Background: The occurrence of exacerbation in these patients may result in worsening quality of life and a faster decline in lung function. Amongst the Indian population the prevalence of COPD is about 3% to 8% in males and 2.5% to 4.5% in females.
Objective: The purpose of this study was to analyze the effectiveness of treatment and the quality of life of those patients who were admitted with a diagnosis of AECOPD to Amrita Institute of Medical Sciences and Research Centre, Kerala between October 2018 and April 2019. The patients selected were those who had received a combination of formoterol with budesonide to the standard treatment of salbutamol with ipratropium bromide nebulization.
Methods: A total of 75 patients with diagnosis of acute exacerbation of COPD met the inclusion criteria. Their dyspnea symptoms, Arterial blood gas (ABG) values, PEFR values and their quality of life were compared on day of admission and day of discharge. The ADRs that occurred were also monitored during their stay at the hospital.
Result: There was a significant change in the effectiveness parameters and the quality of life of the patients who were taking the treatment together. The ADRs observed were not severe
Conclusion: The use of the treatment combination showed a statistical significance in the patient’s condition hence showing that the addition of formoterol and budesonide to the standard therapy may be considered in the treatment of patients suffering from acute exacerbated COPD.
acute exacerbated copd, arterial blood gas, add on therapy, dyspnea, spirometry, st.george respiratory scale
COPD is a multicomponent disease involving emphysema in the lung parenchyma, large central airway inflammation and mucocilliary dysfunction, bronchiolitis and small airway structural changes leading to persistent respiratory symptoms and airflow limitation caused due to either airway or alveolar abnormalities due to significant exposure to noxious particles and gases. These separate factors contribute to this condition and varies from patient to patient [1]. Amongst the Indian population the prevalence of COPD is about 3% to 8% in males and 2.5% to 4.5% in females [2]. The most common and known factor for COPD is cigarette smoking, however there are consistent evidence that COPD may occur in non-smokers as well [3]. Other factors include genetic factors, age and sex of the patient, lung growth and development, exposure to particles, chronic bronchitis and infections [4-24]. An exacerbation of chronic obstructive pulmonary disease (COPD) is “an acute event characterized by a worsening of the patients’ respiratory symptoms that is beyond normal day-to- day variations and leads to a change in medication” [25]. It is associated with worsening quality of life and a faster decline in lung function with a 10% hospital mortality rate and about 25% mortality rate in ICU patients [26]. COPD may be considered in any patient suffering from dyspnea, chronic cough or sputum production or if they have a history of exposure to risk factors for the disease. Spirometry is used to make the diagnosis and the presence of a post-bronchodilator. FEV1/FVC<0.70 confirms presence of persistent airflow limitation and also of COPD in patients with appropriate symptoms and exposure to noxious stimuli. The COPD Assessment test and The Modified MRC Dyspnea scale are the two most commonly used scales for the assessment of symptoms. Stable COPD is managed by regular treatment with one or more long acting bronchodilators. Glucocorticoids are added in case of repeated exacerbations and long-term oxygen therapy is added in case of respiratory failure. The standard treatment of AECOPD is with short acting Beta 2 agonist like Salbutamol with /without short acting muscarinic antagonist like Ipratropium bromide which achieved a synergistic bronchodilatory effect but failed to achieve symptom control, which was overcome using a combination of inhaled corticosteroids and long acting beta 2 agonist which showed significant benefit in respiratory function and restricted activity days [23,27,28]. The use of this combination along with the standard treatment has not been studied yet. So, considering the increasing population who use this combination, studies are required to scientifically and statistically evaluate its effect as an add on therapy.
A total of 75 patients were enrolled in our study with diagnosis of acute exacerbation of COPD and study period was conducted over a span of 1 year and study population consists of patient reporting in pulmonology department. Patients who are unconscious delirious and cognitive impaired patients are excluded from the study. Patients who received add on therapy (formotertol and budesonide) along with standard treatment (salbutamol and ipratropium) and patients who signed informed consent prior to their data being collected, are selected to the study.
The study groups were identified from the population attending pulmonology department based up on the inclusion and exclusion criteria. Their basic information and other demographic details were collected by using standardized data collection form and their medical records.
Efficacy was determined by finding the changes in baseline dyspnea symptoms and dyspnea symptoms were graded according to Modified Medical Research Council (mMRC) dyspnea scale.It mainly consists of 5 statements that describes the entire range of dyspnea from none (grade0 to grade4) which shows incapacity.
