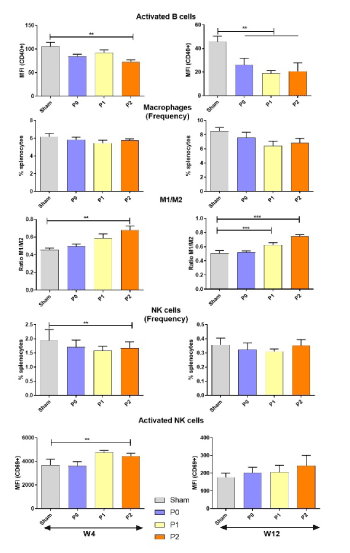
Figure 5. Probiotic treatments influence on B cells, NK cells and macrophages. Flow cytometric characterization of splenic B cell activation, macrophages frequency and polarization and NK cell frequency and activation, at week 4 or week 12. Data represent either the absolute count, the mean florescence intensity of CD69 expression on activated cells or the ratio of M1/M2 macrophages frequency. M1 macrophages were defined as B220−F4/80+CD11b+Ly6CHighCD206- and M2 macrophages as B220−F4/80+CD11b+Ly6cLowCD206+. The ANOVA test with Bonferroni correction was used to detect significant differences between the groups. NS: Non-significant; *p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.001
Probiotic treatment influences the serum markers of inflammation, intestinal permeability and protein oxidation in mice with endometriosis
It has been reported that probiotic treatment is associated with modifications of the intestinal permeability and the systemic markers of inflammation and oxidative stress [30,31].
At W4, the serum level of zonulin, a marker of the intestinal barrier permeability, remained unchanged by P0, while it was significantly reduced by the P1 and the P2 probiotic treatments (p<0.001, Figure 6A). At W12, a drop of the zonulin concentration in the sera of P0, P1 and P2 treated mice was observed (p<0.0001).
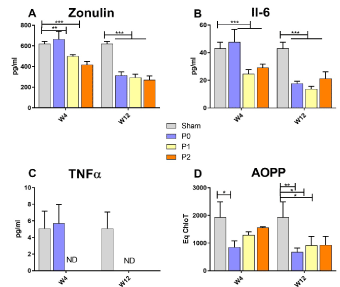
Figure 6. Effects of the probiotic treatment on the serum markers of inflammation, intestinal permeability and protein oxidation in mice with endometriosis. ELISA quantified levels of (A) Zonulin (B) IL6 (C) TNFα in sera from mice treated for 4 weeks or 12 weeks. (D) AOPP levels in sera from mice treated for 4 or 12 weeks. Data are expressed as the mean ± SEM. Statistical analysis of the different groups of mice was carried out by one-way ANOVA followed by a Bonferroni test. The W3-W12 comparison by two way-ANOVA followed by a multiple comparison test. NS, non-significant; ∗P ≤ 0.05; ∗∗P ≤ 0.01; ∗∗∗P ≤ 0.001
Similar tendencies were observed for the pro-inflammatory cytokines IL-6 and TNF-α, with a significant decrease or a non-detection respectively at W4 by P1 and P2 treatments only. At W12, IL-6 cytokine level was significantly reduced in all the tested conditions and TNF-α was only detected in Sham mice (Figures 6B and 6C).
The levels of AOPP in the sera (Figure 6D), that reflect the oxidation of serum proteins, are significantly reduced by the vehicle at W4 and by all the treatments at W12 (Figure 6D).
Endometriosis is an hormone-dependent inflammatory disease in which local and systemic altered immune response are participating to the survival and growth of displaced endometrial tissue in affected women [32]. The role of microbiota in the pathogenesis of endometriosis has been recently highlighted [33]. The microbiota represents all the microorganisms that exist in a particular environment, including bacteria, viruses, fungi and protozoa, that live within the host and regulate several physiological functions [34]. Indeed, a crosstalk exist between the microbiota and the immune system. The influence of the microbiome on the immunomodulation and the development of several inflammatory diseases is well established [35]. It acts by maintaining the integrity of the gastrointestinal epithelial lining, regulating immune homeostasis, preventing bacterial translocation, which can create a systemic low tone inflammation [14,36]. The activation and the function of the immune system are largely influenced by the microbiota [14] and in turn, an altered immune response can induce a modification of the composition and the diversity of the commensal bacteria. Conversely, little is known about the presence and composition of the microbiome along the female reproductive tract and its role in the development of endometriosis or other gynecological pathologies. Endometriosis is developed in an inflammatory gound. Moreover, the microbiome influences estrogen metabolism and estrogen influences the gut microbiota [37]. Postulating that the microbiome could influence endometriosis development is logical. The benefit of probiotics in prevention and treatment of gastrointestinal as well as extraintestinal disorders [38-40] is now established.
