The prevalence of elderly patients with chronic illnesses, associated polypharmacy and a high risk of functional and cognitive impairment has increased in recent years. The care of Complex Chronic Patients (CCP) therefore represents a challenge for health systems in developed countries around the world. This paper presents a study protocol that will explore the effects of an intervention within a pharmaceutical care programme (PCAF) on optimising CCP care and on the suitability of the organisational methods proposed for the PCAF programme.
This is a multicentre experimental study. The setting will be primary health care and community pharmacies in the so-called ‘autonomous community’ of Catalonia in Spain. The participants will be CPP attended to Primary Healthcare Centres with the CCP code in their electronic medical record. The included CCP will be distributed in two strata depending on whether they will be considered likely to receive the arranged medicinal treatment as Monitored Dosage System according to benefits based on criteria or not. They will then be assigned by random stratified sampling at a ratio of 1:1 to the control or intervention groups. A sample size of 860 patients is estimated. Patients will undergo 12 months follow-up. The primary outcome of this study will be the medication adherence measured by the Morisky Green Test and the billing/prescription ratio.
complex chronic patients, community pharmacy, integrated care services, study protocol
In recent years, the prevalence of elderly patients with chronic illnesses, associated polypharmacy and a high risk of functional and cognitive impairment has increased. As a result, hospital admissions and re-admissions have undergone sustained growth [1,2]. In addition, patients with severe illnesses, multimorbidity, polypharmacy, high use of resources and concurrent social risk may generate special needs that complicate management of their illness [1,3]. The care of Complex Chronic Patients (CCP) therefore represents an ever greater challenge for health systems in countries around the world [4,5]. Coderch et al. [6] have noted that an integrated care system focused on primary care, with proactive actions and coordination between care levels, would be beneficial for those CCP requiring different levels of care.
In 1990, Hepler & Strand [7] defined the concept of pharmaceutical care in which the pharmacist is responsible for the use of drugs and for the results of treating patients, identifying and resolving drug-related problems (DRPs). This concept of pharmaceutical care was subsequently adopted by the World Health Organization (WHO) and the International Pharmaceutical Federation (FIP) [8]. Since then, pharmacists’ practice as healthcare professionals has evolved towards collaborative and patient-focused pharmaceutical care [9,10].
The responsibility of the pharmacist regarding use of drugs becomes especially important in CCP for whom the detection and resolution of drug-related problems (DRPs) are a key objective, since such problems are particularly frequent and often preventable [11,12]. For this reason, for some time now, the concept of pharmaceutical care has also been developed and consolidated in many countries in the field of community pharmacies, due to their accessibility, socially communicative nature and territorial balance (by which we mean their well-represented presence throughout a specific region or area), together with professional competence and the population’s trust, thus establishing the community pharmacist as a key element in the global health system [9,13-15].
Consequently, there is currently a variety of professional pharmaceutical care services that are integrated in daily practice and that have been developed to varying extents in different countries, depending on distinct factors of a social and cultural nature, and also on health-policy priorities [16]. Countries such as Canada, Australia, England, Scotland, the United States and the Netherlands have conducted reforms with the aim of expanding the functions and responsibilities of community pharmacists as regards caring for people with multiple chronic conditions [9]. In addition, policies and programmes have been adopted in Canada to give greater responsibility to pharmacists in terms of drug management, including collaboration with other healthcare providers and the medication review services, which are currently being funded in most areas of the country [17].
