Purpose: Recent reports have shown that adjuvant chemotherapy (AC) fails to improve prognosis in patients with high-risk stage II colon cancer. Therefore, we examined the method of identifying cases that could benefit from AC.
Methods: The relation between the number of risk factors and the effects of AC was analyzed using definitions of the National Comprehensive Cancer Network (NCCN), the American Society of Clinical Oncology (ASCO), the European Society for Medical Oncology (ESMO), and the Japanese Foundation for Multidisciplinary Treatment of Cancer (JFMC).
Results: The disease-free survival and overall survival of cases with 1 or 2 risk factors, as defined by NCCN or ESMO, were significantly improved by AC. Improved survival was not observed in cases with more than 3 risk factors according to these definitions. There was no relation between the number of risk factors, as defined by ASCO or JFMC, and the effects of AC.
Conclusions: Patients with stage II colon cancer can be categorized into 3 groups as defined by the NCCN or ESMO guidelines: (A) no risk factor cases, (B) 1 or 2 risk factor cases, and (C) more than 3 risk factor cases. This classification may assist in the selection of AC regimens for patients with stage II colon cancer.
colon cancer, stage II, high-risk cases, adjuvant chemotherapy
Although numerous clinical trials of adjuvant chemotherapy (AC) after colon cancer surgery showed that AC improved prognosis for patients with stage III disease, AC did not affect prognosis for patients with stage II [1-4]. Because some patients with stage II disease experience recurrence, the concept of “high-risk stage II” was developed with the aim of suppressing recurrence and improving prognosis by selecting cases where the risk of recurrence was high [5-8]. A retrospective examination of what kinds of cases were likely to relapse found that the presence of T4 staging, perforation, undifferentiated type, mucinous carcinoma, and fewer than 13 searched lymph nodes (LN) were recommended as high-risk cases according to the 2004 American Society of Clinical Oncology (ASCO) guidelines [9]. Thereafter, the definition of high-risk stage II was also applied to the National Comprehensive Cancer Network (NCCN) and the European Society for Medical Oncology (ESMO) guidelines. The Japanese Foundation for Multidisciplinary Treatment of Cancer (JFMC) conducted phase III clinical trials using its own definition of high-risk stage II based on these recommendations [10-12].
A recent clinical study of patients with high-risk stage II disease found that AC did not improve prognosis in patients with stage II disease (some studies reported that there was an effect for T4 cases) [13-16]. Therefore, the research focus shifted to determining recurrence risk biomarkers using a molecular biological technique. Meanwhile, the search for a method that will identify patients with stage II colon cancer who might benefit from AC continues. Although AC cannot suppress all instances of recurrence, there may be certain patients who can benefit from AC. However, to identify these individuals, we require another method of identifying “high-risk stage II” cases.
Patients considered as “high-risk stage II” possess at least 1 risk factor. The defined risk factors of each guideline are similar, but not identical and it is not clear which definition is appropriate. On the basis of these points, we decided to integrate and analyse cases with stage II disease using available data.
In doing so, we developed a novel selection method for patients with high-risk stage II colon cancer for whom AC may improve prognosis.
Patient information
We collected data (from 2 facilities: Kumamoto City Ueki Hospital and Kumamoto Regional Medical Center) pertaining to patients with stage II colon cancer, who underwent curative surgery from 2000 to 2013. We excluded cases of surgery-related death, cancer of the appendix, advanced cancer history within 3 years, cases with another active cancer, cases that received chemotherapy other than standard regimens, cases with no information of resected LN number, and cases with insufficient AC information. For cases followed by other institutions, we contacted the relevant medical institution and requested cooperation with the recurrence and the prognosis. Clinical stage classifications carried out according to the Union for International Cancer Control classification method. For pre-2013 cases where the description of perineural invasion (PN) was not obligatory in Japan, we used preserved tissues and asked pathologists to determine the presence or absence of PN.
Patient follow up strategy
Patients were followed up every 3 months for the first year after surgery and every 6 months for the next 4 years. They all underwent colonoscopy once a year, and whole-body computed tomography and/or abdominal ultrasound were performed once every 6 months to monitor for recurrence. The introduction of AC was left to the judgment of the attending physician. The regimen of AC was either an oral 5-fluorouracil (5-FU) formulation or 5-fluorouracil/leucovorin (5-FU/LV).
