The global COVID-19 pandemic, caused by the severe acute respiratory syndrome-coronavirus 2 (SARS-CoV-2), spread rapidly across the globe. The lack of available therapies was an essential condition to repurpose available medicines for the treatment or prevention of that worrisome condition. It was our aim to summarize the available knowledge about the mechanism of action of the antirheumatic drugs chloroquine/hydroxychloroquine and the antiviral drug favipiravir. Further, the concomitant therapeutic potential of both drugs is discussed. The multiple mechanisms of action of CQ/HCQ as well as their immunomodulatory properties are still under investigation in COVID-19 treatment. The nucleoside analogue favipiravir is rapidly metabolized in host cells which disrupts viral synthesis and leads to mutagenesis. Clinical trials become available that provide evidence of the beneficial effect of concomitant use of both drugs in mild-COVID-19 patients. Simultaniously, side effects like QTc prolongation or teratogenicity pose risk to extensive community application. Future well designed, large randomized clinical trials are awaited to determine the role if any of those medications in COVID-19 therapy.
chloroquine/hydroxychloroquine, favipiravir, mechanism of action, COVID-19
The current COVID-19 pandemic, a result of the wide spread severe acute respiratory syndrome-coronavirus 2 (SARS-CoV-2) infection across the globe has affected more than 110 million people and has led to approximately 2,5 million fatalities (World Health Organization (WHO), as of 22 Feb 2021). The main route of transmission is via respiratory tract excretions but fecal-oral transmission is also considered. After 4-5 days (up to 11 days) of incubation patients become symptomatic. Clinical features of this highly contagious disease are varied from asymptomatic infection to fever, dry cough, and in some patients breathing difficulties, as well as muscle and/or joint pain, headache, dizziness, decreased sense of smell and taste, diarrhea, and nausea and in some severe cases to acute respiratory distress syndrome, and multi-organ dysfunction. The resolution of the infection is reached by days 8-9 after symptom onset when severe cases of COVID-19 could progress to acute respiratory distress syndrome. The severity of disease is dependent on viral infection and on the host response. In some patients an improper/dysfunctional immune response inflict cytokine storm which could trigger not only lung inflammation but also wide-spread inflammation and multi-organ damage [1].
Up to now, various treatment strategies were adopted in different countries and researchers focus on the repurposing of existing medicines that could prove helpful but a key action to overcome serious and life threatening conditions remains prevention. That is why in attempts to lower the spread of the disease in society the WHO adopted certain precautions to be followed like physical distancing, wearing a mask, keeping rooms well ventilated, cleaning hands, etc. Apart from vaccination, proven preventive measures to overcome infections are quarantine, social distancing and personal hygiene. In case of infection treatment should be started as early as possible with available (repurposed) medicines.
Favipiravir (IUPAC name: 5-fluoro-2-oxo- 1H-pyrazine-3-carboxamide) was initially discovered by phenotypic screening at Research Laboratories of Toyama Chemical Co., Ltd. It was approved in 2014 for the treatment of influenza infection in Japan [2,3]. Since the outbreak of the new CoV infection favipiravir was approved for the treatment of COVID-19 in China. It has a unique mode of action through direct inhibition of viral replication and transcription that is advantageous for further investigation. Favipiravir is a nucleoside analogue that can be triphosphorylated in cells to become active and serves as a substrate of virus RNA-dependent RNA polymerase (RdRp). Favipiravir (T-705) has a wide range of antiviral activity including arenaviruses, bunyaviruses and filoviruses (all causing fatal hemorrhagic fever) as well as influenza viruses, and its sensitive or resistant strains to marketed neuraminidase and M2 inhibitors. Due to its selective inhibitory effect on influenza viruses T-705 was considered a useful compound for the eradication of influenza as confirmed by in vitro and in vivo studies [4]. Additionally, in in vitro studies of RNA + strand viruses (flavaviruses, togaviruses and picornaviruses) favipiravir was also effective in different concentrations.
The main mode of action of favipiravir is exerted ether by (i) induction of lethal mutagenesis or by (ii) RNA chain termination and incorporation of T-705 in the viral RNA strand. The mechanism though seems to be dependent on the type of virus agent involved and its predisposition to mutagenesis that could lead to lethal mutagenesis and to slowed RNA synthesis [5,6]. When tested in in vitro systems favipiravir proved to have inhibitory effect on the replication of viral genome by competing with purine nucleotides. Thus, favipiravir behaves as pseudo purine. The active form of favipiravir (favipiravir ribofuranosyl-5´-triphosphate (favipiravir-RTP)) inhibits viral replication by interacting with viral RNA polymerase. The exact mechanism is not fully understood but it is believed to be connected with mutagenesis [7]. Additionally, SARS-Cov-2 polymerase was found to be 10-fold more active with higher nsp12 elongation rates in in vitro conditions. Transferred to physiological conditions it was suggested that nsp12 could elongate at a rate of 600–700s−1 making it the fastest viral RdRp known [8]. When active forms of favipiravir and ribavirin were compared regarding mode of inosine monophosphate dehydrogenase (IMPDH) inhibition it was observed that favipiravir-RMP was less potent inhibitor of the enzyme [9]. Once the pro-drug enters the cell it undergoes phosphoribosylation followed by phosphorylation to become an active form. Favipiravir-RTP interacts with RdRp, an enzyme crucial for the viral replication. As a result viral replication and assembly are disrupted. Animal models examining the effect of favipiravir showed promising results. Mice infected with influenza virus either of H3N2 (A/Victoria/3/75), H3N2 (A/Osaka/5/70), H5N1 (A/Duck/MN/1525/81) or H5N1 influenza viruses A/Vietnam/UT3040/04 (VN3040) and A/Hanoi/30408/05 clone7 (HN30408cl7) survived after administration of favipiravir compared to controls [10,11]. In an earlier study mice infected with Influenza virus were treated either with favipiravir or with ribavirin. Favipiravir-RTP inhibited influenza virus RNA polymerase activity in a dose-dependent and a GTP-competitive manner and did not affect cellular DNA or RNA synthesis. That mechanism of action of favipiravir was considered important for the low level of toxicity of the drug [12].