Similarly, in Spain, the Strategy to Approach Chronicity in the National Health System has been set up as a coordinated group of General State Administration health services together with those of the autonomous communities or regions. It covers all healthcare functions and services that, by law, are the responsibility of the public authorities. Regarding the autonomous community of Catalonia, in line with its health programme [18] and as a result of the partnership between the Catalan Public Health Agency (ASPCAT), the Department of Health, the Catalan Health Service (CatSalut)—as a public authority responsible for guaranteeing quality public healthcare coverage to the people of Catalonia—and together with the Catalan Council of Pharmacists’ Associations (which represents all such organisations in Catalonia), the need arose to create a pharmaceutical care programme for CCP; this has been termed the “PCAF programme”. This programme forms part of the process in which CatSalut and the Catalan Council of Pharmacists’ Associations provide new healthcare services. One of these new services is the pharmacotherapeutic follow-up (PFU) with monitored dosage systems (MDS), as a system for the repackaging of medicines, once professional judgment and decisions have considered these as the best option to support patient adherence, with follow-up by the community pharmacist. This paper therefore presents a study protocol that will explore the effects of an intervention within the PCAF programme on the optimisation of CCP and the suitability of the organisational methods proposed for this PCAF programme.
Study design
The study is designed as a controlled, randomised, simple blind and multicentre experimental study in the field of primary healthcare and community pharmacies in the autonomous community of Catalonia (Spain). The duration of the study is scheduled to be 12 months from the moment that all patients are included.
Study population and inclusion/exclusion criteria
The inclusion criteria are CCP attending primary healthcare centres (PHC) with the CCP code in their electronic medical record, and who have visited a participant community pharmacy in the study. At present, in the autonomous community of Catalonia, CCP are identified at PHC in accordance with specific criteria established by the Department of Health, which can be viewed in each patient’s electronic medical record [19].
Exclusion criteria include those CCP for whom monitoring could not be guaranteed for various reasons: those that reject the MDS as a system for the conditioning of drugs in order to encourage adherence to follow-up by the community pharmacist, or that are already on an MDS programme; those that have been displaced or transferred but assigned to a PHC; patients with severe cognitive disorder (ISAACS < or = to 25, Pfeiffer > than 3 errors or > than 4 if illiterate, MMT 9-12); or patients on the homecare programme (ATDOM) or that refuse to sign the informed consent form.
Recruitment of GP, community pharmacists and patients
First, the project will be presented to primary healthcare teams that include GP and primary-care nursing staff. The next step is to call upon the community pharmacies located in the basic health areas of the primary care teams that agreed to participate. When the participation of primary care teams is confirmed the Catalan Council of Pharmacists’ Associations will work in coordination with all the pharmaceutical associations in this region (specifically, this means the provinces of Barcelona, Tarragona, Lleida and Girona, which constitute Catalonia) to call an initial informative meeting with the community pharmacies. The community pharmacists that decide to participate will be given a user code and initial password to access a website created by the project and enter all data. The maximum number of patients per community pharmacy has been established by agreement as 10. A specific e-mail address will be set up for the project so that, if there are more patients than the initial prevision, the Catalan Council of Pharmacists’ Associations can be contacted directly in order that it then contact the primary-care teams to prevent sending more patients to the community pharmacy in question.
Regarding the selected patients, these will each be called for an initial meeting with the primary-care team, where they will be informed about the study. The primary-care team will have a list of pharmacies in the area and the patient must confirm whether any of the participant locations are their usual pharmacy. CCP who agree to take part in the study and sign the informed consent form will then constitute the final study sample.
Randomisation of branches of study
The included CCP will be distributed into two strata depending on whether they are considered likely to receive the medication organised as MDS according to benefits based on criteria, or not (Figure 1). The criteria for receiving MDS will be those established by the Department of Health and the Catalan Council of Pharmacists’ Associations’ Guidance for Pharmacists on the pharmacotherapeutic follow-up use of MDS [20]. It is considered that patients who could benefit from monitoring are principally those who meet one or more of the following criteria: polypharmacy patients, undergoing chronic treatment and with a low degree of adherence, with difficulties in fulfilling the treatment, with physical or mental impairments that prevent them from handling medication properly, with alternating, decreasing or irregular or complex dosages, which due to their particular characteristics require adequate monitoring of the medication, or patients referred by hospitals or other services and who require specific monitoring. They will then be assigned by random stratified sampling to the control group or to the intervention group.