Statistical analysis
The relation between disease recurrence and the clinicopathological factors was analysed using the Cox proportional-hazards model. The collected stage II cases were grouped according to the number of risk factors on the basis of the NCCN, ASCO, ESMO and JFMC definitions of high-risk stage II (Table 1). Within these factors, “obstruction” was defined as; cases with symptomatic vomiting, cases where intestinal tract dilation was observed on imaging examination, and cases requiring the insertion of an ileus tube or the construction of a stoma before radical curative surgery. The date of the curative surgery was defined as time zero. The disease-free survival (DFS) and overall survival (OS) curves were calculated using the Kaplan-Meier method and compared by using the log-rank tests. The Cox proportional-hazards model was used for survival analysis. The software R-commander (R-cmdr) was used for all statistical computations. A p-value of < 0.05 was considered significant.
Table 1. High-risk stage II disease risk factors according to various guidelines
NCCN |
ASCO |
ESMO |
JFMC |
T4
perforation
obstruction
por, sig
ly+
v+
LN < 12
PN+
positive margin |
T4
perforation
por, sig, muc
LN < 13 |
T4
perforation
obstruction
por, sig
ly+
v+
LN < 12
PN+ |
T4
perforation
penetration
por, sig, muc
LN < 12 |
NCCN: National Comprehensive Cancer Network , ASCO: American Society of Clinical Oncology, ESMO: European Society for Medical Oncology, JFMC: Japanese Foundation for Multidisciplinary Treatment of Cancer, por: poorly differentiated adenocarcinoma, sig: signet ring cell carcinoma, muc: mucinous adenocarcinoma, ly: lymphatic invasion, v: vascular invasion, LN: lymph node examined, PN: perineural invasion
The Ethics Review Committee of Kumamoto City Ueki Hospital (No. 3) and Kumamoto Regional Medical Center (No. 17-035) approved this study. Each case provided general consent and informed consent before surgery. Each participating institution used an “opt-out” method to remove patients’ data from examination.
Clinicopathological characteristics of collected stage II CRC cases
We collected 246 cases of stage II colon cancer. Of these, there were 5 surgery-related deaths, 1 case of cancer of the appendix, 2 cases of advanced colon cancer within 3 years, 3 cases with another active cancer at the time of diagnosis, 3 cases who received nonstandard chemotherapy (mitomycin C infusion at days 1 and 2 after surgery), 2 cases with no information pertaining to the number of resected LN, and 4 cases with no information concerning AC. After excluding cases for the above-mentioned reasons, there were 226 cases for further analysis.
The clinicopathological characteristics of these cases are shown in table 2. The median follow-up period was 54 months. There were 26 cases (11.5%) of recurrence. The cecum (p = 0.034) and the sigmoid colon (p = 0.016) were significantly related to recurrence. Other significant risk factors or high-risk stage II were perforation, T4 staging, fewer than 12 examined LN (LN <12) and residual tumor (R1) (Table 1). AC was introduced in 50 cases (25.0%) of the recurrence (-) group and in 6 cases (23.1%) of the recurrence (+) group. Within these, the oral 5-FU formulation was introduced in 47 cases of the recurrence (-) group and in 5 cases of the recurrence (+) group. The AC regimen of other cases was 5-FU/LV. AC did not improve the DFS and OS of patients with stage II disease, taken as a whole stage II [sFigure 1].
Table 2. Clinicopathological characteristics of patients with stage II colon cancer
Factors |
recurrence (-)
n=200 |
recurrence (+)
n=26 |
p-value |
Age |
72.7 (31-97) |
71.7 (52-89) |
0.935 |
Gender
male
female |
100
100 |
13
13 |
0.753 |
Location
cecum
ascending
transverse
descending
sigmoid |
14
53
32
14
87 |
4
0
0
3
19 |
0.034
0.997
0.996