The next tool used was peak expiratory flow meter it is used to find out the degree of airway obstructions in patients and is measured by using a peak expiratory flow meter and their flow rate were measured during admission and discharge. Other efficacy parameters including Arterial Blood Gas (ABG), sp02 measurement was also collected on the day of admission and discharge. The mean of these parameters on admission and discharge was taken and their mean difference was calculated. Categorizing the difference of each parameter in individual subjects, the outcome was evaluated. Health-related quality of life improvements was determined by St George’s Respiratory Questionnaire for COPD (SGRQ-C) on the day of admission, during their hospital stay and on the day of discharge. It mainly consists of 14 questions which is mainly divided into 2 parts.
Safety was determined by assessing the nature, incidence and severity of any adverse events (AEs) reported by the patient and it will be graded according to Naranjo causality assessment scale and Hartwig’s severity assessment scale.
Statistical analysis was done by using IBM SPSS 22.0 software. Students paired p test was applied to analyze the effectiveness and quality of life among patients. Ap-value‹0.05 was considered to be statistically significant. The study was approved by institutional and ethics committee of Amrita Institute of Medical Sciences, Kerala, India.
During the study, a total of 75 patients with COPD were hospitalised with an acute exacerbation among which 73(97.3%) of them experienced cough as a common symptom, 65(86.7%) patients complained of shortness of breath while 58 (77.3%) patients had sputum expectoration. A few patients experienced symptoms like myalgia, weakness, weight loss, orthopnoea, giddiness, abdominal distension, chills etc. and were categorized as a whole under other symptoms (Table 1).
Table 1. A report of the general results obtained
Characteristics
|
Category |
No. of patients (% of patients) |
Age |
Mean age ± SD |
69.05±7.8 |
Sex |
Female |
8(10.7) |
Male |
67(89.3) |
Symptoms of AECOPD |
Cough |
73 (97.3) |
Expectoration |
58 (77.3) |
Wheeze |
38 (50.7) |
Shortness of Breath |
65 (86.7) |
Haemoptysis |
2 (2.7) |
Chest Pain |
11 (14.7) |
Fever |
21 (28.0) |
Others |
8 (10.8) |
Risk factors of COPD |
Smoker |
64 (85.3) |
Exposure to dust and smoke |
11 (14.7) |
Chemical agents |
3 (4.0) |
Causes for AECOPD |
Infection |
55 (73.3)
|
Irritants |
9 (12) |
Climate change |
6 (8) |
Exertion |
6 (8) |
Unknown |
4 (5.3) |
Co-morbidities |
HTN |
41 (54.7) |
DM |
29 (38.7) |
CAD |
18 (24) |
TB |
6 (8) |
Dyslipidaemia |
19 (25.3) |
Asthma |
3 (4) |
Cancer |
7 (9.3) |
Respiratory Failure |
17 (22.6) |
Among the 75 patients enrolled in the study 55(73.3%) of them were admitted due to infection while 9(12%) of them were admitted as a result of exposure to irritants.59(78.7%) patients showed an increase in PEFR value and 5(6.7%) of them showed a decrease in PEFR value while there was no change in 11(14.7%) of the patients.65(86.7%) patients showed an increase in spO2 value and 8(10.7%) of them showed a decrease in spO2 value while there was no change in 2(2.7%) of the patients. 69(92%) patients showed an increase in pO2 value and 6(8%) of them showed a decrease in pO2 value.20 (26.7%) patients showed an increase in pCO2 value and 55(73.3%) of them showed a decrease in pCO2 value (Table 2) (Figure 1).