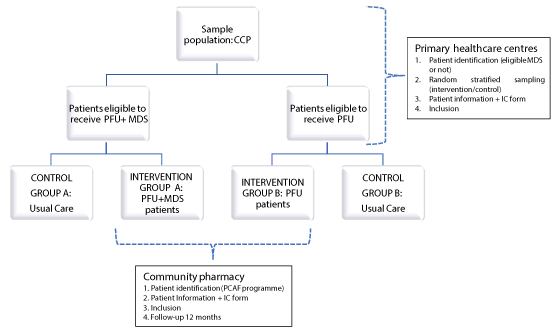
Figure 1. Flow chart of study and study participants
For control-group patients, the usual care will be taken with respect to their problems with adherence and to managing their chronic illness. The inclusion of these subjects in the study will be noted in their clinical records in order for them to be monitored. Monitoring sessions with the primary-care teams will be held at 3 and 6 months, at which time the patient’s clinical and/or therapeutic information is updated. There will also be a final session at 12 months that marks the end of the monitoring period.
Intervention-group patients will be provided with the medication schedule or prescription, which is the document that the patient takes to the pharmacy to collect the drugs prescribed by the GP, along with the referral document that identifies him or her as a patient on the PCAF intervention programme, and which states whether they will be a patient included in the intervention group who receives a pharmacotherapeutic follow-up involving drug-therapy observation, evaluation, and documentation (PFU), or a member of the group who is additionally provided with MDS (PFU+MDS). During the 12 months of the study, there will be one monthly pharmaceutical care visit, or else a pharmaceutical care visit plus MDS at the community pharmacy, respectively.
Intervention
When the patient arrives at the community pharmacy, they will be scheduled for an initial visit, or this will be held at that same moment if convenient for both parties. The patient will be told about the intervention, and if they agree to sign the informed consent for the PFU (or PFU with MDS), the pharmacist will then provide the patient with information on the service, will review the medication and will apply the protocol for PFU and dispensation for the study.
In the pharmaceutical interview, which will be carried out under strict conditions of privacy and confidentiality, the pharmacist will evaluate the knowledge, attitudes and abilities of the subject regarding his or her medication and will also assesses adherence. This is to identify and correct DRPs. The pharmacist will apply the Morisky Green test and the EuroQol-5D, and will record the results on the digital platform.
If a DRP is detected, this must be recorded in the computer application, identifying the type and the drug(s) involved using the national code. Pharmaceutical care will be then conducted in accordance with the criteria of the Forum of Pharmaceutical Care in Community Pharmacies [21] in order to resolve the issue, which will also be recorded on the computer application. Should the pharmacist be unable to resolve the detected DRP or if there is a health requirement whereby referral to the primary care team is advisable, they must issue a message explaining the reasons for the referral and proposing any possible corresponding actions.
For intervention-group patients with MDS, the action will end by providing the medication in solid drug form and potentially re-conditioned with MDS, following the procedures for action and quality control as described in the Guidance for Pharmacists on the pharmacotherapeutic follow-up use of MDS [20].
In the case of an intervention-group patient without MDS, the visit time in minutes, the drug-review time in minutes, the result of the EuroQol-5D test at the first and last visits and the result of the Morisky Green test for each visit will be recorded in the computer application. In the case of an intervention-group patient with MDS, the blister or MDS preparation time (min), the number of blisters used, the number of blisters returned, and the number of unopened blister cells will be also recorded.
If the patient goes to the pharmacy between ordinary visits to make a specific health-related request regarding their treatment or to report a mild or moderate health problem (sign or symptom), this will be recorded in the application as a spontaneous visit, and, if any DRP is detected, the procedure will be the same as for other visits.
Training of community pharmacists
The training of community pharmacists will involve a 3.5-hour face-to-face session divided into the following sections: presentation of the PCAF programme; description of the protocol; registration and communication of the data. In addition, some time will be devoted to clearing up possible doubts at the end of the session.
Study variables
Primary variables: These will be the number of patients who are shown to be adherent (Morisky Green questionnaire) and the billing/prescription ratio.