Here, we show that probiotic treatments can be effective to alleviate the clinical severity of the endometriosis lesions in a surgically induced endometriosis mouse model. Saccharomyces boulardiiis a probiotic yeast often used for the treatment of gastrointestinal tract disorders such as diarrhea symptoms.Itpresents phenotypic traits and physiological properties that underlie its success as probiotic, such as optimal growth temperature, resistance to the gastric environment and viability at low pH. Saccharomyces boulardii probiotic activity has been elucidated as a conjunction of multiple pathways, ranging from improvement of gut barrier function, pathogen competitive exclusion, production of antimicrobial peptides, immune modulation, and trophic effects [41]. Lactobacillus acidophilus is a lactic acid bacteria able to exert neuroprotective effects that may be associated with gut microbiota remodeling in traumatic brain injury mice [42]. Lactobacillushas a long history of being safely added to dairy products, and it is particularly recognized for enhancing intestinal barrier function and regulating the gut microbiota [43,44]. Disruptions of the gut microbiota contribute to intestinal barrier impairment, inducing bacterial translocation, systemic inflammatory response, and sepsis [45,46].
In our mice model of endometriosis, after 4 and 12 weeks of probiotic treatments P1 (18 mg/kg Saccharomyces Boulardii) or P2 (18 mg/kg Saccharomyces Boulardii + 9 mg/kg Lactobacillus Acidophilus), we observed that volume and size of the lesions are significantly lower than in P0 untreated mice. Clinically, heat sensitivity is significantly lower in P1-treated mice from W4 while tactile sensitivity is decreased by P2 treatment. Nevertheless, the expression of the Nerve Growth Factor (NGF), a potent regulator of growth, maintenance, proliferation, and survival of certain target neurons [47,48] was significantly increased by P1 treatment at W12. In women with cul-de-sac/uterosacral endometriosis, NGF has been associated with deep dyspareunia and sexual pain. COX-2/PGE2 axis is believed to mediate this association may be mediated by increased nerve bundle density and by COX-2/PGE2 stimulation via NGF/Trk receptor [49].
At the histological level, the glandular aspect, a hallmark of active endometriotic lesions, was markedly attenuated in mice receiving P1 or P2 treatment. In endometriosis, the glands observed in the tissue are hormone-sensitive, responds to the same cyclic variations as the eutopic endometrium and are responsible for the pain [20]. Probiotics have been tested successfully to alleviate the hyperplasia of glandular structure associated with gastric Helicobacter pylori infection and to reduce the overexpression of COX-2, which is also involved in the pathogeny of endometriosis [50,51].
Immunologically, we observed a substantial decrease of the activation of CD4+ and CD8+ T-cells subsets and B-lymphocytes in endometriotic mice treated with probiotics. It has been previously reported that there is a greater frequency of activated T-cells in the ectopic endometrium of women with endometriosis [52]. This observation is less clear in peripheral blood of endometriosis patients, where some studies showed an increase, while others presented a reduction of the frequency and the activation of the T-cells [53]. B cells, which are important players of the immune system, are increased in the blood and peritoneal cavity of women with endometriosis [54]. A polyclonal activation of B cells and the presence of anti-endometrial autoantibodies [55,56] have been described. Here, the probiotic treatment reduced the activation of B cells in mice with endometriosis. Interestingly, it has been reported that inactivation of the B cells by Ibrutinib, a Bruton’s tyrosine kinase inhibitor, prevents endometriosis progression in mice [57].
Local and systemic changes in NK cell phenotype and function have been reported in women with endometriosis (Wilson TJ, et al: Decreased natural killer cell activity in endometriosis patients: relationship to disease pathogenesis. Fertil Steril 1994; 62:1086–1088). Indeed, several studies have reported an altered NK cell phenotype and function with a decreased expression of the activation markers CD69 and CD107a whereas the inhibitory receptors are upregulated. Interestingly, it has been established that probiotics increase NK cell activation and enhance their cytotoxicity [58]. In our mouse model, the probiotic treatment induced an increase of NK cells activation at W4. An improvement of the NK cell capacity of the detection and the clearance of abnormal cells, early upon the surgery, may contribute to the immunological control of the endometriotic fragments in our model.