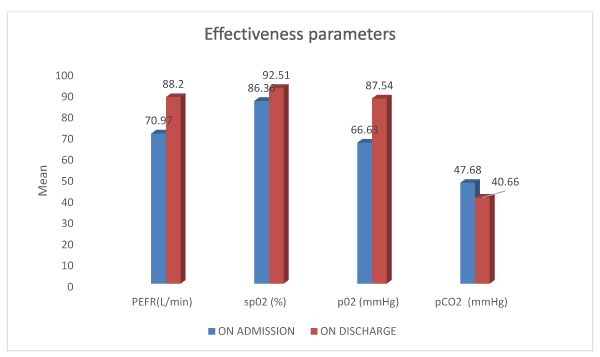
Figure 1. Graphical representation of Effectiveness parameters
Table 2. A report of the results obtained pertaining to effectiveness of the treatment
Effectiveness
|
ON ADMISSION
|
ON DISCHARGE
|
P value
|
Mean ± S D |
Mean ± S D
|
PEFR(L/min)
|
70.97±12.23 |
88.20±20.51 |
<0.001 |
PEFR%
|
16.36±2.96 |
20.30±4.59
|
sp02 (%)
|
86.36±8.49 |
92.51±6.30 |
p02 (mmHg)
|
66.63±19.85 |
87.54±19.14 |
pCO2 (mmHg)
|
47.68±13.98 |
40.66±8.64 |
MMRC
|
3.08±0.67
|
1.37±0.97 |
<0.001 |
The total numbers of ADRS reported in the study populations (N=75) was found to be 23(30.6%) and the most common ADR reported was palpitation (8%) followed by hypokalaemia (6.7%), tachycardia (5.3%), dry mouth (4%), oral candidiasis (4%) and tremor (2.7%) (Table 3) (Figure 2).
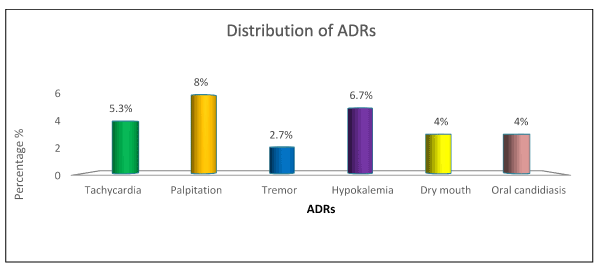
Figure 2. Graphical presentation of ADR
Table 3. ADRS observed during the course of the study
ADRS |
Frequency
(n=75) |
Percentage (%) |
Tachycardia |
4 |
5.3 |
Palpitation |
6 |
8 |
Tremor |
2 |
2.7 |
Hypokalaemia |
5 |
6.7 |
Dry Mouth |
3 |
4 |
Oral candidiasis |
3 |
4 |
No ADR |
52 |
69.3 |
There was remarkable change in the components of the questionnaire as well as in the total score on admission and on discharge.Patients taking the treatment had a mean decrease in the following : Sypmtom score % of -47.131±22.607, Activity score % of -3188.430±14.17, Impact % of -35.143 ±13.346 and an overall total % score of -36.264±11.002 which is statistically significant with a p value <0.001 (Table 4) (Figure 3).
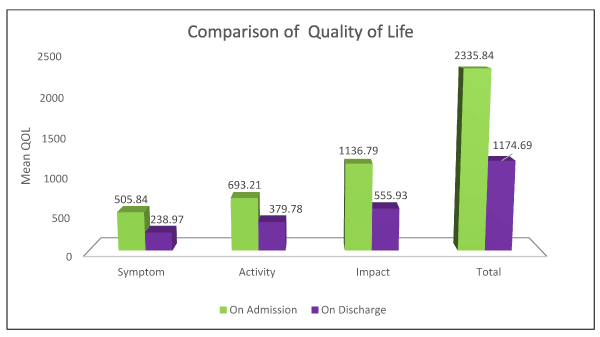
Figure 3. Graphical presentation of the Quality of life of study participants on admission and on discharge
Table 4. Quality of life assessment using the St.George Respiratory C questionnaire
Quality of Life Score
|
ON ADMISSION |
ON DISCHARGE
|
P value
|
Mean± S D |
Mean± S D
|
Symptom |
505.84±72.90 |
238.97±97.44 |
<0.001
|
Activity |
693.21±143.60 |
379.78±132.13 |
Impact |
1136.79±237.88 |
555.93±188.70 |
Total |
2335.84±402.61 |
1174.69±343.77 |
Acute exacerbated COPD (AECOPD) has been shown to have a 10% hospital mortality rate and up to 25% mortality in case of those patients admitted in the ICU. It is associated with worsening quality of life and faster decline in lung function. Thus, necessitating an emphasis on the comprehensive and stepwise approach towards its management.
On conducting this study, it was found that the disease was more predominant in males than in females and majority of the patients were in their seventies. Larger prevalence of smoking might be a reason as to why more males are inclined to the disease than females. On the other hand, female patients experienced AECOPD when they were exposed to smoke and dust particles or when they had an encounter with extreme temperatures. The same was observed in a study conducted by Tamara Shikowski et al. [29].