0.487
0.016 |
Perforation |
0 |
2 |
<0.001 |
Obstruction |
15 |
4 |
0.241 |
Penetration |
5 |
2 |
0.070 |
Tumor marker
CEA
CA19-9 |
9.8 (1.1-229.0)
32.2 (1.0-495.0) |
17.2 (1.5-161.4)
32.7 (1.0-160.4) |
0.311
0.903 |
Complication |
55 |
7 |
0.715 |
Histology
por/sig
muc
well/mod/pap |
5
12
183 |
0
1
25 |
0.997
0.724
0.479 |
T-factor
T3
T4 |
177
23 |
16
10 |
<0.001 |
Examined LN number
LN< 12
LN<13 |
95
107 |
18
18 |
0.026
0.132 |
Lymphatic invasion
ly-
ly+ |
131
91 |
10
16 |
0.134 |
Vascular invasion
v-
v+
unknown |
45
153
2 |
5
20
1 |
0.751 |
Perineural invasion
PN-
PN+
unknown |
190
5
5 |
21
2
3 |
0.100 |
Residual tumor
R0
R1
unknown |
181
14
5 |
17
9
0 |
<0.001 |
Adjuvant
present
absent |
50
150 |
6
20 |
0.368 |
Procedure
laparo
open |
2
198 |
0
26 |
0.694 |
CEA: carcinoembryonic antigen, CA19-9: carbohydrate antigen 19-9, por: poorly differentiated adenocarcinoma, sig: signet ring cell carcinoma, muc: mucinous adenocarcinoma, well: well differentiated adenocarcinoma, mod: moderately differentiated adenocarcinoma, pap: papillary adenocarcinoma. LN: lymph node. ly: lymphatic invasion, v: vascular invasion, PN: perineural invasion

sFigure 1: Adjuvant chemotherapy did not improve the disease-free survival (a) and the overall survival (b) of patients with stage II disease
Analysis of the ordinarily high-risk stage II cases
After referring to the risk factors for each guideline (Table 1), we selected high-risk cases using clinical trial methods (even 1 risk factor is regarded as a high-risk case). The number of high-risk cases according to each definition was NCCN, 205 (90.7%); ASCO, 148 (65.5%); ESMO, 205 (90.7%); JFMC, 140 (61.9%). Within these high-risk cases, those that received AC were NCCN, 53; ASCO, 38; ESMO, 54; JFMC, 37. The DFS and OS of these high-risk cases did not significantly differ relative to AC [sFigures 2 and 3]. We further analysed DFS and OS by each risk factor. No factor was associated with improved prognosis by following administration of AC [sTable 1]. These results are similar to those of recent reports [13-16].

sFigure 2: Adjuvant chemotherapy did not improve the disease-free survival of the ordinarily high-risk stage II cases (even 1 risk factor is regarded as a high-risk case). (a) NCCN definition, (b) ASCO definition, (c) ESMO definition, (d) JFMC definition
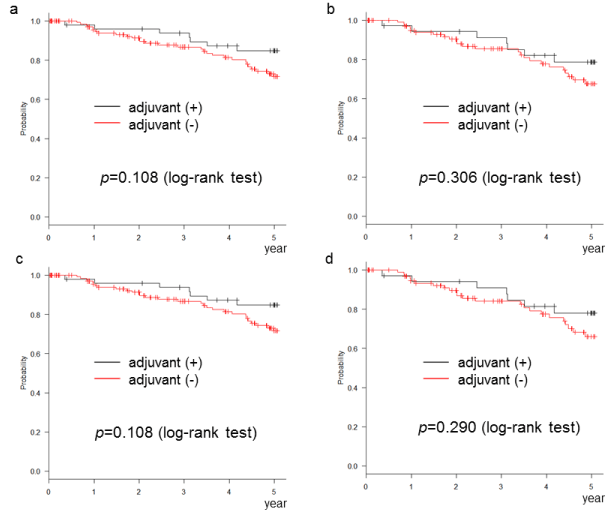
sFigure 3: Adjuvant chemotherapy did not improve the overall survival of the ordinarily high-risk stage II cases (even 1 risk factor is regarded as a high-risk case). (a) NCCN definition, (b) ASCO definition, (c) ESMO definition, (d) JFMC definition
sTable 1. DFS and OS of each risk factor by adjuvant chemotherapy
Factors |
Cases |
HR |
95% CI |
p-value |
T4
perforation
obstruction
penetration
por/sig
por/sig/muc
ly+
v+
LN <12
LN <13
PN+
R1 |
33
2
19
7
5
18
107
173
113
125
7
23 |
0.636
─
0.747
0.543
─
0.625
0.624
0.658
0.507
0.572
─
0.793 |
0.218-1.855
─
0.147-3.802
0.044-6.642
─
0.063-6.172
0.278-1.400
0.322-1.346
0.208-1.235
0.248-1.315
─
0.250-2.511 |
0.407
─
0.725
0.633
─
0.688
0.252
0.252
0.135
0.188
─
0.693 |
Factors |
Cases |
HR |
95% CI |
p-value |
T4
perforation
obstruction
penetration
por/sig
por/sig/muc
ly+
v+
LN <12
LN <13
PN+
R1 |
33
2
19
7
5
18
107
173
113
125
7
23 |
1.002
─
0.438
0.712
─
0.584
0.694
0.625
0.608
0.689
─
0.689 |
0.250-4.021
─
0.072-2.655
0.063-8.022
─
0.060-5.717
0.271-1.779
0.367-1.458
0.228-1.626
0.277-1.712
─
0.164-2.894 |
0.998
─
0.369
0.783
─
0.644
0.447
0.277
0.322
0.422
─
0.611 |
por: poorly differentiated adenocarcinoma, sig: signet ring cell carcinoma, muc: mucinous adenocarcinoma, well: well differentiated adenocarcinoma, mod: moderately differentiated adenocarcinoma, pap: papillary adenocarcinoma. LN: lymph node, PN: Perineural invasion, R: Residual tumor, ─: calculation impossible due to the cases.