Probiotic treatment was accompanied by a disbalance in M1 and M2 macrophages phenotype with an important increase of the M1/M2 ratio in mice with endometriosis treated by the probiotics. Studies in human and mice evidenced the key role of M2 macrophages in the pathogenesis of endometriosis lesions establishment and growth. Indeed, it was shown an elevation of the proportion of M2 macrophages in the peritoneal fluid of women with endometriosis, compared with controls [59]. In addition, adoptive intraperitoneal transfer of M2 macrophages enhanced lesion growth and neovascularization in mice [60]. The probiotics can exert an immunomodulatory effect on macrophage polarization [61]. Lactobacillus Acidophilus that is contained in our P2 probiotic treatment has been shown to promote the production of the IL-12 cytokine that favors the M1 polarization through a shift toward Th1 T cells response [62,63]. Moreover, it has been shown that Lactobacillus acidophilus inhibits nitric oxide and TNF-α production while it stimulates the IL-10 production in the macrophages line RAW264.7 cells [64]. TNF-α is a potent pro-inflammatory cytokine mainly produced by the macrophages, monocytes, and activated T cells.It has been involved in the pathophysiology of endometriosis [65]. Its level is increased in peritoneal fluid of women with endometriosis [66], correlated with the disease severity [67,68]. Serum TNFα level is also increased, and monocytes from patients with endometriosis release more TNF-αin vitrocompared with monocytes from control women [69]. The anti-inflammatory effect of blocking TNF-α by monoclonal antibodies (e.g. infliximab) or by soluble TNF-α receptors (e.g. etanercept) has been demonstratedin vivoin animal models and in women. In baboons with laparoscopically confirmed endometriosis, TNF-α blockade with p55 soluble TNF-α receptors results in inhibition of the development and growth of endometriotic implants [70]. The size of peritoneal red lesions was decreased in comparison with a control group [71]. As well in rats with ectopically transplanted endometrial tissue, the administration of recombinant human TNF-α binding protein-1 resulted in defective development of implants compared with controls [72]. A decrease of serum TNF-α level with our probiotic treatments is of major interest. Indeed, it has been reported that the expression of TNF-α is modulated by probiotics in a strain-dependent manner as they can inhibit its production by normal and inflamed mucosa [73].
As endometriosis is characterized by a disrupted immune function, we believe that the probiotic treatment may exert potent immunomodulatory effects to dampen the excess of inflammation as reflected by a drop of the serum TNF-α and IL-6 levels, to modify the subsets ratio of M1/M2 macrophages and to reduce the B-cells activation
Zonulin, is a marker of permeability of the intestinal barrier. A growing number of publications focused on human genetics, the gut microbiome, and proteomics, suggesting that loss of mucosal barrier function, particularly in the gastrointestinal tract, may substantially affect antigen trafficking, ultimately causing chronic inflammation, including autoimmunity, in genetically predisposed individuals [74]. The gut mucosa works as a semipermeable barrier in that it permits nutrient absorption and regulates immune surveillance while retaining potentially harmful microbes and environmental antigens within the intestinal lumen.Enteric infections have been implicated in the pathogenesis of several pathological conditions, including allergic, autoimmune, and inflammatory diseases, by increasing the paracellular permeability of the intestinal barrier [75]. In our hands Zonuline is significantly reduced in sera by P1 and P2 at W4 and by M, P1 and P2 at W12, sign of a preserved intestinal barrier.
It has been demonstrated that the alteration of the gut mucosal barrier is associated with a neuro-endocrine dysfunction and an increased expression of the NGF protein in the rectosigmoid tissue [21-23]. In our experiment, an alteration of the intestinal permeability as reflected by the increased zonulin level in the sera of the endometriotic mice treated with probiotics may explain the variations we observed in the Nfg mRNA expression.