Favipiravir is approved for the treatment of new or recurrent influenza in Japan [13] and for COVID-19 in China. Different dosing regimens were adopted for treatment of affected patients. The usual dosage consists of 1200 mg/time on the first day, twice a day and 400 mg/time from the 2nd to 5th days, twice a day. Since the EC50 of T-705 is higher in COVID-19 compared to influenza it is recommended to administer 1800 mg loading dose BID on day 1 followed by 800 mg BID from day 2 to a maximum duration of 14 days [14]. Another regimen described in the literature consists of two doses of 1600 mg on day 1 followed by 600 mg twice daily on days 2–10 or until SARS-CoV-2 RNA negative but it needs further evaluation [15]. In adult patients with mild-to-moderate coronavirus disease 2019 a loading dose of 1800 mg favipiravir plus standard supportive care followed by 800 mg BID plus standard treatment until day 14 led to suspension of viral shedding by day 5 of treatment and reduced duration of clinical signs and symptoms [16].
One experimental clinical study compared the effect of favipiravir versus lopinavir/ritonavir. Patients with laboratory-confirmed COVID-19 received oral favipiravir plus interferon-α by aerosol inhalation for 14 days. The other group was treated with lopinavir/ritonavir plus interferon-α by aerosol inhalation for the same duration. Favipiravir group showed shorter viral clearance median time as well as significant improvement in chest CT of patients [17].
The efficacy of favipiravir and umifenovir was also compared. The recovery rate was similar after seven days of treatment. Favipiravir reduced pyrexia and cough and exhibited mild adverse effects [18]. One Chinese study examined the effect of favipiravir and baloxavir acid in hospitalized adult COIVID-19 patients. Patients were randomized into three groups: favipiravir, baloxavir marboxil and control group. While baloxavir acid showed antiviral activity similar to arbidol and lopinavir, favipiravir proved to have low antiviral activity up to 100 μM. The reason for that was associated with insufficient concentrations of the drugs relative to their antiviral activities [19].
The effect of favipiravir alone, tocilizumab or a combination of both in severe COVID-19 patients was studied. Favipiravir administered orally at a dose of 1600 mg, twice a day on the first day, and 600 mg, twice a day from the second day to the seventh day; after that continuation was evaluated on individual basis. Tocilizumab was applied via intravenous infusion at a dose of 4-8 mg/kg and repeated on next day in case of temperature. In the combination group pulmonary inflammation was significantly lower as compared with favipiravir alone group, but opposite effect was noted for the tocilizumab group. Additionally, combination therapy contributed to clinical symptoms relieve and blood parameters normalization which was indicative of the therapeutic potential of tocilizumab plus favipiravir combination in COVID-19 treatment [20].
Favipiravir has both embryotoxic and teratogenic effects and should be prescribed with caution. Apart from that, favipiravir possesses relatively safe profile with main adverse reactions including rising of uric acid, diarrhea, neutropenia, increased hepatic enzymes, psychiatric symptoms, tachycardia, and QT prolongation - as a consequence of drug interactions of favipiravir with concomitant drugs [21,22]. Due to its teratogenic potential the wide spread use of favipiravir for the treatment or prophylaxis of the current pandemic is still under debate and needs further evaluation [23]. Uric acid elevation by favipiravir is due to the decreased excretion of uric acid into urine. The probable mechanism is mediated by urate transporter 1 (URAT1). It ensures the reabsorption of uric acid on the apical membrane of proximal tubules in the kidney. Organic anion transporter 1 and 3 (OAT1 and OAT3) participate in urate excretion on the basolateral side. The inactive metabolite of T-705 inhibits slightly OAT1 and OAT3 and promotes uric acid reuptake in tubules via URAT1 leading to blood uric acid elevation [24]. Until more clinical data are available on the dosing and antiviral efficacy of favipiravir in eradication of SARS-CoV-2 viral load it is of immense importance to implement careful use of the drug in clinical settings [25].
Chloroquine (CQ) and its derivative hydroxychloroquine (HCQ) are anti-parasitic drugs that present anti-inflammatory properties. They accumulate in the lysosomes of host cells that leads to increased pH via protonation. Hydroxychloroquine is approved for the treatment of rheumatoid arthritis (RA), antiphospholipid syndrome (APS), systemic lupus Erythematosus (SLE) and primary Sjogren syndrome [26]. The exact mechanism of action (MOA) of CQ/HCQ is still under investigation. Now it is known that the mechanism is related to autophagy, lysosomal activity, receptor binding and membrane fusion. Nicol MR et al proposed that the mechanism of action of both CQ and HCQ in COVID-19 is direct antiviral, immunomodulatory and anti-inflammatory. The direct antiviral MOA is related to the pH of endosomes which affects viral/cellular fusion. The anti-inflammatory effect is connected with improvements of skin rashes and arthritis. Immunomodulation is leading to inhibition of antigen presentation to dendritic cells, reduced cytokine production in macrophages and reduced signaling of both B and T cells [27]. Mechanisms of action of HCQ and favipiravir are presented schematically in Figure 1.