Evaluation of cases by the number of risk factors
The relation between the number of risk factors, according to each definition, and the rate of recurrence is shown in table 3. The recurrence rate increased as the number of risk factors increased. The DFS and OS of cases with 1 or 2 risk factors, as per the NCCN or ESMO definition, were significantly improved by AC (Figures 1a,1c,2a,2c). This phenomenon was not observed in cases with 1 risk factor, as per the ASCO or JFMC definition (Figures 1b,1d,2b,2d). In cases with no risk factors, no significant improvement with AC was found in any of the definitions (Figures 3 and 4). Similarly, no significant improvement was found by AC in cases with more than 3 risk factors, as per the NCCN or ESMO definition or more than 2 risk factors, as per the ASCO and JFMC definition (Figures 5 and 6).

Figure 1. Disease-free survival of cases with (a) 1 or 2 risk factors according to the NCCN definition, (b) 1 risk factor according to the ASCO definition, (c) 1 or 2 risk factors according to the ESMO definition, (d) 1 risk factor according to the JFMC definition

Figure 2. Overall survival of cases with (a) 1 or 2 risk factor according to the NCCN definition, (b) one risk factor according to the ASCO definition, (c) 1 or 2 risk factors according to the ESMO definition, (d) 1 risk factor according to the JFMC definition

Figure 3. Disease-free survival of cases with (a) no risk factors according to the NCCN definition, (b) no risk factors according to the ASCO definition, (c) no risk factors according to the ESMO definition, (d) no risk factors according to the JFMC definition

Figure 4. Overall survival of cases with (a) no risk factors according to the NCCN definition, (b) no risk factors according to the ASCO definition, (c) no risk factors according to the ESMO definition, (d) no risk factors according to the JFMC definition

Figure 5. Disease-free survival of cases with (a) more than 3 risk factors according to the NCCN definition, (b) more than 2 risk factors according to the ASCO definition, (c) more than 3 risk factors according to the ESMO definition, (d) more than 2 risk factors according to the JFMC definition

Figure 6. Overall survival o cases with (a) more than 3 risk factors according to the NCCN definition, (b) more than 2 risk factors according to the ASCO definition, (c) more than 3 risk factors according to the ESMO definition, (d) more than 2 risk factors according to the JFMC definition
Table 3. Relation between the number of risk factors and recurrence
Guideline |
factors |
cases |
recurrence |
rate (%) |
NCCN |
0
1-2
≥ 3 |
21
123
82 |
1
8
17 |
4.76
6.50
20.73 |
ASCO |
0
1
≥ 2 |
78
119
29 |
4
13
9 |
5.13
10.92
31.03 |
ESMO |
0
1-2
≥ 3 |
21
131
74 |
1
10
15 |
4.76
7.63
20.27 |
JFMC |
0
1
≥ 2 |
86
112
28 |
4
13
9 |
4.65
11.61
32.14 |
NCCN: National Comprehensive Cancer Network, ASCO: American Society of Clinical Oncology, ESMO: European Society for Medical Oncology, JFMC: Japanese Foundation for Multidisciplinary Treatment of Cancer
We sought to identify patients with high-risk stage II disease to identify those at high-risk of recurrence and improve prognosis by introducing AC [5-8]. As the high-risk factors related to recurrence in stage II differ for each guideline, there is no list of established factors that are monitored in clinical settings. This leads to difficulty in judging the indications for AC. In addition, if at least 1 risk factor is placed in the category of the high-risk group, most cases become “high-risk” cases. Because AC does not improve prognosis for all patients with stage II disease, it is no wonder that there is no prognostic effect if most cases fall within the same category as the high-risk group [13-16]. The methods of identifying high-risk stage II patients require reconsideration. We therefore examined the relation between the number of risk factors and the effects of AC.