The decrease rate of oxidation of serum proteins (AOPP) by P0 at W4 and by P0, P1 and P2 at W12 is thus not a surprise. Previous studies have implicated reactive oxygen species (ROS) and cytokines in the regulation of permeability. The vascular endothelium regulates the passage of fluids, solutes, and cells from blood into tissues. Disruption of vascular permeability contributes to the pathogenesis of a wide range of diseases, including atherosclerosis, inflammatory tissue injury, and acute respiratory distress syndrome. Three cytokines have been implicated in the regulation of barrier function in inflammatory states. Tumor necrosis factor-α (TNF-α), interleukin (IL)-1, and IL-6 are increased in blood [76,77] after tissue injury.Pro-inflammatory cytokines could contribute to the reversible changes in endothelial permeability observed during periods of prolonged hypoxia. Studies have begun to implicate reactive oxygen species (ROS) in the cellular responses to inflammatory cytokines [78,79], whereas other works have demonstrated that ROS directly participate in the intracellular signaling initiated during physiological hypoxia [80,81]. The involvement of ROS in both suggests that cytokines and hypoxia may interact in the regulation of endothelial barrier function during inflammation. A major functional consequence of ROS production during hypoxia is the increase in IL-6 secretion, which contributes to the changes in endothelial permeability [82].
In our study, even though there is no significant difference in the expression of inflammation, fibrosis, and angiogenesis markers (cox 2, col1 and cd31 respectively) they presented a tendency to decrease with the P2 treatment on W12. P1 reduces significantly Igf1 after a W12 treatment. COX-2 is an inducible enzyme that catalyzes the production of prostaglandin E2 as a cellular response to inflammation. COX-2 overexpression has a pleiotropic and multifaceted role in inflammation and carcinogenesis. It shapes the structure and function of the extracellular matrix in primary and metastatic breast tumors [83]. It is now recognized that endometriotic lesions have high COX-2 and COX-2-derived prostaglandin E biosynthesis compared with the normal endometrium. COX-2 downregulation in a triple negative breast cancer cells induce decreased Col1 fiber density [83].
All together, these results evidence that probiotics are beneficial nonpathogenic bacteria that have been used as a nutritional approach for the prevention or treatment of some diseases [84,85].Treatment with one (P1) or two (P2) probiotics, have both favorable effects on clinical, immune, and physiologic parameters in endometriosis. Because of its greater ease of handlin and its effects observed on pain, Saccharomyces boulardii (P1) seems to be more suited to be used as a new therapeutic strategy for endometriosis. Nevertheless, P2 treatment remains also an interesting alternative because of the potential effects of Lactobacillus acidophilus on peripheric neuronal functions and thus on pain, described in patients.
This work was supported by GYNOV and Iprad society
The authors would like to thank the PIV and Histim facilities of Cochin Institute, Paris, for ultrasonography monitoring and histology techniques.
- Scheerer C, Bauer P, Chiantera V, Sehouli J, Kaufmann A, et al. (2016) Characterization of endometriosis-associated immune cell infiltrates (EMaICI). Arch Gynecol Obstet 294: 657‑664.
- Ahn SH, Monsanto SP, Miller C, Singh SS, Thomas R, et al. (2015) Pathophysiology and immune dysfunction in endometriosis. BioMed Res Int 2015: 795976. [Crossref]
- Björk E, Vinnars MT, Nagaev I, Nagaeva O, Lundin E, et al. (2020) Enhanced local and systemic inflammatory cytokine mRNA expression in women with endometriosis evokes compensatory adaptive regulatory mRNA response that mediates immune suppression and impairs cytotoxicity. Am J Reprod Immunol 84: e13298. [Crossref]
- Gmyrek GB, Sieradzka U, Goluda M, Gabrys M, Sozanski R, et al. (2008) Flow cytometric evaluation of intracellular cytokine synthesis in peripheral mononuclear cells of women with endometriosis. Immunol Invest 37: 43‑61. [Crossref]
- Nishimura H, Honjo T (2001) PD-1: an inhibitory immunoreceptor involved in peripheral tolerance. Trends Immunol 22: 265‑268.
- Chen L (2004) Co-inhibitory molecules of the B7-CD28 family in the control of T-cell immunity. Nat Rev Immunol 4: 336‑347. [Crossref]
- Kvaskoff M, Mu F, Terry KL, Harris HR, Poole EM, et al. (2015) Endometriosis: a high-risk population for major chronic diseases? Hum Reprod Update 21: 500‑516.
- Jess T, Frisch M, Jørgensen KT, Pedersen BV, Nielsen NM (2012) Increased risk of inflammatory bowel disease in women with endometriosis: a nationwide Danish cohort study. Gut 61: 1279‑1283.