Secondary variables: These will be the EuroQol-5D score [22]; the number of deaths due to chronic pathology; the number of visits to the primary-care team registered in one year; the number of visits to specialist care registered in one year; the total number of admissions in one year; the average number of days of admission excluding extreme cases; the number of visits to the emergency services registered; the monetary value of the basic-care units (a GP and a nurse) consumed in primary, emergency or specialised care; and the cost of medications, the number of hypertensive (>140/90 mmHg), diabetics (HbA1<7%), cholesterol (<200 mg/dl) patients; the number of DRPs and types during the 12 months of monitoring; the types of action performed by the pharmacist during the 12 months of monitoring; the number of referrals during the 12 months of monitoring; the drugs involved in DRPs in the form of their national code; time spent on visits in minutes; time spent on producing, revising and dispensing the MDS; and the number of monitoring visits made.
Statistical analysis
Sample size: On an accepted proportion of 50% non-compliance for a unilateral hypothesis with an alpha error of 0.05, a power of 0.09 and to detect intergroup differences of 10%, and for a percentage loss estimated at 10%, a sample size of 860 patients is estimated. These will be randomly assigned at a ratio of 1:1 to the two control and intervention groups.
Analysis strategy: The objective is to perform one analysis per intention to treat. For both primary and secondary variables, a descriptive analysis of frequencies (proportions) will be performed for nominal and ordinal variables, and to calculate the mean and standard deviation for parametric quantitative variables. For non-parametric continuous variables (Kolmogrov-Smirnov test), the position measurements (mean, q1 and q3) will be calculated.
Inferential analysis will also be conducted depending on the type of variable and design. The qualitative variables for independent groups will be analysed by means of Pearson’s chi-squared (χ2) test; for paired groups, the McNemar test will be used. For the parametric quantitative variables, Student’s t test will be used, as well as for the non-parametric variables (Mann Whitney U test, for independent groups and the Wilcoxon t test for paired data). Contrast of the hypothesis with a level of significance is 0.05.
Monitoring of data recorded by pharmacies
The Catalan Council of Pharmacists’ Associations will set up a technical office to monitor the data recorded by pharmacies. An e-mail address will also be created for sending relevant information about the project and to resolve any issues that pharmacists might have in terms of registration, protocol, etc.
Limitations of the study protocol
One of the limitations of the protocol is that patients could be lost if their pharmacy of reference is not participating in the study. Another limitation, which also relates to the loss of patients initially included, is that there will be no exhaustive control of all those who do not get to the pharmacy.
Ethics
Informed consent shall be obtained from patients both by the primary care teams and by the community pharmacists, which the patients shall only sign if they agree to be included in the study and all that it implies.
The study protocol has already been approved by the IDIAP Jordi Gol Clinical Research Ethics Committee (IRB00005101) and by the University of Barcelona’s Bioethics Commission (IRB00003099).
In the Spanish context, no studies have been published in which primary-care teams and community pharmacies are integrated into the design with the aim to compare pharmaceutical care (or pharmaceutical care with the development of MDS) with established usual care. The conSIGUE study conducted in Spain [23] determined the impact of PFU with respect to the number of drugs, control of health problems and identification and resolution of DRPs, as well as the cost-effectiveness of health problems in polypharmacy patients. However, in the protocol presented here, the selection of pharmacies will be determined by the participating primary-care teams and the inclusion and randomisation of patients will be done via these teams. In addition, the fact that it forms part of the existing agreement between CatSalut and the Catalan Council of Pharmacists’ Associations could facilitate the provision of this service through a public body, such as CatSalut, in the autonomous community in which this study will be conducted.
Another study also published in our area [24] sought to assess whether a pharmaceutical care programme in chronic patients with heart failure and/or chronic obstructive pulmonary disease improved clinical development and quality of life, and diminished health resources. This study, however, included patients in hospitals due to decompensation and/or exacerbation of heart failure and/or chronic obstructive pulmonary disease, in which the diagnosis for admission was made via internal medical services, although it also included primary care and community pharmacists.