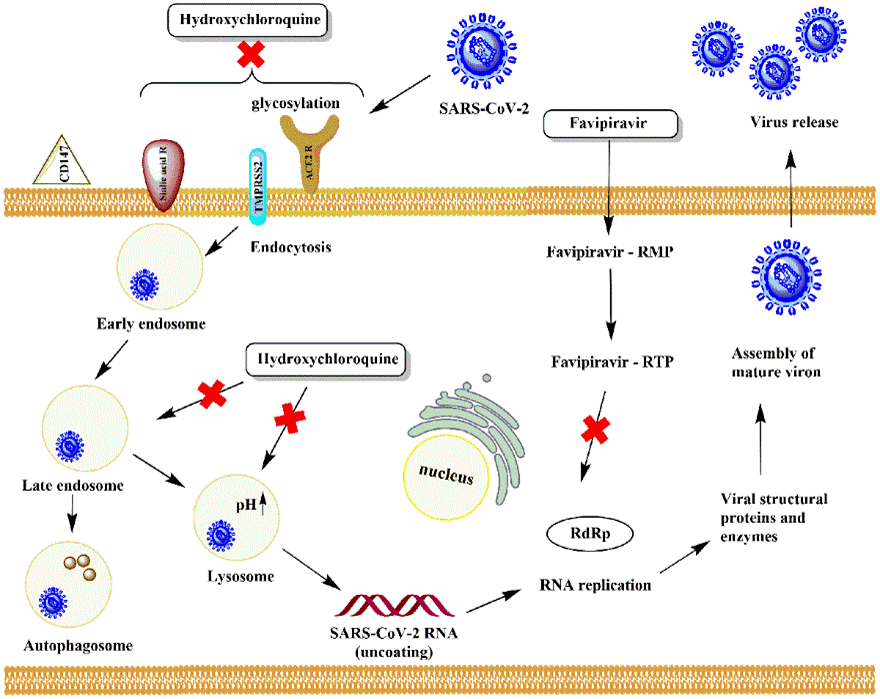
Figure 1. Effects of hydroxychloroquine and favipiravir on viral infection induced by the new SARS-CoV-2. HCQ prevents viral entry by endosomal acidification, glycosylation of receptors and proteolytic processing. Favipiravir acts on RdRp to prevent replication and translation processes
The process of autophagy is a conserved transport pathway aiming to sequester, mature and deliver targeted structures into lysosomes for degradation. By their lysosomotropism CQ and HCQ block lysosomal degradation which results in inhibition of autophagy. Simultaneously, endosomal trafficking and Golgi complex organization get affected. Once the pH of lysosomes and endosomes is increased it leads to dysregulation of virus fusion with host cell and prevents further virus replication [28]. On the other hand, the neutralization of pH in endosomes and lysosomes results in obstruction in the effects of proteases which affects S protein cleavage and entry of the virus into the host cell. Further HCQ prevents viral genome release by preventing endosomes to transform into early lysosomes. Instead, autophagosomes are formed that break S protein of the virus.
Receptor binding: ACE2 receptor is a cell surface receptor most abundantly found in organs like heart, lungs and kidney. It is considered as one point of entry of SARS-CoV-2 virus. The binding is through the S1 and S2 proteins on the surface of the virus. In order to be active ACE2 needs to be glycosylated and the cellular protease like transmembrane serine protease II (TMPRSS2) takes part in the process. In presence of HCQ in the cell the terminal glycosylation of ACE2 receptor is disrupted thus interfering with the binding of SARS-CoV-2 to the ACE2 receptor [29]. Another possible explanation of the inhibition of SARS-CoV-2 entry into host cell is through its anesthetic-like mechanism of action. Suggestion is that in the plasma membrane ACE2 associates primarily with GM1 lipid rafts in presence of high cholesterol levels and in normal cholesterol levels the receptor is associated with the phosphatidylinositol 4,5-bisphosphate (PIP2). In presence of HCQ ACE2 exits from the GM1 raft and PIP2 domains and moves into a generic disordered region of the cell membrane. Thus, dependent on its location ACE2 receptor dictates the efficacy of viral entry [30].
Binding of α 2–6-linkage and α 2–3-linkage sialic acid receptors with SARS-CoV-2 is believed to be another entry pathway in the host organism. The 2–6-linkage is highly abundant in the epithelia of the conjunctiva and cornea and the nasolacrimal region possesses both these receptors. Consequently, the entry in the eye or the respiratory tract may lead to its successful entry into the host cells [31]. Using in silico approaches it was shown that in presence of both CQ and HCQ the SARS-CoV-2 S protein is not able to bind sialic acid receptors and gangliosides on host cell surface diminishing the initial attachment of virus particles to the respiratory tract surface epithelium [32]. That was in accordance with the idea of initial therapy of COVID-19 preferably with HCQ.
The role of CD147 (knows also as basigin) as SARS-CoV-2 entry pathway is still controversial. CD147 a transmembrane glycoprotein of the immunoglobulin superfamily that participates in malaria parasite Plasmodium invasion, tumor development, and bacterial and virus infection. It interacts directly with the spike protein of SARS-CoV-2 thus favoring the entrance of the virus in the host cell by endocytosis. Consequently, in cells that lack ACE2 receptor the presence of CD147 receptor offers an alternative route for cell invasion [33,34]. No evidence of such interaction though is reported in the work of Shilts et al but still the role of basigin as indirect mediator of COVID-19 clinical progression due to immune system development is proposed [35]. No role of CQ/HCQ was documented regarding CD147 entry pathway.
Immunomodulatory mode of action of HCQ: in lysosomes both CQ and HCQ lead to increase of pH and thus dysregulate the activity of antigen presenting cells (APCs), which include plasmacytoid dendritic cells (pDCs) and B cells. Further, they prevent antigen processing and major histocompatibility complex (MHC) class II –mediated autoantigen presentation to T-cells. As a result T-cell activation is declined as well as the differentiation and expression of co-stimulatory proteins and cytokines produced by T-cells and B cells (IL-1, IL-6, and TNF-alpha). Not only the innate immune system is attenuated but HCQ also affects the adaptive immune system. Altered endosomal pH disrupts binding to TLR7/9 that affects the release of cytokines like interferon, IL-6, and IL-12. Moreover, CQ/HCQ lower the numbers of rapidly proliferating T-cells and reduces the number of Th17 and Th1 cells. Since the immunomodulatory effects of HCQ are not fully understood it remains to be elucidated what the response of T- and B-cells towards SARS-CoV-2 in presence of the latter drug is [36]. Moreover, HCQ can efficiently block TLR9 and RNA facilitated activation of TLR 7 processing via association with nucleic acids which leads to reduction of cytokines production. By inhibiting the interaction of DNA/RNA with Toll-like receptors (TLRs) and the nucleic acid sensor cyclic GMP-AMP (cGAMP) synthase (cGAS), HCQ limits the transcription of pro-inflammatory genes which leads to lowering of cytokines production and prevention of the cytokine storm [31].