There are 3 possible prognostic categories for patients with stage II colon cancer. Group 1: some cases are cured only by surgical therapy. These cases do not experience recurrence, regardless of whether AC is introduced or not. The prognosis of these cases is good, and there is no prognostic difference due to the introduction of AC. Group 2: in some cases, recurrence is suppressed by AC. These patients’ prognosis will vary, depending on the presence or absence of AC. Group 3: some patients experience recurrence even after AC is introduced. These patients have a poor prognosis, and there is no prognostic value to introducing AC.
In this study, the prognosis of patients with stage II colon cancer worsened as the number of risk factors included in each case increased. Kim et al. also reported this phenomenon [17]. Cases where a cure is obtained only by surgical therapy might be those with no risk factors: in other words, a ‘low-risk group’. Most patients where recurrence is suppressed by AC might be included in the group with 1 or 2 risk factors according to the NCCN or ESMO definition. Most cases with recurrence despite AC might be included in the group with more than 3 risk factors according to the NCCN or ESMO definition.
Although the cases analysed in this study spanned a relatively long period, they all had stage II disease and their AC regimen was either an oral 5-FU formulation or 5-FU/LV. Therefore, the AC regimen used appeared not to change prognosis [18]. Therefore, results generalizability might be limited to patients who only received oral 5-FU or 5-FU/LV. This suggests that cases with 1 or 2 risk factors according to the NCCN or ESMO definition, may benefit from oral 5-FU or 5-FU/LV. Stated differently, oral 5-FU or 5-FU/LV may be insufficient to suppress recurrence in cases with 3 or more risk factors according to the NCCN or ESMO definition. The effect of adding oxaliplatin against stage II colon cancer is controversial [19-22]. However, in these reports, the authors did not extract and evaluate populations with recurrence rates equivalent to those of patients with stage III colon cancer. According to the Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines [23], the 5-year OS of patients with colon cancer with any T N1 (JSCCR defines these cases as stage IIIa) is 76.1%. In this study, the OS values of cases with more than 3 risk factors, according to the NCCN and ESMO definitions were 78.0% and 77.0%, respectively. This is considered comparable to stage IIIa. This suggests that the prognosis of patients with more than 3 risk factors according to the NCCN or ESMO guidelines, can be improved when oxaliplatin is added to the AC regimen.
These results led us to consider classifying patients with stage II colon cancer into 3 categories instead of into 2 types (low and high -risk). Referring to the NCCN and ESMO guidelines, the following treatment methods may be considered: follow-up observation without AC for cases with no risk factors, AC with oral 5-FU or 5-FU/LV for cases with 1 or 2 risk factors, an oxaliplatin augmented regimen for cases with 3 or more risk factors. As expected, accurate verification is impossible without a prospective clinical trial. However, if possible, recent clinical trial should be reviewed when considering this method of stratification.
The authors thank the members of the Kumamoto City Medical Association for their cooperation with the patient's prognoses survey. The authors also thank Ms. Miki Noda, Ms. Tomomi Fukuoka, and Ms. Yumi Yamamoto for their kind support in collecting patients’ information.
This study was supported by a Grant-in-Aid from the Ministry of Education, Culture, and Science of Japan (No. 25462062).
- Gill S, Loprinzi CL, Sargent DJ, Thome SD, Alberts SR, et al. (2004) Pooled analysis of fluorouracil-based adjuvant therapy for stage II and III colon cancer: who benefits and by how much? J Clin Oncol 22: 1797-1806. [Crossref]
- André T, Boni C, Mounedji-Boudiaf L, Navarro M, Tabernero J, et al. (2004) Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med 350: 2343-51. [Crossref]
- Kuebler JP, Wieand HS, O’Connel MJ, Smith RE, Colonagelo LH, et al. (2007) Oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: results from NSABP C-07. J Clin Oncol 25: 2198-2204. [Crossref]
- Sargent DJ, Wieand HS, Haller DG, Gray R, Bebedetti JK, et al. (2005) Disease-free survival versus overall survival as a primary end point for adjuvant colon cancer studies: individual patient data from 20,898 patients on 18 randomized trials. J Clin Oncol 23: 8664-70. [Crossref]
- Merkel S, Wein A, Günther K, Papadopoulos T, Hohenberger W, et al. (2001) High-risk groups of patients with Stage II colon carcinoma. Cancer 92: 1435-43. [Crossref]
- Chung KY, Kelsen D (2006) Adjuvant therapy for stage II colorectal cancer: Who and with what? Curr Treat Options Gastroenterol 9: 272-80. [Crossref]
- André T, Sarget D, Tabernero J, O'Connell M, Buyse M, et al. (2006) Current issues in adjuvant treatment of stage II colon cancer. Ann Surg Oncol 13: 887-98. [Crossref]
- Quah HM, Chou JF, Gonen M, Shia J, Schrag D, et al. (2008) Identification of patients with high-risk stage II colon cancer for adjuvant therapy. Dis Colon Rectum 51: 503-7. [Crossref]
- Benson AB 3rd, Schrag D, Somerfield MR, Cohen AM, Figueredo AT, et al. (2004) American Society of Clinical Oncology recommendations on adjuvant chemotherapy for stage II colon cancer. J Clin Oncol 22: 3408-19. [Crossref]
- Benson AB, Venook AP, Bekaii-Saab T, Chan E, Chen YJ, et al. (2016) National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology: Colon Cancer. (Version 2). Available at: https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf.
