Abstract
Background: Implantation of an embryo in ART is a complex process. With the advent of ERA, a diagnostic tool was supposedly developed to identify a receptive endometrium based on the array of 238 genes which are expressed at different times in a cycle. Case: The current case describes a patient with three previously failed IVF cycles, who underwent an ERA testing in an HRT cycle, with diagnosis of a post receptive endometrium. However, an embryo transfer was performed on day 5 after progesterone rise in a natural cycle, resulting in successful achievement of pregnancy, despite timing the embryo transfer outside the assumed window of implantation as diagnosed by ERA. Conclusion: This puts into perspective the question whether ERA can be regarded as the ultimate tool of endometrial receptivity assessment.
Key words
endometrial receptivity array, hormone replacement therapy, repeated implantation failure
Teaching points
- A displaced window of implantation after an ERA in an HRT cycle may not be replicated in a natural cycle.
- An accurately timed natural cycle, correlating ultrasound findings with hormonal profile is crucial for a successful embryo transfer.
- Further large prospective studies are needed to confirm that a difference exists between an HRT and a natural cycle.
Introduction
Human endometrium is a dynamic tissue that undergoes cyclical physiological changes in response to steroid hormones [1]. The endometrium is receptive to a potentially implanting embryo during a specific time in the menstrual cycle, known as the “window of implantation”. For implantation to occur, a synchronous coordination must exist between the embryo development stage and the endometrial status, therefore lately endometrial receptivity has become a focus for research [2].
Noyes et al initially defined a set of morphological criteria to evaluate endometrial development and for histological dating of endometrium [3,4]. Studies have since concluded that histological endometrial dating is not useful when determining the management of women with reproductive failure [5].
After numerous studies it became apparent that the study of a few molecules, when investigating the complex role of the endometrium in implantation was insufficient and new approaches were taken utilizing genomics, proteomics and metabolomics and finally lead to a molecular diagnostic test, named Endometrial Receptivity Assay (ERA) which is based on a customized array of 238 genes. A specific transcriptomic signature identifies the receptive endometrium 7 days after the luteinizing hormone peak (LH+7) in natural cycles or 5 days after progesterone administration (P+5) in a Hormone Replacement Therapy (HRT) cycle [6,7]. The ERA test has demonstrated that the “window of implantation” can be displaced,either delayed or advanced [8] and therefore facilitates the concept of a personalized embryo transfer as a therapeutic strategy in recurrent implantation failure [9]. For a test to be of clinical value, it must be reproducible in the same patient. We report a case where an abnormal ERA test in an HRT cycle was not replicable in an accurately conducted natural cycle.
Case report
A 41-year-old patient with primary unexplained infertility of 10 years attended our clinic after three previous failed IVF treatments in other clinics. Her AMH concentration was 1.8 ng/ml.
During the first treatment under our care, no euploid embryos were obtained after PGT-A (Preimplantation Genetic Testing for aneuploides). In the subsequent trial, two euploid blastocysts were transferred without success. As aneuploid embryos are the most common cause of implantation failure [10] and despite the fact that the transfer of 2 euploid embryos failed to result in implantation, an ERA test was recommended to exclude a displaced window of implantation. The ERA protocol for an HRT cycle was followed correctly, the ERA result identified a post- receptive endometrium.
As a result of one further stimulation, one euploid embryo was obtained. Taking the ERA result into consideration and the recent evidence suggesting that natural cycles are associated with improved endometrial receptivity than artificial cycles, the decision was taken to transfer the euploid embryo in a spontaneous natural cycle, neglecting the ERA result due to lack of scientific evidence of ERA in accurately conducted natural cycle [11]. Throughout the patients’ natural cycle, periodical ultrasound scans were performed to monitor follicular growth in conjunction with the monitoring of serum FSH, LH, estradiol and progesterone levels measured with an automated Elecsys® immunoanalyzer (Roche Diagnostics, Mannheim, Germany). Frozen-thawed embryo transfer was performed 5 days following the progesterone rise on day 21 of the cycle [12] as shown in the graph.
Serum β-hCG (human chorionic gonadotropin) level was positive 12 days following embryo transfer. An ongoing pregnancy was confirmed at 7 weeks gestation by the presence of a single intrauterine gestational sac with foetal pole showing heart activity (Figure 1).