In Vero E6 cells HCQ in combination with azithromycin was tested in presence of SARS-CoV-2. The latter combination had significant inhibitory effect on viral replication [37]. In vitro studies were performed in ACE2hi cells to analyze binding of CQ/HCQ and ACE2 receptor. Both drugs bind their corresponding receptor with good affinity and suppress viral entrance of the COVID-19 Spike pseudotype virus. Due to their structural difference HCQ appears to be more potent inhibitor on the activity of ACE2hi cells as confirmed by molecular docking. Both drugs did not affect cell apoptosis within the 24 h period of observation but induced LC3-mediated autophagy. Consequently, both drugs could have impact on the clinical treatment of COVID-19 [38].
CQ/HCQ exert action on iron homeostasis in cells. Previous studies indicate that CQ/HCQ may promote cellular iron starvation. The exact mechanism is believed to be through Tf/transferrin receptor 1 (TFR1) complex endocytosis. By raising the pH of endocytic vesicles CQ/HCQ cause tight binding of Tf to Fe3+ which possibly inhibits iron removal from Tf inside the endocytic vesicles. Further the ion transporter channels (divalent metal-ion transporter 1 (DMT1), mucolipin 1 (TRPML1/MCOLN1) and others) get affected by the low pH and ions cannot be released into the cytosol. Ferritin, the main storage protein of iron in the cell gets affected by CQ and ferritinophagy is inhibited that contributes to iron starvation of cells. Further evidence on the role of iron homeostasis in immune cells proves that macrophages with low levels of iron contribute to the lowering of inflammation. Future studies will reveal the role of all those mechanisms in SARS-CoV-2 life cycle [39].
Despite all advantages still there is lack of agreement whether to include HCQ in COVID-19 therapies or not. Consequently, the use of the drug should be approached carefully depending on the individual case. The molecular mechanism of action of HCQ involves multiple steps in the viral pathway. In interaction studies between HCQ and double strained DNAs using gel electrophoresis and force spectroscopy two binding models were identified. It was observed that HCQ interacts strongly with DNA and there was presence of two binding mechanisms dependent of drug concentration. The binding mode of the second mechanism was determined as intercalation which raised questions on the drug concentrations used in practice [40].
Molecular coupling techniques proved as useful model to predict stable complex formation. One such study showed that affinity energies of chloroquine, hydroxychloroquine and favipiravir had the most potent values of affinity to complex with the crystalline structure of SARS-CoV-2 main protease. Consequently, chloroquine and hydroxychloroquine had more stable anchored structure compared to other drugs studied and thus better values of biological activity which makes them a useful option for drug redirection purposes especially during the current global pandemic [41]. These calculations combined with the relatively safe profile of hydroxychloroquine and favipiravir could make them good candidates for concomitant use in prophylaxis and early treatment purposes of COVID-19 (Figure 1).
The use of both CQ and HCQ in the treatment of a wide variety of diseases is reviewed extensively in the work of Ben-Zvi I, et al. Among conditions treated are malaria, rheumatoid arthritis, granuloma annulare, lichen planus, eosinophilic fasciitis, systemic lupus erythematosus (SLE) and others. Additionally, beneficial effects on metabolism and cardiovascular system were described that include improvement of glycemic control in RA and SLE patients, reduction of glycosylated hemoglobin in diabetes patients, improved lipid profile in SLE and RA patients, antithrombotic effects and antineoplastic effects [42,43].
Expert consensus statement has recommended HCQ as possible antiviral treatment in China. In clinical practice against COVID-19 hydroxychloroquine was given to patients >50 kg, 500 mg/time, twice a day for 7 days; in patients ≤ 50 kg, 500 mg/time, twice a day for the first and second days, 500 mg/time, once a day for the third to seventh days. Doses above 2.3 mg/kg body weight/day were considered high risk [44]. Besides, some studies consider HCQ as safe and reliable agent for the treatment of SARS-CoV-2 viral infection in short term [45]. HCQ as less toxic derivative of CQ was advised for post exposure prophylaxis in health care workers in doses of 400 mg twice daily for the first day and then 400 mg once weekly for 3 to eight weeks, with strict monitoring of safety parameters [46].
Favipiravir dose for the treatment of COVID-19 in adults is set to 1800 mg orally twice daily on first day followed by 800 mg orally twice daily, up to maximum of 14 days. The main disadvantage of such dose regimen appears to be the high pill burden-for some manufacturers the loading dose consists of 18 tablets and 8 tablets thereafter until the end of the treatment course [47].
Main toxicological observations after CQ/HCQ treatment include retinopathy, neuromyopathy, cardiomyopathy and gastrointestinal changes after long term use. Since both drugs are slowly excreted it is mandatory to monitor side effects, since keratopathy and continued maculopathy on the retina may be delayed in occurrence. Mechanisms involved in the development of neuromyopathy and cardiomyopathy are a result of vacuolization of cardiac and skeletal muscle cells [48].