- Schmoll HJ, Van Cutsem E, Stein A, Valentini V, Glimelius B, et al. (2012) ESMO Consensus Guidelines for management of patients with colon and rectal cancer. a personalized approach to clinical decision making. Ann Oncol 23: 2479-516. [Crossref]
- Sadahiro S, Morita S, Sasaki K, Sakamoto K, Ohge H, et al. (2015) Treatment Rationale and Study Design for Clinical Trial on the Efficacy of UFT/LV for Stage II Colorectal Cancer with Risk Factors for Recurrence (JFMC46-1201). Clin Colorectal Cancer 14: 277-80. [Crossref]
- Kumar A, Kennecke HF, Renouf DJ, Lim HJ, Gill S, et al. (2015) Adjuvant chemotherapy use and outcomes of patients with high-risk versus low-risk stage II colon cancer. Cancer 121: 527-34. [Crossref]
- Casadaban L, Rauscher G, Aklilu M, Villenes D, Freels S, et al. (2016) Adjuvant chemotherapy is associated with improved survival in patients with stage II colon cancer. Cancer 122: 3277-87. [Crossref]
- Booth CM, Nanji S, Wei X, Peng Y, Biagi JJ, et al. (2017) Adjuvant Chemotherapy for Stage II Colon Cancer: Practice Patterns and Effectiveness in the General Population. Clin Oncol (R Coll Radiol) 29: e29-e38. [Crossref]
- Park JS, Chon HJ, Jeung HC, Shin SJ, Rha SY, et al. (2016) High-risk clinicopathological features and their predictive significance in Korean patients with stage II colon cancer. J Cancer Res Clin Oncol 142: 2051-9. [Crossref]
- Kim MK, Won DD, Park SM, Kim T, Kim SR, et al. (2018) Effect of Adjuvant Chemotherapy on Stage II Colon Cancer: Analysis of Korean National Data. Cancer Res Treat 50: 1149-63. [Crossref]
- Sakamoto J, Ohashi Y, Hamada C, Buyse M, Burzykowski T, et al. (2004) Efficacy of oral adjuvant therapy after resection of colorectal cancer: 5-year results from three randomized trials. J Clin Oncol 22: 484-92. [Crossref]
- Tournigand C, André T, Bonnetain F, Chibaudel B, Lledo G, et al. (2012) Adjuvant therapy with fluorouracil and oxaliplatin in stage II and elderly patients (between ages 70 and 75 years) with colon cancer: subgroup analyses of the Multicenter International Study of Oxaliplatin, Fluorouracil, and Leucovorin in the Adjuvant Treatment of Colon Cancer trial. J Clin Oncol 30: 3353-60. [Crossref]
- Hatano S, Ishida H, Ishibashi K, Kumamoto K, Haga N, et al. (2013) Identification of risk factors for recurrence in high-risk stage II colon cancer. Int Surg 98: 114-21. [Crossref]
- Teufel A, Gerken M, Hartl J, Itzel T, Fichtner-Feigl S, et al. (2015) Benefit of adjuvant chemotherapy in patients with T4 UICC II colon cancer. BMC Cancer 15: 419. [Crossref]
- Meyers BM, Cosby R, Quereshy F, Jonker D (2016) Adjuvant systemic chemotherapy for stages II and III colon cancer after complete resection: a clinical practice guideline. Curr Oncol 23: 418-24. [Crossref]
- Watanabe T, Muro K, Ajioka Y, Hashiguchi Y, Ito Y, et al. (2017) Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2016 for the treatment of colorectal cancer. Int J Clin Oncol 23: 1-34. [Crossref]
View Supplementary Data









