Due to the increase in the use of bioprosthetic valves, structural valve degeneration will become prevalent. Transcatheter aortic valve replacement (TAVR) represents a safe and effective alternative to redo surgery. We present a case of valve in valve (ViV) implantation with an unfortunate result.
Aortic stenosis, transcatheter aortic valve replacement, valve in valve implantation
TAVR: Transcatheter aortic valve replacement; ViV: Valve in valve replacement; SAVR: Surgical aortic valve replacement; HF: Heart failure; NYHA: New york heart association; LVEF: left ventricular ejection fraction; AS: Aortic stenosis; PG: Pressure gradient; AVA: Aortic valve area; CTA: Computerized tomography angiography; CPR: Cardiopulmonary Resuscitation; THV: Transcatheter heart valve
An 83 years old female patient, with a history of hypertension and surgical aortic valve replacement (SAVR) with bioprosthetic Sorin Freedom Stentless 21 mm (Sorin Biomedica Spa, Saluggio, Italy), 9 years ago, was admitted to our hospital because of recurrent heart failure (HF) symptoms (NYHA III-IV) the last 4 months, due to degenerated aortic bioprothesis. The echocardiography showed normal left ventricular ejection fraction (LVEF) 60%, degeneration of bioprosthesis with severe aortic stenosis (AS) (mean pressure gradient-PG 47mmHg, peak gradient 74 mmHg, aortic valve area-AVA 1cm2) and severe aortic regurgitation. Coronary angiography revealed normal coronary arteries. Due to patient’s high logistic Euroscore (34.73%), our heart team’s decision was a valve in valve (ViV) procedure.
Following the standard protocol of the appropriate workup of patients who are being considered for TAVR, Computerized Tomography Angiography (CTA) measurements was performed to the patient and based on the results (Sinus of valsava diameter 26.1 mm, 26.5 mm, 25.1 mm for left, right and non-coronary irrespectively, sinus of Valsalva height 16.9 mm, 17.3 mm, 17 mm for left, right and non-coronary irrespectively and coronary ostia height 9.8 mm, 11.1 mm for left and right irrespectively) we decide to oppose a 26 mm valve (Medtronic CoreValve™).
During the first attempt of the valve positioning patient became quickly hemodynamically unstable, due to obstruction of the coronary arteries after the complete expansion of the valve (Figure 1a). Cardiopulmonary Resuscitation (CPR) and intubation of the patient were immediately performed. Subsequently the valve was removed from the initial position using an En Snare Catheter (Merit Medical Europe) (Figure 1b). Spontaneous circulation of the coronary arteries was achieved immediately, and the patient became hemodynamically stable (Figure 1c). High risk surgery score and the unstable condition of the patient led us to decide a second try of ViV procedure. We decided to oppose a CoreValve 23 mm (Medtronic) a little deeper at the aortic annulus, trying to change the relative anatomical position of the frame of the valve, avoiding the abrupt closure of the coronaries. An initial interim angiography confirmed the patency of the coronary arteries, (Figure2a) but after the full expansion of the second valve the patient became again hemodynamically unstable. At this time, we noticed occlusion of only the left coronary artery (Figure 2b) and we tried unsuccessfully to remove the second valve using again the En Share catheter. The second valve was blocked by the first one as it is shown characteristically (Figure 2c). Finally, the resuscitation was unsuccessful.
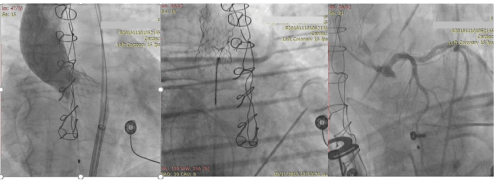
Figure 1. a) Fully expanded CoreValve, which seems to be deeper than expected into the left ventricular outflow tract. The absence of coronary vessels’ flow is also evident b) With the En Snare catheter the valve has been removed into the thoracic aorta. The grey horizontal lines indicate the position of the automatic cardiopulmonary resuscitation device. c) Coronary angiography depicts the patency of the left coronary system, after removal of the Core Valve.