One clinical study examined the effect of low- and high- doses of CQ on patients with severe SARS-CoV-2 infection. The observation was that higher doses of CQ could have hazardous effect on critically ill hospitalized patients due to QTc interval prolongation. The drug was not recommended for simultaneous use with azithromycin and oseltamivir [49]. On the contrary, Million M and co-workers in their retrospective study evidenced of the positive effect of HCQ in combination with azithromycin in the treatment of mild COVID-19 before signs of severity of disease appear. Rhythmic cardiac events or sudden deaths were not reported [50]. Rosenberg, et al. evaluated the risk of in-hospital mortality in patients on hydroxychloroquine, azithromycin or both. Treatments were not significantly associated with differences in in-hospital mortality but the combination hydroxychloroquine + azithromycin was significantly associated with cardiac arrest [51]. According to Gao J, et al. treatment of more than 100 patients with COVID-19 with CQ resulted in exacerbation of pneumonia and overall improvement of their health. Additionally, no severe adverse reactions were noted in those patients [52]. Patients with documented SARS-CoV-2 pneumonia who needed oxygen therapy but not intensive care were given HCQ and were compared to control group. Observation was in favor of control group and HCQ was not recommended for COVID-19 patients who require oxygen [53]. CQ showed significant antiviral activity against SARS and MERS coronaviruses. Hydroxychloroquine though seems to be the promising candidate for preventive treatment due to its lower toxicity levels. Additionally, the drug proved to be more effective in eradicating COVID-19 from the nasopharyngeal region which could be helpful for the reduction of viral load during the initial stages of the disease [54]. When HCQ was compared to usual care in hospitalized patients with COVID-19 prolonged hospitalization, invasive mechanical ventilation or death were noted. Consequently, T-705 was not associated with effective COVID-19 treatment in hospitalized patients and its prophylactic role in the community was not addressed in the trial. Based on the understanding of a lack of benefit the World Health Organization (WHO) and the National Institutes of Health have ceased trials with CQ and HCQ in hospitalized COVID-19 patients [55].
Favipiravir and hydroxychloroquine are listed in many guidelines for the treatment of the new coronavirus disease. Among countries that implement favipiravir and HCQ in their treatment guidelines are China, Turkey, Spain, and Iran. T-705 is also used for the treatment of COVID-19 in China and Russia based on experimental and clinical observations performed mainly in China [56]. France, Italy, the Netherlands and South Korea also permit the use of CQ and CHQ for the treatment of certain hospitalized patients [57]. McCullough reported a treatment algorithm for COVID-19-like and confirmed COVID-19 cases in ambulatory patients at home that consisted of immediate intake of 2 or more antivirals (HCQ plus azithromycin or doxycycline or favipiravir) right after symptom onset and in case of symptoms worsening [58]. One retrospective study compared the effect of favipiravir, HCQ or the combination of favipiravir and CHQ in mild to moderately ill patients with COVID-19. No statistically significant difference between HCQ and HCQ plus favipiravir group was discovered. On the other hand, intensive care unit (ICU) admission was higher for the favipiravir only treatment group [59]. To add, pharmacokinetic studies in critically ill patients with COVID-19 showed that drug concentrations were lower than the lower limit of quantification (1 μg/mL) and half-maximal effective concentration (9.7 μg/mL) against SARS-CoV-2 as determined in vitro. T-705 trough concentration in critically ill patients was much lower than that of healthy subjects which questions the use of favipiravir in critically ill COVID-19 patients [60].
Favipiravir alone in different dose regimens was compared to standard of care in hospitalized patients with moderate COVID-19 pneumonia. The drug presented with high treatment potential and enabled viral clearance in about 62% of the moderately ill patients [61]. Another study examined the effect of T-705 on asymptomatic to mildly symptomatic COVID-19 patients. The aim was to assess the efficacy of favipiravir in achieving viral clearance. Patients were divided into early and late treatment group. Viral clearance by day 6 was achieved in 66.7% of early treatment group patients compared to 56.1% in the late treatment group. The most prevalent adverse event encountered was hyperuricemia which was classified as dose dependent rather than cumulative. Thus, favipiravir again proved to be connected with earlier viral clearance [62]. Treatment with favipiravir in combination with anti-inflammatory and anti-coagulant medicines was initiated in severely ill COVID-19 patients. Favipiravir had some benefit on patients’ health probably due to relatively late administration of T-705 in their therapy [63]. Moolasart V et al described the use of concomitant medication with favipiravir, hydroxychloroquine, and lopinavir/ritonavir in a newborn infant assuring that favipiravir-based regimen could be the therapy of choice in COVID-19 infected newborns which needed further evaluation [64]. Favipiravir was combined with inhaled interferon beta-1b in adult hospitalized patients with moderate to severe COVID-19 and compared to treatment with HCQ in another clinical study. No differences in clinical outcomes and no serious side effects were observed between the two treatment groups which was indicative of the lack of clinical response due to timing of therapy initiation [65].
Favipiravir has no effect on adsorption and release stage of the virus. As noted in one mathematical model of Ebola virus infection T-705 could have capability to reduce viral load in humans on the basis as early as possible. Thus, in patients with advanced stage of disease favipiravir might prove useless to apply because after the viremia peak there could be not enough sufficient time to achieve high levels of intracellular triphosphates needed for the maximal effectiveness of the drug [66]. Whether this mathematical model could be extrapolated for the current SARS-CoV-2 pandemic remains to be elucidated.
In terms of QTc interval prolongation one single-center retrospective study found that favipiravir was a safe option for the treatment of COVID-19 patients compared to hydroxychloroquine [67]. There were three groups in the study: hydroxychloroquine, favipiravir and combination group of favipiravir and hydroxychloroquine. ECG recordings of patients were not affected days after treatment with favipiravir or favipiravir plus hydroxychloroquine which showed a safety in terms of QT prolongation. Additionally, other clinical trials in healthy subjects and in COVID-19 patients in hospitals in Russia found that favipiravir did not affect QT interval whereas previous Ebola studies showed it led to QT prolongation [68,69].
Many candidate drugs are under investigation for their potential prophylactic and therapeutic application in COVID-19. It is of great importance to identify high-risk populations in order to start preventive therapy as soon as possible. The dose should be adjusted having in mind the stage of the disease and the health status of the patient. Experimental and clinical data are available for the antiviral activity of favipiravir against SARS-CoV-2 virus. Despite the fact that some studies contradict each other it is important to determine the exact dose to be applied in the treatment of COVID-19. Limited studies are available that demonstrate the efficacy of favipiravir on asymptomatic and moderate mode of disease. In patients with severe COVID-19 infection studies on T-705 efficacy are unavailable. Future studies should include careful evaluation of safety parameters (uric acid levels, development of gouty arthritis, QT prolongation). Concomitant use of favipiravir and hydroxychloroquine is already under investigation but the synergistic mode of action of both drugs needs further evaluation. Future studies involving T-705 plus HCQ need to answer many question like what is the correct moment to therapy initiation since symptom onset/prophylaxis, what is the correct dose to be applied, how safe are those substances and what clinical signs should clinicians monitor so as to benefit the patients. Thus, well designed, large randomized clinical trials are awaited to determine the role if any of those medications in COVID-19 therapy.