- Huijs E, Nap A (2020) The effects of nutrients on symptoms in women with endometriosis: a systematic review. Reprod Biomed Online 41: 317‑328. [Crossref]
- Azad MAK, Sarker M, Li T, Yin J (2018) Probiotic species in the modulation of gut microbiota: An overview. BioMed Res Int 2018: 9478630. [Crossref]
- Carding S, Verbeke K, Vipond DT, Corfe BM, Owen LJ (2015) Dysbiosis of the gut microbiota in disease. Microb Ecol Health Dis 26: 26191. [Crossref]
- Scarpellini E, Ianiro G, Attili F, Bassanelli C, De Santis A, et al. (2015) The human gut microbiota and virome: Potential therapeutic implications. Dig Liver Dis 47: 1007‑1012. [Crossref]
- Yoo JY, Groer M, Dutra SVO, Sarkar A, McSkimming DI (2020) Gut microbiota and immune system interactions. Microorganisms 8: 1587. [Crossref]
- Belkaid Y, Hand T (2014) Role of the microbiota in immunity and inflammation. Cell 157: 121‑41. [Crossref]
- Rinninella E, Raoul P, Cintoni M, Franceschi F, Miggiano GAD, et al. (2019) What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganisms 7: 14. [Crossref]
- Solt I (2015) The human microbiome and the great obstetrical syndromes: A new frontier in maternal–fetal medicine. Best Pract Res Clin Obstet Gynaecol 29: 165‑175. [Crossref]
- Hilton E, Isenberg HD, Alperstein P, France K, Borenstein MT (1992) Ingestion of yogurt containing lactobacillus acidophilus as prophylaxis for candidal vaginitis. Ann Intern Med 116: 353‑357. [Crossref]
- Reid G, Bruce AW, Fraser N, Heinemann C, Owen J, et al. (2001) Oral probiotics can resolve urogenital infections. FEMS Immunol Med Microbiol 30: 49‑52. [Crossref]
- Morotti M, Vincent K, Brawn J, Zondervan KT, Becker CM (2014) Peripheral changes in endometriosis-associated pain. Hum Reprod Update 20: 717‑736.
- Stratton P, Berkley KJ (2011) Chronic pelvic pain and endometriosis: translational evidence of the relationship and implications. Hum Reprod Update 17: 327‑346. [Crossref]
- As-Sanie S, Harris RE, Napadow V, Kim J, Neshewat G, et al. (2012) Changes in regional gray matter volume in women with chronic pelvic pain: a voxel-based morphometry study. Pain 153: 1006‑1014. [Crossref]
- Rousseaux C, Thuru X, Gelot A, Barnich N, Neut C, et al. (2007) Lactobacillus acidophilus modulates intestinal pain and induces opioid and cannabinoid receptors. Nat Med 13: 35‑37.
- Ngô C, Chéreau C, Nicco C, Weill B, Chapron C, et al. (2009) Reactive oxygen species controls endometriosis progression. Am J Pathol 175: 225‑234. [Crossref]
- Marcellin L, Santulli P, Chouzenoux S, Cerles O, Nicco C, et al. (2017) Alteration of Nrf2 and Glutamate Cysteine Ligase expression contribute to lesions growth and fibrogenesis in ectopic endometriosis. Free Radic Biol Med 110: 1‑10. [Crossref]
- Santulli P, Marcellin L, Chouzenoux S, Boulard V, Just PA, et al. (2016) Role of the protein kinase BRAF in the pathogenesis of endometriosis. Expert Opin Ther Targets 20: 1017‑1029.
- Deuis JR, Dvorakova LS, Vetter I (2017) Methods used to evaluate pain behaviors in rodents. Front Mol Neurosci 10: 284. [Crossref]
- Minett MS, Eijkelkamp N, Wood JN (2014) Significant determinants of mouse pain behaviour. PloS One 9: e104458.