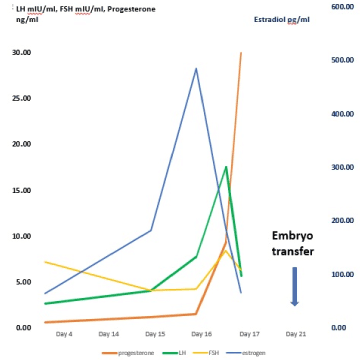
Figure 1. Embryo transfer in natural cycle
Discussion
This case demonstrates the successful achievement of pregnancy, despite timing the embryo transfer outside the assumed window of implantation, as diagnosed by the ERA test result during an HRT cycle. This case report raises two issues, first, the question of whether the ERA test performed during an HRT cycle can be replicated in a true natural cycle and second, what is the optimal means of preparing the endometrium in frozen-thawed cycles?
In recent years the number of frozen-thawed embryo transfer (FET) cycles has increased significantly. The common treatment protocols for frozen embryo transfers are natural cycles with or without hCG trigger or endometrial preparation with hormonal treatment (artificial cycles), with or without pituitary down regulation with Gonadotrophin – releasing hormone agonist. During an artificial FET cycle, estradiol and progesterone are administered to simulate the changes in hormonal profile that take place during a natural cycle. Recent systematic reviews and meta-analysis that have compared different cycle regimens for FET concluded that the evidence available is insufficient to support the use of one protocol in preference to another. However, the number of randomised controlled trials is limited and comprise only small patient numbers [13,14]. Programmed cycles are advantageous for patients and fertility units due to limited ultrasound monitoring requirements and the flexibility of scheduling. In contrast, natural cycles require increased monitoring which may be inconvenient for patients and offer little flexibility when scheduling the embryo transfer. In natural cycles, ovulation is the marker for timing the thawing and transfer. Ovulation can be estimated by detection of the luteinizing hormone (LH) surge in either urine or blood or following triggering of ovulation of the dominant follicle using hCG (modified natural cycle). The accuracy of the timing is crucial to the success of a natural cycle FET. Recent studies comparing artificial and natural cycles, concluded that the optimal means of endometrial preparation for frozen- thawed cycle remains unclear and both options may be offered to women with regular ovulatory cycles [15,16].
Hence, the validity of studies on natural cycles for FET is determined by the method used to detect ovulation correctly as the LH surge is crucial to avoid embryo- endometrial asynchrony on the day of embryo transfer. Recent studies report ovulation based on ultrasound findings alone, which means that a premature LH or progesterone rise would be missed [16,17]. In addition, ovulation detection using LH urine kits is well recognised to be a sub-optimal means of accurately detecting ovulation [18,19].
Contrary to the previous publications, in the herein presented case report ovulation was detected by a combination of ultrasound monitoring and serial measurement of LH, estradiol and progesterone, which is recognised to be the most accurate method of correctly identifying ovulation [14,20]. The LH surge was deemed to have commenced with a rise in LH by 180% and continued to rise thereafter. A decrease in estradiol levels was noted one day after the LH rise, with an increased progesterone (>1.5 ng/ml) confirmed ovulation [14]. A recent study has demonstrated that natural cycles are associated with better endometrial receptivity than artificial cycles [13]. Artificial cycles appeared to have a stronger negative effect on the expression of genes and pathways crucial for endometrial receptivity [13].
In the case presented here, pregnancy was achieved in an appropriately conducted natural cycle despite timing the embryo transfer outside the assumed window of implantation, as diagnosed by an ERA test in an HRT cycle. The successful embryo transfer took place at LH+7 days and therefore in a time period judged to be post-receptive by the previously performed ERA test. In light of this result, the question arises, whether an ERA performed during an HRT cycle can reliably predict the window of implantation in a natural cycle?
Further studies of natural cycles with both, ultrasound and hormonal monitoring to correctly identify ovulation for frozen thawed embryo transfer are merited and it might be that in future the ART could go back to a natural cycle.
Conflict of interest
The authors report no conflict of interest.
No funding was received for the case report.
Ethical approval
Approval from patient was obtained for the case report.
Acknowledgement
We thank Dr Carol Coughlan and Dr Leif Bungum for linguistic revision.