All authors contributed equally to study design, data collection and paper preparation.
The authors declare that VP, SH and KU are employees of Tchaikapharma High Quality Medicines Inc. TV declares no competing interests.
This manuscript is a review article and does not involve a research protocol requiring approval by the relevant institutional review board or ethics committee.
Tchaikapharma High Quality Medicines Inc. funded this review article.
- Tay MZ, Poh CM, Renia L, MacAry PA, Ng LFP (2020) The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol 20: 363-374. [Crossref]
- Hayden FG, Shindo N (2019) Influenza virus polymerase inhibitors in clinical development. Curr Opin Infect Dis 32: 176-186. [Crossref]
- Delang L, Abdelnabi R, Neyts J (2018) Favipiravir as a potential countermeasure against neglected and emerging RNA viruses. Antiviral Res 153: 85-94. [Crossref]
- Furuta Y, Takahashi K, Fukuda Y, Kuno M, Kamiyama T, et al. (2002) In Vitro and In Vivo Activities of Anti-Influenza Virus Compound T-705. Antimicrob Agents Chemother 46: 977-981. [Crossref]
- Jochmans D, van Nieuwkoop S, Smits LS, Neyts J, Fouchier RAM, et al. (2016) Antiviral Activity of Favipiravir (T-705) against a Broad Range of Paramyxoviruses In Vitro and against Human Metapneumovirus in Hamsters. Antimicrob Agents Chemother 60: 4620 -4629. [Crossref]
- Arias A, Thorne L, Goodfellow I (2014) Favipiravir elicits antiviral mutagenesis during virus replication in vivo. Elife 3: e03679. [Crossref]
- Furuta Y, Komeno T, Nakamura T (2017) Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase. Proc Jpn Acad Ser B 93: 449-463. [Crossref]
- Shannon A, Selisko B, Le NT, Huchting J, Touret F, et al. (2020) Rapid incorporation of Favipiravir by the fast and permissive viral RNA polymerase complex results in SARS-CoV-2 lethal mutagenesis. Nat Commun 11: 4682.
- Smee DF, Hurst BL, Egawa H, Takahashi K, Kadota T, et al. (2009) Intracellular metabolism of favipiravir (T-705) in uninfected and influenza A (H5N1) virus-infected cells. J Antimicrob Chemother 64: 741-746. [Crossref]
- Furuta Y, Gowen BB, Takahashi K, Shiraki K, Smee DF, et al. (2013) Favipiravir (T-705), a novel viral RNA polymerase inhibitor. Antiviral Res 100: 446-454. [Crossref]
- Kiso M, Takahashi K, Sakai-Tagawa Y, Shinya K, Sakabe S, et al. (2010) T-705 (favipiravir) activity against lethal H5N1 influenza A viruses. PNAS 107: 882-887. [Crossref]
- Furuta Y, Takahashi K, Kuno-Maekawa M, Sangawa H, Uehara S, et al. (2005) Mechanism of Action of T-705 against Influenza Virus. Antimicrob Agents Chemother 49: 981-986. [Crossref]
- De Clercq E, Li G (2016) Approved Antiviral Drugs over the Past 50 Years. Clin Microbiol Rev 29: 695-747. [Crossref]
- Joshi S, Parkar J, Ansari A, Vora A, Talwar D, et al. (2021) Role of favipiravir in the treatment of COVID-19. Int J Infect Dis 102: 501-508. [Crossref]
- Fu D, Cao R, Zhao L, Li W, Zhong W, et al. (2020) Oral favipiravir for patients with delayed SARS-CoV-2 viral RNA clearance: a case series. Crit Care 24: 578. [Crossref]
- Udwadia ZF, Singh P, Barkate H, Patil S, Rangwala S, et al. (2021) Efficacy and safety of favipiravir, an oral RNA-dependent RNA polymerase inhibitor, in mild-to-moderate COVID-19: A randomized, comparative, open-label, multicenter, phase 3 clinical trial. Int J Infect Dis 103: 62-71. [Crossref]
- Cai Q, Yang M, Liu D, Chen J, Shu D, et al. (2020) Experimental Treatment with Favipiravir for COVID-19: An Open-Label Control Study. Engineering (Beijing) 10: 1192-1198. [Crossref]
- Chen C, Zhang Y, Huang J, Yin P, Cheng Z, et al. (2020) Favipiravir versus Arbidol for COVID-19. A Randomized Clinical Trial. MedRxiv.