- Szóstek-Mioduchowska AZ, Baclawska A, Okuda K, Skarzynski DJ (2019) Effect of proinflammatory cytokines on endometrial collagen and metallopeptidase expression during the course of equine endometrosis. Cytokine 123: 154767. [Crossref]
- Fusco R, D’amico R, Cordaro M, Gugliandolo E, Siracusa R, et al. (2018) Absence of formyl peptide receptor 1 causes endometriotic lesion regression in a mouse model of surgically-induced endometriosis. Oncotarget 9: 31355‑31366. [Crossref]
- Rao RK, Samak G (2013) Protection and restitution of gut barrier by probiotics: Nutritional and clinical implications. Curr Nutr Food Sci 9: 99‑107. [Crossref]
- Mohammadi AA, Jazayeri S, Khosravi-Darani K, Solati Z, Mohammadpour N, et al. (2015) Effects of probiotics on biomarkers of oxidative stress and inflammatory factors in petrochemical workers: A randomized, double-blind, placebo-controlled trial. Int J Prev Med 6: 82. [Crossref]
- Herington JL, Bruner-Tran KL, Lucas JA, Osteen KG (2011) Immune interactions in endometriosis. Expert Rev Clin Immunol 7: 611‑626.
- Leonardi M, Hicks C, El-Assaad F, El-Omar E, Condous G (2020) Endometriosis and the microbiome: a systematic review. BJOG 127: 239‑249. [Crossref]
- Cani PD (2018) Human gut microbiome: hopes, threats and promises. Gut 67: 1716‑1725. [Crossref]
- Blaser MJ (2014) The microbiome revolution. J Clin Invest 124: 4162‑4165. [Crossref]
- Wu HJ, Wu E (2012) The role of gut microbiota in immune homeostasis and autoimmunity. Gut Microbes 3: 4‑14. [Crossref]
- Baker JM, Al-Nakkash L, Herbst-Kralovetz MM (2017) Estrogen–gut microbiome axis: Physiological and clinical implications. Maturitas 103: 45‑53. [Crossref]
- Hajela N, Ramakrishna BS, Nair GB, Abraham P, Gopalan S, et al. (2015) Gut microbiome, gut function, and probiotics: Implications for health. Indian J Gastroenterol 34: 93‑107. [Crossref]
- Hori T, Matsuda K, Oishi K (2020) Probiotics: A dietary factor to modulate the gut microbiome, host immune system, and gut–brain interaction. Microorganisms 8: 1401.
- Quigley EMM, Gajula P (2020) Recent advances in modulating the microbiome. F1000Res.
- Pais P, Almeida V, Yılmaz M, Teixeira MC (2020) Saccharomyces boulardii: What makes it tick as successful probiotic? J Fungi Basel Switz.
- Ma Y, Liu T, Fu J, Fu S, Hu C, et al. (2019) Lactobacillus acidophilus exerts neuroprotective effects in mice with traumatic brain injury. J Nutr 149: 1543‑1552. [Crossref]
- Lépine AFP, de Wit N, Oosterink E, Wichers H, Mes J, et al. (2018) Lactobacillus acidophilus attenuates salmonella-induced stress of epithelial cells by modulating tight-junction genes and cytokine responses. Front Microbiol 9:1439. [Crossref]
- Wu D, Lewis ED, Pae M, Meydani SN (2018) Nutritional modulation of immune function: analysis of evidence, mechanisms, and clinical relevance. Front Immunol 9: 3160. [Crossref]
- Stevens BR, Goel R, Seungbum K, Richards EM, Holbert RC, et al. (2018) Increased human intestinal barrier permeability plasma biomarkers zonulin and FABP2 correlated with plasma LPS and altered gut microbiome in anxiety or depression. Gut 67: 1555‑1557. [Crossref]
- Lee S, Keirsey KI, Kirkland R, Grunewald ZI, Fischer JG, et al. (2018) Blueberry supplementation influences the gut microbiota, inflammation, and insulin resistance in high-fat-diet-fed rats. J Nutr 148: 209‑219. [Crossref]
- Denk F, Bennett DL, McMahon SB (2017) Nerve growth factor and pain mechanisms. Annu Rev Neurosci 40: 307‑325. [Crossref]
- Sørensen LB, Gazerani P, Sluka KA, Graven-Nielsen T (2020) Repeated injections of low-dose nerve growth factor (NGF) in healthy humans maintain muscle pain and facilitate ischemic contraction-evoked pain. Pain Med 21: 3488-3498. [Crossref]
- Peng B, Zhan H, Alotaibi F, Alkusayer GM, Bedaiwy MA, et al. (2018) Nerve growth factor is associated with sexual pain in women with endometriosis. Reprod Sci 25: 540‑549.