On evaluating the symptoms that were faced by the study population we sought out that the most common symptom was cough. The other symptoms included breathlessness, expectoration of sputum, wheeze, fever, chest pain and hemoptysis as o served in fig. All of these symptoms were in recognition of a typical episode of AECOPD. Myalgia, weakness, weight loss, orthopnea, giddiness, abdominal distention, chills etc. were a few other symptoms that were experienced to a lesser extent. Analogous symptoms were reported in a study conducted by Terrenece Seemungal et al. [30]
Smoking turned out to be the major risk factor for COPD. Exposure to dust and smoke and subjection to chemical agents (fig) were the other risk factors. Our data was similar to that of a study conducted by Johanna R Feary et al. [31].
Certain factors are responsible for inducing an exacerbation in COPD patients of which infection was found to be the major triggering factor. Other triggering factors noticed in our study included irritants, climate change and exertion. Studies conducted by Terrenece Seemungal et al portrayed the same triggering factors [30].
During the course of our study we observed hypertension, diabetes mellitus, CAD, dyslipidemia, TB, asthma and cancer to be a few of the co-morbidities that accompanied COPD. There were 17patients with respiratory failure that may have occurred as a complication to acute exacerbation of COPD as a result of low blood oxygen levels and raised blood carbon dioxide levels. Comparable co-morbidities were detailed by P J Barnes et al in his studies [32].
It would be appropriate to mention that there have not been studies conducted to find out the safety and efficacy of nebulized budesonide and formoterol combination as an add on therapy to nebulized salbutamol and ipratropium bromide combination in the management of AECOPD, yet to our knowledge. However previous studies have been conducted showing the safety and efficacy of either a combination of salbutamol and ipratropium bromide or a combination of formoterol and budesonide.
The combination of salbutamol and ipratropium bromide gained its popularity in the treatment of AECOPD for its unique ability to provide faster relief from exacerbation. However, in a recent study by Josiah Gordon et al states that the use of this combination was relatively inconvenient due to their short half-lives and the necessity in dosing four times daily in comparison to newer long acting bronchodilators like formoterol, aclidinium, salmeterol, indacterol etc [33]. The GOLD guidelines recommend a stepwise increase in the pharmacologic treatment of COPD. Mild COPD may be managed with a short acting bronchodilator while the use of LABA or anticholinergic may be initiated individually and then in combination as the airflow worsens, and symptoms like dyspnea and functional impairment progresses. The next step advised to be initiated is the addition of ICS. Furthermore, in a study carried out by The Combivent Inhalation Aerosol Study Group it was stated that the advantage of the combination of salbutamol and ipratropium was apparent only during the first four hours after administration [34]. In order to overcome this minor setback, the addition of nebulized budesonide and formoterol may be used. The combination of formoterol and budesonide can maintain the benefit of treatment optimization, stabilize lung function and also helps delays exacerbations more effectively as per a study by P M Calverley et al and this finding was also backed by studies carried out by Tobias Welte et al and W.Szafranski et al. The effectiveness of the treatment was measured using the following parameters like PEFR, spO2, ABGand mMRC score [35-37].
PEFR allows measuring the airflow through the bronchi and thus the degree of obstruction in the airways. In our study, the mean PEFR percentage on admission was 16.36±2.96 and on discharge was 20.30±4.59, with a p value <0.001.A mean PEFR change of 17.23±17.15 and a mean PEFR % change of 3.940±3.88 was observed with a p value <0.001. A majority of the patients had a PEFR % that ranged from 0 to 5 in which 59(78.7%) of them showed an increase in PEFR value and 5(6.7%) of them showed a decrease in PEFR value while there was no change in 11(14.7%) of the patients showing that there was a significant improvement with PEFR measurement.
The improvement in the PEFR may be attributed to the administration of nebulized formoterol and budesonide as an add on therapy to salbutamol and ipratropium bromide which relivesinflammation, scarring, bronchospasm, mucus and stretched airways thus airflow through the bronchi and the degree of obstruction in the airways improved. A study conducted by W.Szafranski et al showed an improvement in the PEFR values when the study population was treated with budesonide and formoterol [37].