- Lou Y, Liu L, Yao H, Hu X, Su J, et al. (2021) Clinical Outcomes and Plasma Concentrations of Baloxavir Marboxil and Favipiravir in COVID-19 Patients: An Exploratory Randomized, Controlled Trial. Eur J Pharm Sci 157: e105631. [Crossref]
- Zhao H, Zhu Q, Zhang C, Li J, Wei M, et al. (2021) Tocilizumab combined with favipiravir in the treatment of COVID-19: A multicenter trial in a small sample size. Biomed Pharmacother 133: e110825. [Crossref]
- Wang D, Li Z, Liu Y (2020) An overview of the safety, clinical application and antiviral research of the COVID-19 therapeutics. J Infect Public Health 13: 1405-1414. [Crossref]
- Kaur RJ, Charan J, Dutta S, Sharma P, Bhardwaj P, et al. (2020) Favipiravir Use in COVID-19: Analysis of Suspected Adverse Drug Events Reported in the WHO Database. Infect Drug Resist 13: 4427-4438. [Crossref]
- Pilkington V, Pepperrell T, Hill A (2020) A review of the safety of favipiravir – a potential treatment in the COVID-19 pandemic? J Virus Erad 6: 45-51. [Crossref]
- Mishima E, Anzai N, Miyazaki M, Abe T (2020) Uric Acid Elevation by Favipiravir, an Antiviral Drug. Tohoku J Exp Med 251: 87-90. [Crossref]
- Shrestha DB, Budhathoki P, Khadka S, Shah PB, Pokharel N, Rashmi P (2020) Favipiravir versus other antiviral or standard of care for COVID-19 treatment: a rapid systematic review and meta-analysis. Virol J 17: 141. [Crossref]
- Schrezenmeier E, Dörner T (2020) Mechanisms of action of hydroxychloroquine and chloroquine: implications for rheumatology. Nat Rev Rheumatol 16: 155-166. [Crossref]
- Nicol MR, Joshi A, Rizk ML, Sabato PE, Savic RM, et al. (2020) Pharmacokinetics and Pharmacological Properties of Chloroquine and Hydroxychloroquine in the Context of COVID-19 Infection. Clin Pharmacol Ther 108: 1135-1149. [Crossref]
- Sahu M, Kumar A, Shankar SH, Vishwakarma V, Sahoo P, et al. (2020) Insights into the Mechanisms of action of Chloroquine and Hydroxychloroquine and its use in COVID-19 for chemoprophylaxis. Authorea.
- Pahan P, Pahan K (2020) Smooth or Risky Revisit of an Old Malaria Drug for COVID-19? J Neuroimmune Pharmacol 15: 174-180. [Crossref]
- Yuan Z, Pavel MA, Wang H, Hansen SB (2020) Hydroxychloroquine: mechanism of action inhibiting SARS-CoV-2 entry. bioRxiv. [Crossref]
- Satarker S, Ahuja T, Banerjee M, Balaji EV, Dogra S, et al. (2020) Hydroxychloroquine in COVID-19: Potential Mechanism of Action against SARS-CoV-2. Curr Pharmacol Rep 6: 203-211. [Crossref]
- Fantini J, Di Scala C, Chahinian H, Yahi N (2020) Structural and molecular modelling studies reveal a new mechanism of action of chloroquine and hydroxychloroquine against SARS-CoV-2 infection. Int J Antimicrob Agents 55: 105960. [Crossref]
- Wang K, Chen W, Zhang Z, Deng Y, Lian JQ, et al. (2020) CD147-spike protein is a novel route for SARS-CoV-2 infection to host cells. Sig Transduct Target Ther 5: 283. [Crossref]
- Tripathy S, Dassarma B, Roy S, Chabalala H, Matsabisa MG (2020) A review on possible modes of action of chloroquine/hydroxychloroquine: repurposing against SARS-CoV-2 (COVID-19) pandemic. Int J Antimicrob Agents 56: 106028. [Crossref]
- Shilts J, Crozier TWM, Greenwood EJD, Lehner PJ, Wright GJ (2021) No evidence for basigin/CD147 as a direct SARS-CoV-2 spike binding receptor. Sci Rep 11: 413.
- Meyerowitz EA, Vannier AGL, Friesen MGN, Schoenfeld S, Gelfand JA, et al. (2020) Rethinking the role of hydroxychloroquine in the treatment of COVID-19. FASEB J 34: 6027-6037. [Crossref]
- Andreani J, Le Bideau M, Duflot I, Jardot P, Rolland C, et al. (2020) In vitro testing of combined hydroxychloroquine and azithromycin on SARSCoV- 2 shows synergistic effect. Microb Pathog 145: e104228. [Crossref]
- Wang N, Han S, Liu R, Meng L, He H, et al. (2020) Chloroquine and hydroxychloroquine as ACE2 blockers to inhibit viropexis of 2019-nCoV Spike pseudotyped virus. Phytomedicine 79: 153333.
- Roldan EQ, Biasiotto G, Magro P, Zanella I (2020) The possible mechanisms of action of 4-aminoquinolines (chloroquine/hydroxychloroquine) against Sars-Cov-2 infection (COVID-19): A role for iron homeostasis? Pharmacol Res 158: Article e104904. [Crossref]
- Bazoni RF, Moura TA, Rocha MS (2020) Hydroxychloroquine Exhibits a Strong Complex Interaction with DNA: Unraveling the Mechanism of Action. J Phys Chem Lett 11: 9528-9534.
- Silva Arouche TD, Reis AF, Martins AY, Costa JF, Carvalho Junior RN, et al. (2020) Interactions Between Remdesivir, Ribavirin, Favipiravir, Galidesivir, Hydroxychloroquine and Chloroquine with Fragment Molecular of the COVID-19 Main Protease with Inhibitor N3 Complex (PDB ID: 6LU7) Using Molecular Docking. J Nanosci Nanotechnol 20: 7311-7323. [Crossref]
- Ben-Zvi I, Kivity S, Langevitz P, Shoenfeld Y (2012) Hydroxychloroquine: From Malaria to Autoimmunity. Clinic Rev Allerg Immunol 42: 145-153. [Crossref]
- Misra DP, Gasparyan AY, Zimba O (2020) Benefits and adverse effects of hydroxychloroquine, methotrexate and colchicine: searching for repurposable drug candidates. Rheumatol Int 40: 1741-1751. [Crossref]
- Marmor MF, Kellner U, Lai TY, Melles RB, Mieler WF (2016) American Academy of Ophthalmology. Recommendations on Screening for Chloroquine and Hydroxychloroquine Retinopathy. Ophthalmology 123: 1386-1394. [Crossref]
- Sun X, Ni Y, Zhang M (2020) Rheumotologitsts’ view on the use of hydroxychloroquine to treat COVID-19. Emerg Microbes Infect 9: 830-832. [Crossref]
- Otdelenov VA, Krykov AV, Sychev DA (2020) Possibilities for the use of hydroxychloroquine for pre- and postexposure prophylaxis of SARS-CoV-2 infection among exposed contacts and healthcare personnel. Kachestvennaya Klinicheskaya Praktika = Good Clin Pract S4: 81-86.