- Brzozowski T, Konturek PC, Mierzwa M, Drozdowicz D, Bielanski W, et al. (2006) Effect of probiotics and triple eradication therapy on the cyclooxygenase (COX)-2 expression, apoptosis, and functional gastric mucosal impairment in helicobacter pylori-infected mongolian gerbils. Helicobacter 11: 10‑20. [Crossref]
- Lai ZZ, Yang HL, Ha SY, Chang KK, Mei J, et al. (2019) Cyclooxygenase-2 in endometriosis. Int J Biol Sci 15: 2783‑2797. [Crossref]
- Witz CA, Montoya IA, Dey TD, Schenken RS (1994) Characterization of lymphocyte subpopulations and T cell activation in endometriosis. Am J Reprod Immunol 32: 173‑179. [Crossref]
- Riccio L da GC, Santulli P, Marcellin L, Abrão MS, Batteux F, et al. (2018) Immunology of endometriosis. Best Pract Res Clin Obstet Gynaecol 50: 39‑49.
- Riccio LGC, Baracat EC, Chapron C, Batteux F, Abrão MS (2017) The role of the B lymphocytes in endometriosis: A systematic review. J Reprod Immunol 123: 29‑34. [Crossref]
- Wild RA, Shivers CA (1985) Antiendometrial antibodies in patients with endometriosis. Am J Reprod Immunol Microbiol 8: 84‑86. [Crossref]
- Fernàndez-Shaw S, Hicks BR, Yudkin PL, Kennedy S, Barlow DH, et al. (1993) Anti-endometrial and anti-endothelial auto-antibodies in women with endometriosis. Hum Reprod 8: 310‑315.
- Riccio LGC, Jeljeli M, Santulli P, Chouzenoux S, Doridot L, et al. (2019) B lymphocytes inactivation by Ibrutinib limits endometriosis progression in mice. Hum Reprod 34: 1225‑1234.
- Jeung I, Cheon K, Kim MR (2016) Decreased cytotoxicity of peripheral and peritoneal natural killer cell in endometriosis. BioMed Res Int.
- Hudson QJ, Ashjaei K, Perricos A, Kuessel L, Husslein H, et al. (2020) Endometriosis patients show an increased M2 response in the peritoneal CD14+low/CD68+low macrophage subpopulation coupled with an increase in the T-helper 2 and T-regulatory cells. Reprod Sci 27: 1920‑1931. [Crossref]
- Bacci M, Capobianco A, Monno A, Cottone L, Di Puppo F, et al. (2009) Macrophages are alternatively activated in patients with endometriosis and required for growth and vascularization of lesions in a mouse model of disease. Am J Pathol 175: 547‑556. [Crossref]
- Wang Y, Liu H, Zhao J (2020) Macrophage polarization induced by probiotic bacteria: a concise review. Probiotics Antimicrob Proteins 12: 798‑808. [Crossref]
- Quinteiro-Filho WM, Brisbin JT, Hodgins DC, Sharif S (2015) Lactobacillus and Lactobacillus cell-free culture supernatants modulate chicken macrophage activities. Res Vet Sci 103: 170‑175.
- Amar Y, Rizzello V, Cavaliere R, Campana S, De Pasquale C, et al. (2015) Divergent signaling pathways regulate IL-12 production induced by different species of Lactobacilli in human dendritic cells. Immunol Lett 166: 6‑12. [Crossref]
- Kim DH, Kim S, Lee JH, Kim JH, Che X, et al. (2019) Lactobacillus acidophilus suppresses intestinal inflammation by inhibiting endoplasmic reticulum stress. J Gastroenterol Hepatol 34: 178‑185. [Crossref]
- Agic A, Xu H, Finas D, Banz C, Diedrich K, et al. (2006) Is endometriosis associated with systemic subclinical inflammation? Gynecol Obstet Invest 62: 139‑147.
- Bedaiwy MA, Falcone T, Sharma RK, Goldberg JM, Attaran M, et al. (2002) Prediction of endometriosis with serum and peritoneal fluid markers: a prospective controlled trial. Hum Reprod 17: 426‑431. [Crossref]
- Richter ON, Dorn C, Rösing B, Flaskamp C, Ulrich U (2005) Tumor necrosis factor alpha secretion by peritoneal macrophages in patients with endometriosis. Arch Gynecol Obstet 271: 143‑147. [Crossref]
- Bullimore DW (2003) Endometriosis is sustained by tumour necrosis factor-alpha. Med Hypotheses 60: 84‑88.