The mean spO2 on admission was 86.36±8.49 and on discharge was 92.51±6.30, with a p value <0.001. Furthermore, a majority of 46(61.3%) patients had an improvement in their spO2 in the range of 0 to 10. The study population was found to have a mean change in spO2 % of 6.15±6.57 .In which 65(86.7%) of them showed an increase in spO2 value and 8(10.7%)of them showed a decrease in spO2 value while there was no change in 2(2.7%) of the patients. This data suggested that there was significant improvement in the oxygen saturation in those responding to the treatment.
The next parameter ABG comprised of pO2 and pCO2.
Here, the mean pO2 on admission was found to be 66.63±19.85 and on discharge was 87.54±19.14, with a p value <0.001. Majority of the patients experienced an improvement in the pO2 in the range of 20 to 40. It was observed that the patients had a mean change in pO2 of 20.91±20.56 in which 69(92%) of them showed an increase in pO2 value and 6(8%) of them showed a decrease in pO2 value. In a study conducted by Jill P.Karpel et al, the mean pO2 on admission was 68.5±4, after 30 minutes was found to be 74.5±4.3 with a mean change of 5.8±3 mmHg showing that there was a significant rise in pO2 [38] .
On the other hand, the mean pCO2 on admission was 47.68±13.98 and on discharge was 40.66±8.64 with a p value<0.001. The patients were found to have a mean change in pCO2 of -7.02±11.45 in which 20(26.7%) of them showed an increase in pCO2 value and 55(73.3%) of them showed a decrease in pCO2 value in which a majority of the patients had a decline in the range of -20 to 0.
The combination of inflammatory insults at the level of bronchial and bronchiolar airways and the loss of the alveoli and capillaries inevitably leads to a progressive inefficiency of the intrapulmonary gas exchange regarding spO2, pO2 and pCO2 that was improved with the help of the treatment on which our study in based.
The mMRC scale uses symptom scores experienced by the study population in which the decline in the score states an improvement in the symptom of the patient. On calculating the mMRC score it was found that there was a mean change of -1.92±0.358.
in which 73(97.3%) of them showed a decline in mMRC value and 2(2.7%) of them showed no change. The decrease in the overall score of this scale showed an improvement in the symptoms experienced by the patients. On comparing these results, to a study conducted by Donald P. Tashkin et al, in finding out the safety and efficacy of budesonide and formoterol, the mean change in the score was -0.33±0.58 [39].
Taking into consideration the safety profile, out of the 75 patients, there were 23 (30.6%) reported ADRS. The most commonly occurring ADRs were palpitation (8%), hypokalemia (6.7%), tremors (2,2.7%) , dry mouth (3,4%) ,oral candidiasis (3,4%) and tachycardia (5.3%) .The bronchodilators used in our study, salbutamol, formoterol and ipratropium bromide may have caused the ADRS like tachycardia, palpitation, tremor, dry mouth and hypokalemia. These findings are upheld in studies conducted by Jill P.Karpel et al and K.M Lulich et al. The occurrence of oral candidiasis may be due to administration of corticosteroid (budesonide). Donald P. Tashkin et al. conducted a study in which similar results were stated [38-40].
On carrying out the Naranjo Causality Assessment, we found that the ADRS observed among the study population was probable (69.56%) and possible (30.44%). We also utilized the Hartwig Severity scale to assess the severity of ADRS which showed that 78.26% of the observed ADRS were moderate and that none were severe.
In order to assess the QOL of the study population taking the treatment, we used the St.George Respiratory –C Scale and found out that there was a significant change in all the components into which the questionnaire was categorised into with a Sypmtom score % of -47.131±22.607, Activity score % of -3188.430±14.17, Impact % of -35.143 ±13.346 and an overall total % score of -36.264±11.002 which was significant with a p value <0.001. This showed that there was a considerable improvement in the patient’s QOL.A similar pattern of occurrence was traced in a study conducted by Donald P. Tashkin et al. They used the same questionnaire to find the QOL of patients who were treated with formoterol and budesonide [39]. Their Symptom score % -7.38±20.10, Activity score % -4.37±14.06 and Impact score %-3.41±14.02 and an overall total score of -4.32±12.17.