- Agrawal U, Raju R, Udwadia ZF (2020) Favipiravir: A new and emerging antiviral option in COVID-19. Med J Armed Forces India 76: 370-376. [Crossref]
- Pereira BB (2020) Challenges and cares to promote rational use of chloroquine and hydroxychloroquine in the management of coronavirus disease 2019 (COVID-19) pandemic: a timely review. J Toxicol Environ Health B Crit Rev 23: 177-181. [Crossref]
- Borba MGS, Val FFA, Sampaio VS, Alexandre MAA, Melo GC, et al. (2020) Effect of High vs Low Doses of Chloroquine Diphosphate as Adjunctive Therapy for Patients Hospitalized with Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection: A Randomized Clinical Trial. JAMA Netw Open 3: e208857. [Crossref]
- Million M, Lagier JC, Gautret P, Colson P, Fournier PE, et al. (2020) Early treatment of COVID-19 patients with hydroxychloroquine and azithromycin: A retrospective analysis of 1061 cases in Marseille, France. Travel Med Infect Dis 35: e101738. [Crossref]
- Rosenberg ES, Dufort EM, Udo T, Wilberschied LA, Kumar J, et al. (2020) Association of Treatment with Hydroxychloroquine or Azithromycin with In-Hospital Mortality in Patients with COVID-19 in New York State. JAMA 323: 2493-2502. [Crossref]
- Gao J, Tian Z, Yang X (2020) Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends 14: 72-73. [Crossref]
- Mahevas M, Tran VT, Roumier M, Chabrol A, Paule R, et al. (2020) Clinical efficacy of hydroxychloroquine in patients with covid-19 pneumonia who require oxygen: observational comparative study using routine care data. BMJ 369: m1844. [Crossref]
- Shamim S, Khan M, Kharaba ZJ, Ijaz M, Murtaza G (2020) Potential strategies for combating COVID-19. Arch Virol 165: 2419-2438. [Crossref]
- Horby P, Mafham M, Linsell L, Bell JL, Staplin N, et al. (2020) Effect of Hydroxychloroquine in Hospitalized Patients with Covid-19. N Engl J Med 383: 2030-2040.
- Matveev AV, Kiselev YuYu, Sychev DA (2020) Current and future use of favipiravir in patients with COVID-19. Kachestvennaya Klinicheskaya Praktika = Good Clin Pract S4:106-114.
- Dagens A, Sigfrid L, Cai E, Lipworth S, Cheng V, et al. (2020) Scope, quality, and inclusivity of clinical guidelines produced early in the covid-19 pandemic: rapid review. BMJ 369: m1936. [Crossref]
- McCullough PA (2020) Favipiravir and the Need for Early Ambulatory Treatment of SARS-CoV-2 Infection (COVID-19). Antimicrob Agents Chemother 64: 02017-02020. [Crossref]
- Guner R, Hasanoglu I, Kayaaslan B, Aypak A, Akinci E, et al. (2021) Comparing ICU admission rates of mild/moderate COVID-19 patients treated with hydroxychloroquine, favipiravir, and hydroxychloroquine plus favipiravir. J Infect Public Health 14: 365-370.
- Irie K, Nakagawa A, Fujita H, Tamura R, Eto M, et al. (2020) Pharmacokinetics of Favipiravir in Critically Ill Patients With COVID-19. Clin Transl Sci 13: 880-885. [Crossref]
- Ivashchenko AA, Dmitriev KA, Vostokova NV, Azarova VN, Blinow AA, et al. (2020) AVIFAVIR for treatment of patients with moderate COVID-19: interim results of a phase II/III multicenter randomized clinical trial. Clin Infect Dis 73: 531-534. [Crossref]
- Doi Y, Hibino M, Hase R, Yamamoto M, Kasamatsu Y, et al. (2020) Prospective, Randomized, Open-Label Trial of Early versus Late Favipiravir Therapy in Hospitalized Patients with COVID-19. Antimicrob Agents Chemother 64: e01897-20. [Crossref]
- Yamamura H, Matsuura H, Nakagawa J, Fukuoka H, Domi H, et al. (2020) Effect of favipiravir and an anti-inflammatory strategy for COVID-19. Crit Care 24: 413. [Crossref]
- Moolasart V, Wongsawat J, Phokhom P, Thienthong V (2020) Favipiravir-based regimen for coronavirus disease 2019 pneumonia for a 47-day-old male newborn. SAGE Open Med Case Rep Article 8: 2050313X20964046. [Crossref]
- Khamis F, Al Naabi H, Al Lawati A, Ambusaidi Z, Al Sharji M, et al. (2021) Randomized controlled open label trial on the use of favipiravir combined with inhaled interferon beta-1b in hospitalized patients with moderate to severe COVID-19 pneumonia. Int J Infect Dis 102: 538-543. [Crossref]
- Madelain V, Oestereich L, Graw F, Nguyen TH, de Lamballerie X, et al. (2015) Ebola virus dynamics in mice treated with favipiravir. Antiviral Res 123: 70-77. [Crossref]
- Çap M, Bilge Ö, Isik F, Burak C, Karagöz A, et al. (2020) The effect of favipiravir on QTc interval in patients hospitalized with coronavirus disease 2019. J Electrocardiol 63:115-119. [Crossref]
- Kiselev YuYu, Matveev AV, Mirzaev KB, Sychev DA (2020) Monitoring of safety using favipiravir: risk management of adverse drug reactions in clinical practice. Kachestvennaya Klinicheskaya Praktika = Good Clin Pract S4: 115-119.
- Chinello P, Petrosillo N, Pittalis S, Biava G, Ippolito G, et al. (2017) QTc interval prolongation during favipiravir therapy in an Ebolavirus-infected patient. PLoS Negl Trop Dis 11: e0006034. [Crossref]