References
- Garrido-Gomez T, Ruiz-Alonso M, Blesa D, Diaz- Gimeno P, Vilella F, et al. (2013) Profiling the gene signature of endometrial receptivity: clinical results. Fertil Steril 99: 1078-1085. [Crossref]
- Coughlan C, Ledger W, Wang Q, Liu F, Demirol A, et al. (2014) Recurrent Implantation Failure: definition and management. Reprod Biomed Online 28: 14-38. [Crossref]
- Noyes RW, Hertig AT, Rock J (1950) Dating the endometrial biopsy. Fertil Steril 1: 3-25. [Crossref]
- Noyes RW, Haman JO (1953) Accuracy of endometrial dating; correlation of endometrial dating with basal body temperature and menses.Fertil Steril4: 504-517. [Crossref]
- Coutifaris C, Myers ER, Guzick DS, Diamond MP, Carson SA, et al. (2004) Histological dating of timed endometrial biopsy tissue is not related to fertility status. Fertil Steril 82: 1264-1272. [Crossref]
- Ruiz-Alonso M, Blesa D, Simón C (2012) The genomics of the human endometrium.Biochim Biophys Acta1822: 1931-1942. [Crossref]
- Altmae S, Martinez-Conejero JA, Salumets A, Simon C, Horcajadas JA, et al. (2010) Endometrial gene expression analysis at the time of embryo implantation in women with unexplained infertility. Mol Hum Reprod 16: 178-187. [Crossref]
- Blesa D, Ruiz-Alonso M, Simon C (2014) Clinical Management of Endometrial Receptivity. Semin Reprod Med 32: 410-413. [Crossref]
- Ruiz-Alonso M, Blesa D, Diaz-Gimeno P, Gomez E, Fernandez-Sanchez M, et al. (2013) The endometrial receptivity array for diagnosis and personalized embryo transfer as a treatment for patients with repeated implantation failure. Fertil Steril 100: 818- 824. [Crossref]
- Brosens JJ, Salker MS, Teklenburg G, Nautiyal J, Salter S, et al. (2014) Uterine selection of human embryos at implantation.Sci Rep4: 3894. [Crossref]
- Altmae S, Tamm-Rosenstein K, Esteban F, Simm J, Kolberg L, et al. (2016) Endometrial transcriptome analysis indicates superiority of natural over artificial cycles in recurrent implantation failure patients undergoing frozen embryo transfer. Reprod Biomed Online 32: 597-613. [Crossref]
- Fatemi H, Kyrou D, Bourgain C, Van den Abbeel E, Griesinger G, et al. (2010) Cryopreserved - thawed human embryo transfer: spontaneous natural cycle is superior to human chorionic gonadotropin-induced natural cycle. Fertil Steril 94: 2054-2058. [Crossref]
- Bourgain C, Devroey P (2003) The endometrium in stimulated cycles for IVF.Hum Reprod Update9: 515-522. [Crossref]
- Ghobara T, Gelbaya TA, Ayeleke RO (2017) Cycle regimens for frozen-thawed embryo transfer. Cochrane Database Syst Rev 5: CD003414. [Crossref]
- Groenewoud E, Cohlen B, Macklon N (2018) Programming the endometrium for deferred transfer of cryopreserved embryos: hormone replacement versus modified natural cycles. Fertil Steril 109: 768-774. [Crossref]
- Groenewoud E, Cohlen B, Al-Oraiby A, Brinkhuis E, Broekmans F, et al. (2016) A randomized controlled, non-inferiority trial of modified natural versus artificial cycle for cryo-thawed embryo transfer. Hum Reprod 31: 1483-1492. [Crossref]
- Shi Y, Sun Y, Hao C, Zhang H, Wei D, et al. (2018) Transfer of Fresh versus Frozen Embryos in Ovulatory Women.N Engl J Med378: 126-136. [Crossref]
- O'Connor K, Brindle E, Miller RC, Shofer JB, Ferrell RJ, et al. (2006) Ovulation detection methods for urinary hormones; precision, daily and intermittent sampling and a combined hierarchical method. Hum Reprod 21: 1442-1452. [Crossref]
- Park SJ, Goldsmith LT, Skurnick JH, Wojtczuk A, Weiss G (2007) Characteristics of the urinary luteinizing hormone surge in young ovulatory women. Fertil Steril 88: 684-690. [Crossref]
- Irani M, Robles A, Gunnala V, Reichman D, Rosenwaks Z (2017) Optimal parameters for determining the LH surge in natural cycle frozen-thawed embryo transfers. J Ovarian Res 10: 70. [Crossref]