Additionally, a recent meta-analysis [25] and another published study [26] demonstrated that coordinated action among different care levels, and integrating community pharmacies, improved the management of chronic diseases. It should therefore be noted that the cross-cutting strategy for addressing community health projects [27] framed within the Catalan Health Plan 2016-2020 [18] refers to the community pharmacy as a set of professionals and services that provide outpatient care and services to the population of a region, the nucleus or basis of which are the PHC and the primary-care teams. Consequently, this protocol study is designed to discern whether these interventions will be efficacy and safe with respect to the usual care established at present, with the ultimate objective of determining a protocol for multidisciplinary integrated management.
Conflict of interest: The authors declare that there are no conflicts of interest.
Funding: Pharmacists will be paid using the funds of the Catalan Council of Pharmacists’ Associations.
Authors’ contributions: BT-N, PG, PR, RG-E and AC collectively drafted the study protocol and sought ethical approval. BT-N drafted and approved the final submitted manuscript. PM and ELM contributed expertise and drafted and approved the final submitted manuscript. PG, PR, RG-E and AC approved the final submitted manuscript. All the authors reviewed and agreed on the submitted version of the manuscript.
- Gual N, Yuste A, Enfedaque B, Blay C, Martín R, et al. (2016) Profile and evolution of chronic complex patients in a subacute unit. Aten Primaria 49: 510-517. [Crossref]
- Campins L, Serra-Prat M, Palomera E, Bolibar I, Martínez MÀ et al. (2019) Reduction of pharmaceutical expenditure by a drug appropriateness intervention in polymedicated elderly subjects in Catalonia (Spain). Gac Sanit 33: 106-111. [Crossref]
- Masnoon N, Shakib S, Kalisch-Ellett L, Caughey GE (2017) What is polypharmacy? A systematic review of definitions. BMC Geriatr 17: 230. [Crossref]
- Latif A, Mandane B, Anderson E, Barraclough C, Travis S (2018) Optimizing medicine use for people who are homebound: an evaluation of a pilot domiciliary Medicine Use Review (dMUR) service in England. Integr Pharm Res Pract 7: 33-40. [Crossref]
- Mubarak N, Hatah E, Aris MAM, Shafie AA, Zin CS (2019) Consensus among healthcare stakeholders on a collaborative medication therapy management model for chronic diseases in Malaysia; A Delphi study. PLoS One 14: 1-28. [Crossref]
- Coderch J, Pérez-Berruezo X, Sánchez-Pérez I, Sánchez E, Ibern P, et al. (2018) Assessment of the effectiveness of a proactive and integrated healthcare programme for chronic complex patients. Gac Sanit 32: 18-26. [Crossref]
- Hepler CD, Strand LM (1990) Opportunities and responsibilities in pharmaceutical care. Am J Hosp Pharm 47: 533-543. [Crossref]
- World Health Organization (2011) Joint FIP/WHO guidelines on good pharmacy practice: standards for quality of pharmacy services. (WHO) [Internet] WHO technical report series, No. 961, Annex 8, [accessed 2020 Sept 1]. Available from: https://apps.who.int/medicinedocs/en/m/abstract/Js18676en/.
- Mossialos E, Courtin E, Naci H, Benrimoj S, Bouvy M, et al. (2015) From “retailers” to health care providers: Transforming the role of community pharmacists in chronic disease management. Health Policy 119: 628-639. [Crossref]
- Perraudin C, Bugnon O, Pelletier-Fleury N (2016) Expanding professional pharmacy services in European community setting: Is it cost-effective? A systematic review for health policy considerations. Health Policy 120: 1350-1362. [Crossref]
- Alton S, Farndon L (2018) The impact of community pharmacy-led medicines management support for people with COPD. Br J Community Nurs 23: 214-219. [Crossref]
- Torres A, Fite B, Gascon P, Barau M, Guayta-Escolies R, et al. (2009) Effectiveness of a pharmaceutical care program in the improvement of blood pressure monitoring in poorly controlled hypertensive patients. PressFarm Study (in Spanish). Hipertens y Riesgo Vasc 27: 13-22.