We have now been able to paint a picture on the use of budesonide and formoterol as an add on therapy to salbutamol and ipratropium bromidewhich was found to be an effective treatment in the management of AECOPD . Our studsy points out that ,this treatment was effective in providing early and sustained improvement in lung function which was observed from the improvement in PEFR , spO2 and ABG values. With the aid of the mMRC dyspnoea scale, a discernable improvement was observed in the symptomatology. The ADRS noticed in the study subjects were not severe. The quality of life of the patients taking this treatment was improved considerably . It can be concluded that the addition of nebulised budesonide and formoterol combination leads to significant benefit in respiratory function and restricted activity days.Hence this treatment appears to be a promising therapy in the management of AECOPD.
- Vestbo J, Anderson W, Coxson HO, Crim C, Dawber F, et al. (2008) Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE). Eur Respir J 31: 869-873. [Crossref]
- Bhome AB (2012) COPD in India: Iceberg or volcano? J Thorac Dis 4: 298-309. [Crossref]
- Lopez AD, Shibuya K, Rao C (2006) Chronic obstructive pulmonary disease: current burden and future projections. Eur Respir J 27: 397-412.
- Stoller JK, Aboussouan LS (2005) Alpha1-antitrypsin deficiency. Lancet 365: 2225-2236. [Crossref]
- Mc Closkey SC, Patel BD, Hinchliffe SJ, Reid ED, Wareham NJ, et al. (2001) Siblings of patients with severe chronic obstructive pulmonary disease have a significant risk of airflow obstruction. Am J Respir Crit Care Med. 164: 1419-1424.
- Mercado N, Ito K, Barnes PJ (2015) Accelerated ageing of the lung in COPD: new concepts. Thorax 70: 482-489. [Crossref]
- Landis SH, Muellerova H, Mannino DM et al. (2014) Continuing to Confront COPD International Patient Survey: methods, COPD prevalence, and disease burden in 2012-2013. Int J Chron Obstruct Pulmon Dis 9: 597.
- Regan EA, Lynch DA, Curran-Everett D (2015) Clinical and radiologic disease in smokers with normal spirometry. JAMA Intern Med 175: 1539-1549.
- Stern DA, Morgan WJ, Wright AL, Guerra S, Martinez FD (2007) Poor airway function in early infancy and lung function by age 22 years: a non-selective longitudinal cohort study. The Lancet 370: 758-764.
- Kohansal R, Martinez-Camblor P, Agustí A, Buist AS, Mannino DM, (2009) The natural history of chronic airflow obstruction revisited: an analysis of the Framingham offspring cohort. Am J Respir Crit Care Med 180: 3-10.
- Yin P, Jiang CQ, Cheng KK (2007) Passive smoking exposure and risk of COPD among adults in China: the Guangzhou Biobank Cohort Study. The Lancet 370: 751-757.
- Tager IB, Ngo L, Hanrahan JP (1995) Maternal smoking during pregnancy. Effects on lung function during the first 18 months of life. Am J Respir Crit Care Med 152: 977-983.
- Paulin LM, Diette GB, Blanc PD (2015) Occupational exposures are associated with worse morbidity in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 191: 557-565.
- Eisner MD, Anthonisen N, Coultas D (2010) An official American Thoracic Society public policy statement: Novel risk factors and the global burden of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 182: 693-718.
- Marchetti N, Garshick E, Kinney GL (2014) Association between occupational exposure and lung function, respiratory symptoms, and high-resolution computed tomography imaging in COPDGene. Am J Respir Crit Care Med 190: 756-762.
- Orozco-Levi M, Garcia-Aymerich J, Villar J, Ramirez-Sarmiento A, Anto JM (2006) Wood smoke exposure and risk of chronic obstructive pulmonary disease. Eur Respir J 27: 542-546.
- Gan WQ, FitzGerald JM, Carlsten C, Sadatsafavi M, Brauer M (2013) Associations of ambient air pollution with chronic obstructive pulmonary disease hospitalization and mortality. Am J Respir Crit Care Med 187: 721-727.
- Zhou Y, Zou Y, Li X (2014) Lung function and incidence of chronic obstructive pulmonary disease after improved cooking fuels and kitchen ventilation: a 9-year prospective cohort study. PLoS med 11: e1001621.
- Allinson JP, Hardy R, Donaldson GC, Shaheen SO, Kuh D, et al. (2016) The presence of chronic mucus hypersecretion across adult life in relation to chronic obstructive pulmonary disease development. Am J Respir Crit Care Med 193: 662-672.