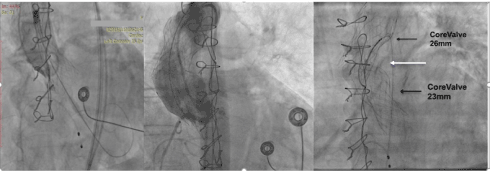
Figure 2. a) At an early stage of expansion of the second valve, the left main coronary artery is depicted. b) At a more advanced stage of the expansion the right coronary artery is the only shown patent, while the left coronary artery is absent. c) The thinner upper black arrow depicts the first removed valve into the proximal part of the aortic arch. The white arrow shows the En Share Catheter used to remove the second valve. The thick black arrow shows the second valve which is trapped by the first valve.
During the last decade, the relative use of surgical bioprosthetic valves has increased by nearly 80% and this observation likely explained by improved surgical techniques, valve durability, avoidance of anticoagulation and patient’s preference [1]. Nevertheless, surgical bioprosthetic valves are more durable comparing to the past, but they are known to fail as well [1]. Freedom from reoperation for a failing bioprosthetic valve is 95%, 90% and 70% at 5, 10 and 15 years, respectively [1]. The gold-standard treatment for a failing surgical bioprosthetic valve is a redo operation [1,2]. The operative mortality following an elective redo operation in low-to-intermediate risk patients is 2-7% [1,3,4]. In high surgical risk or non-elective cases, however, the mortality can be as high as 30% [1,3,4]. Even in low-risk and elective redo scenarios, the risks of wound infection, blood transfusions, postoperative pain and delayed functional recovery are not negligible [1,3,5,6].
Coronary obstruction following aortic ViV procedures is a life-threatening complication that requires immediate cardiopulmonary resuscitation and reinstitution of coronary blood flow [7]. Left coronary ostial occlusion is most common, although right coronary occlusion may rarely occur [8,9]. The majority of recognized events have been associated with immediate hemodynamic collapse and fatality [8]. Most of the patients having ostial left-main obstruction died during their hospital stay [8]. The propensity for this complication is clearly related to the spatial geometry of the surgical valve leaflets inside the aortic sinuses and seems to be more common within the Mitroflow and Freedom valves [8]. The Mitroflow leaflets are unique, being relatively long (≈13 mm) and mounted externally over the stent rather than internally, as in most other stented bioprosthesis [8]. The reported frequency of 3.5% in the global registry experience is much higher than associated with native valve procedures (0.7%) [8,9]. It is possible that this may be an underestimate when coronary obstruction is partial, bypass grafts are present, or manifestations occur late [8]. Rarely, coronary obstruction may result from the transcatheter heart valve (THV) sealing cuff overlying a coronary ostium or sinotubular ridge [8]. This might occur with high implantation of a CoreValve device where the sealing cuff might well extend above the bioprosthesis, but it is unlikely with the shorter SAPIEN device [8]. Besides, CoreValve device has some distinct characteristics making the exact position of the valve being unpredictable [10]. A more common scenario is for the displaced leaflets of the failing bioprosthetic valve to come in direct contact with the coronary ostia or with the sinotubular junction overlying the coronary ostia, a concern common to all THV designs [8].