- Braun DP, Gebel H, House R, Rana N, Dmowski NP (1996) Spontaneous and induced synthesis of cytokines by peripheral blood monocytes in patients with endometriosis. Fertil Steril 65: 1125‑1129. [Crossref]
- D’Hooghe TM, Nugent NP, Cuneo S, Chai DC, Deer F, et al. (2006) Recombinant human TNFRSF1A (r-hTBP1) inhibits the development of endometriosis in baboons: a prospective, randomized, placebo- and drug-controlled study. Biol Reprod 74: 131‑136. [Crossref]
- Barrier BF, Bates GW, Leland MM, Leach DA, Robinson RD, et al. (2004) Efficacy of anti-tumor necrosis factor therapy in the treatment of spontaneous endometriosis in baboons. Fertil Steril 81 Suppl 1: 775‑779. [Crossref]
- D’Antonio M, Martelli F, Peano S, Papoian R, Borrelli F (2000) Ability of recombinant human TNF binding protein-1 (r-hTBP-1) to inhibit the development of experimentally-induced endometriosis in rats. J Reprod Immunol 48: 81‑98. [Crossref]
- Habil N, Al-Murrani W, Beal J, Foey A (2011) Probiotic bacterial strains differentially modulate macrophage cytokine production in a strain-dependent and cell subset-specific manner. Benef Microbes 2: 283‑293.
- Valitutti F, Fasano A (2019) Breaking down barriers: How understanding celiac disease pathogenesis informed the development of novel treatments. Dig Dis Sci 64: 1748‑1758.
- El Asmar R, Panigrahi P, Bamford P, Berti I, Not T, et al. (2002) Host-dependent zonulin secretion causes the impairment of the small intestine barrier function after bacterial exposure. Gastroenterology 123: 1607‑1615. [Crossref]
- Ertel W, Morrison MH, Ayala A, Chaudry IH (1995) Hypoxemia in the absence of blood loss or significant hypotension causes inflammatory cytokine release. Am J Physiol-Regul Integr Comp Physiol 269: R160‑R166. [Crossref]
- Schütte H, Lohmeyer J, Rosseau S, Ziegler S, Siebert C, et al. (1996) Bronchoalveolar and systemic cytokine profiles in patients with ARDS, severe pneumonia and cardiogenic pulmonary oedema. Eur Respir J 9: 1858‑1867. [Crossref]
- Chua CC, Hamdy RC, Chua BHL (1998) Upregulation of vascular endothelial growth factor by H2O2 in rat heart endothelial cells. Free Radic Biol Med 25: 891‑897. [Crossref]
- Simon AR, Rai U, Fanburg BL, Cochran BH (1998) Activation of the JAK-STAT pathway by reactive oxygen species. Am J Physiol-Cell Physiol 275: C1640‑C1652.
- Chandel NS, Maltepe E, Goldwasser E, Mathieu CE, Simon MC, et al. (1998) Mitochondrial reactive oxygen species trigger hypoxia-induced transcription. Proc Natl Acad Sci U S A 95: 11715‑11720. [Crossref]
- Duranteau J, Chandel NS, Kulisz A, Shao Z, Schumacker PT (1998) Intracellular signaling by reactive oxygen species during hypoxia in cardiomyocytes. J Biol Chem 273: 11619‑11624.
- Ali MH, Schlidt SA, Chandel NS, Hynes KL, Schumacker PT, et al. (1999) Endothelial permeability and IL-6 production during hypoxia: role of ROS in signal transduction. Am J Physiol 277: L1057-L1065.
- Krishnamachary B, Stasinopoulos I, Kakkad S, Penet MF, Jacob D, et al. (2017) Breast cancer cell cyclooxygenase-2 expression alters extracellular matrix structure and function and numbers of cancer associated fibroblasts. Oncotarget 8: 17981‑17994.
- Bruce-Keller AJ, Salbaum JM, Berthoud HR (2018) Harnessing gut microbes for mental health: Getting from here to there. Biol Psychiatry 83: 214‑223. [Crossref]
- Shen NT, Maw A, Tmanova LL, Pino A, Ancy K, et al. (2017) Timely use of probiotics in hospitalized adults prevents clostridium difficile infection: A systematic review with meta-regression analysis. Gastroenterology 152: 1889-1900. [Crossref]