- Rhalimi M, Rauss A, Housieaux E (2018) Drug-related problems identified during geriatric medication review in the community pharmacy. Int J Clin Pharm 40: 109-118. [Crossref]
- Ayorinde AA, Porteous T, Sharma P (2013) Screening for major diseases in community pharmacies: A systematic review. Int J Pharm Pract 21: 349-361. [Crossref]
- Shaw J, Harrison J, Harrison J (2014) A community pharmacist-led anticoagulation management service: attitudes towards a new collaborative model of care in New Zealand. Int J Pharm Pract 22: 397-406. [Crossref]
- Lowres N, Krass I, Neubeck L, Redfern J, McLachlan AJ, et al. (2015) Atrial fibrillation screening in pharmacies using an iPhone ECG: a qualitative review of implementation. Int J Clin Pharm 37: 1111-1120. [Crossref]
- Dolovich L, Consiglio G, MacKeigan L, Abrahamyan L, Pechlivanoglou P, et al. (2016) Uptake of the MedsCheck annual medication review service in Ontario community pharmacies between 2007 and 2013. Can Pharm J 149: 293-302. [Crossref]
- Department of Health. Government of Catalonia (2016) Catalan Health Plan 2016-2020, [accessed 2020 Sept 1]. Available from: http://salutweb.gencat.cat/web/.content/_departament/pla-de-salut/Pla-de-salut-2016-2020/documents/Pla_salut_Catalunya_2016_2020.pdf.
- Department of Health. Government of Catalonia (2019) Chronicity healthcare process in primary care network (in Catalan), [accessed 2020 Sept 1]. Available from: http://salutweb.gencat.cat/web/.content/_ambits-actuacio/Linies-dactuacio/Estrategies-de-salut/enapisc/enapisc-cronicitat-complexa.pdf.
- Catalan Council of Pharmacists’ Associations and Catalan Department of Health (2012) Guidance for Pharmacists on the pharmacotherapeutic follow-up use of Monitored Dosage Systems (MDS) (in Catalan), 1st Edition, Barcelona, pp: 1- 64.
- General Council of Official Pharmaceutical Associations (2004) Forum of pharmaceutical care in community pharmacy, Practical Guide to Pharmaceutical Care Services in Community Pharmacy, [accessed 2020 Sept 1]. Available from: https://www.portalfarma.com/inicio/serviciosprofesionales//forofarmaciacomunitaria/Documents/ATFC_Guia%20FORO.pdf.
- Badia X, Roset M, Montserrat S, Herdman M, Segura A (1999) The Spanish version of EuroQol: a description and its applications. European Quality of Life scale. Med Clin 112: 79-85. [Crossref]
- Jódar-Sánchez F, Malet-Larrea A, Martín JJ, García-Mochón L, López Del Amo MP, et al. (2015) Cost-utility analysis of a medication review with follow-up service for older adults with polypharmacy in community pharmacies in Spain: the conSIGUE program. Pharmacoeconomics 33: 599-610. [Crossref]
- Gorgas Torner MQ, Pez Vives F, Camós Ramió J, de Puig Cabrera E, Jolonch Santasusagna P, et al. (2012) Integrated pharmaceutical care programme in patients with chronic diseases (in Spanish). Farm Hosp 36: 229-239.
- Mubarak N, Hatah E, Khan TM, Zin CH (2019) A systematic review and meta-analysis of the impact of collaborative practice between community pharmacist and general practitioner on asthma management. J Asthma Allergy 12: 109-153. [Crossref]
- Ensing HT, Koster ES, Dubero DJ, van Dooren AA, Bouvy ML (2019) Collaboration between hospital and community pharmacists to address drug-related problems: The HomeCoMe-program. Res Soc Adm Pharm 15: 267-278. [Crossref]
- Department of Health Government of Catalonia (2017) Strategy for addressing community health projects (in Catalan), [accessed 2020 Sept 1]. Available from: http://salutpublica.gencat.cat/web/.content/minisite/aspcat/promocio_salut/salut_comunitaria/links_sueltos_relacionats/Estrategia-Transversal-Salut-Comunitaria.pdf