A recent publication described two cases of coronary obstruction during treatment of a degenerated Mitroflow (Sorin) bioprostheses [7]. This may suggest that certain surgical bioprostheses may increase the risk of coronary obstruction in the setting of TAV-in-SAV implantation [7]. Coronary obstruction occurs more commonly than in the setting of native aortic valve stenosis as a result of an interplay between the aortic root, the bioprosthesis, and the transcatheter valve; all must be considered [8,11]. The type of bioprosthesis (leaflet profile and relationship to a valve stent), valve configuration (stented/Stentless), position (intra-annular/supra-annular), mode of failure, and leaflet bulkiness are important in the evaluation of risk [8]. Several strategies can be considered when an increased risk of coronary obstruction is suspected.8 Selection of a relatively smaller THV implant will result in less displacement of the bioprosthetic leaflets, as will low implantation [8]. A retrievable THV may allow prompt removal, at least while the delivery system is still attached. Transesophageal echocardiography may be helpful in evaluating the presence of coronary obstruction or other causes of procedural hypotension [8]. One strategy has been to place a bailout angioplasty wire, balloon, or stent within the artery at risk, offering the potential to displace an occlusive bioprosthetic leaflet or THV away from the coronary ostia. The reliability of this approach is unclear, and the potential need for cardiopulmonary support and a surgical solution should be evaluated. Perhaps the safest strategy in patients at high risk would be to avoid a ViV procedure altogether or a transapical valve in valve procedure avoiding the high risk of a new surgery [8].
VIV implantations are consisted of really demanding and challenging cases. Precautionary measures and extremely careful technique during procedure could be the most important element avoiding lethal cases.
- Piazza N, Bleiziffer S, Brockmann G, Hendrick R, Deutsch MA, et al. (2011) Transcatheter aortic valve implantation for failing surgical aortic bioprosthetic valve from concept to clinical application and evaluation (Part 1). JACC Cardiovasc Interv 4: 721-732. [Crossref]
- Nalluri N, Atti V, Munir AB, Karam B, Patel NJ, et al. (2018) Valve in valve transcatheter aortic valve implantation (ViV-TAVI) versus redo-Surgical aortic valve replacement (redo-SAVR): A systematic review and meta-analysis. J Interv Cardiol 31: 661-671. [Crossref]
- Wernly B, Zappe AK, Unbehaun A, Sinning JM, Jung C, et al. (2019) Transcatheter valve-in-valve implantation (VinV-TAVR) for failed surgical aortic bioprosthetic valves. Clin Res Cardiol 108: 83-92. [Crossref]
- Lange R, Piazza N (2013) Transcatheter aortic valve-in-surgical aortic valve implantation: current status and future perspectives. Eur J Cardiothorac Surg 44: 403-406. [Crossref]
- Tsigkas G, Despotopoulos S, Makris A, Koniari I, Armylagos S (2018) Transcatheter versus surgical aortic valve replacement in severe, symptomatic aortic stenosis. J Geriatr Cardiol 15: 76-85. [Crossref]
- Bapat V, Davies W, Attia R, Hancock J, Bolter K, et al. (2014) Use of balloon expandable transcatheter valves for valve-in-valve implantation in patients with degenerative stentless aortic bioprostheses: Technical considerations and results. J Thorac Cardiovasc Surg 148: 917-922. [Crossref]
- Piazza N, Bleiziffer S, Brockmann G, Hendrick R, Deutsch MA, et al. (2011) Transcatheter aortic valve implantation for failing surgical aortic bioprosthetic valve from concept to clinical application and evaluation (Part 2). JACC Cardiovasc Interv 4: 733-742. [Crossref]
- Stanger O, Gisler F, Londono MC (2015) Valve-in-valve: Estimating the risk of ostium obstruction in failed sorin stentless valves. J Thorac Cardiovasc Surg 149: 644-645. [Crossref]
- Webb JG, Dvir D (2013) Transcatheter aortic valve replacement for bioprosthetic aortic valve failure: the valve-in-valve procedure. Circulation 127: 2542-2550. [Crossref]
- Dvir D, Webb J, Brecker S, Bleiziffer S, Hildick-Smith D, et al. (2012) Transcatheter aortic valve replacement for degenerative bioprosthetic surgical valves: results from the global valve-in-valve registry. Circulation 126: 2335-2344. [Crossref]
- Grubitzsch H, Zobel S, Christ T, Holinski S, Stangl K, et al. (2010) Redo procedures for degenerated stentless aortic xenografts and the role of valve-in-valve transcatheter techniques. Eur J Cardiothorac Surg 140: 248-249. [Crossref]