- Guerra S, Sherrill DL, Venker C, Ceccato CM, Halonen M, et al. (2009) Chronic bronchitis before age 50 years predicts incident airflow limitation and mortality risk. Thorax 64: 894-900.
- Kim V, Han MK, Vance GB (2011) The chronic bronchitic phenotype of COPD: an analysis of the COPDGene Study. Chest 140: 626-633.
- de Marco R, Accordini S, Marcon A (2011) Risk factors for chronic obstructive pulmonary disease in a European cohort of young adults. Am J Respir Crit Care Med 183: 891-897.
- Drummond MB, Kirk GD (2014) HIV-associated obstructive lung diseases: insights and implications for the clinician. Lancet Respir Med 2: 583-592.
- Byrne AL, Marais BJ, Mitnick CD, Lecca L, Marks GB (2015) Tuberculosis and chronic respiratory disease: a systematic review. Int J Infect Dis 32: 138-146.
- American Lung Association Epidemiology and Statistics Unit. Trends in COPD (Chronic Bronchitis and Emphysema): Morbidity and Mortality.
- Menezes AM, Jardim JR, Pérez-Padilla R et al. (2005) Prevalence of chronic obstructive pulmonary disease and associated factors: the PLATINO Study in São Paulo,Brazil. Cad Saude Publica 5: 1565-1573.
- Mathers CD, Loncar D (2006) Projections of global mortality and burden of disease from 2002 to 2030. PLoS medicine 3: e442.
- Halbert RJ, Natoli JL, Gano A, Badamgarav E, Buist AS (2006) Global burden of COPD: systematic review and meta-analysis. Eur Respir J 28: 523-532.
- Schikowski T, Sugiri D, Ranft U, Gehring U, Heinrich J, et al. (2005) Long-term air pollution exposure and living close to busy roads are associated with COPD in women. Respir Res 6: 152.
- Seemungal T, Harper-Owen R, Bhowmik A, Moric I, Sanderson G, et al. (2001) Respiratory viruses, symptoms, and inflammatory markers in acute exacerbations and stable chronic obstructive pulmonary disease. Am J Respir Crit Care Med 164: 1618-1623.
- Feary JR, Rodrigues LC, Smith CJ, Hubbard RB, Gibson JE (2010) Prevalence of major comorbidities in subjects with COPD and incidence of myocardial infarction and stroke: a comprehensive analysis using data from primary care. Thorax.
- Barnes PJ, Celli BR (2009) Systemic manifestations and comorbidities of COPD. Eur Respir J 33: 1165-1185.
- Gordon J, Panos RJ (2010) Inhaled albuterol/salbutamol and ipratropium bromide and their combination in the treatment of chronic obstructive pulmonary disease. Expert Opin Drug Metab Toxicol 6: 381-392.
- Combivent Inhalation Aerosol Study Group (1994) In COPD, a combination of ipratropium and albuterol is more effective than either agent alone: an 85-day multicenter trial. Chest 105: 1411-1419.
- Calverley PM, Boonsawat W, Cseke Z, Zhong N, Peterson S (2003) Maintenance therapy with budesonide and formoterol in chronicobstructive pulmonary disease. Eur Respir J 22: 912-919.
- Welte T, Miravitlles M, Hernandez P, Eriksson G, Peterson S, et al. (1994) Efficacy and tolerability of budesonide/formoterol added to tiotropium in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 180: 741-750.
- Szafranski W, Cukier A, Ramirez A, Menga G, Sansores R, et al. (2003) Efficacy and safety of budesonide/formoterol in the management of chronic obstructive pulmonary disease. Eur Respir J 21: 74-81.
- Karpel JP, Pesin J, Greenberg D, Gentry E (1990) A comparison of the effects of ipratropium bromide and metaproterenol sulfate in acute exacerbations of COPD. Chest 98: 835-839.
- Tashkin DP, Rennard SI, Martin P, Ramachandran S, Martin UJ, et al. (2008) Efficacy and safety of budesonide and formoterol in one pressurized metered-dose inhaler in patients with moderate to very severe chronic obstructive pulmonary disease. Drugs 68: 1975-2000.
- Lulich KM, Goldie RG, Ryan G, Paterson JW (1986) Adverse reactions to ß 2-agonist bronchodilators. J Med Toxicol 1: 286-299.



