Inflammatory myofibroblastic tumour is a rare benign entity that behaves like a malignancy especially in abdomen, commonly seen in paediatric age group and is known to have high chances of recurrence.
Our infant came with breathlessness,abdominal distension, fever and jaundice. After stabilization, child underwent laparotomy where multiple masses were removed amounting to 90-95% of total masses including a large mass (15x12 cm) adherent to the mesentery and the large intestine, and a colostomy was needed. Histopathology confirmed IMT(ALK positive). 6 months after discharge colostomy closure is planned.
Due to features of varying presentations, multiple focal deposits and satellite lesions, recurrence, recorded malignant transformation, local invasion together with lack of medical literature and overall rarity, makes this entity difficult to diagnose and treat.
Complete resection is the most preferable treatment, without which chances of recurrence is high. Various treatment modalities has been tried but with inconsistent results. Usage of combination of modalities especially with newer therapeutic agents is proving beneficial in cases where complete resection is difficult either due to local tissue invasion or recurrence.
Our case had significant local invasion and multi-focal deposits with multiple satellite lesions but more conservative treatment modalities such as chemo, radio and immunomodulation therapy could not be utilized due to lack of safety and efficacy studies in infants with IMTs or otherwise.
inflammatory myofibroblastic tumour, infant, intra-abdominal, ALK positive
Inflammatory Myofibroblastic Tumour
A rare intra-abdominal mass: Inflammatory Myofibroblastic Tumour (IMT) is a rare benign neoplasm(less than 1 case per million people) [1]. It is more commonly seen in children and adolescents, and it commonly involves pulmonary and gastrointestinal tracts [2,3]. Extra pulmonary IMTs account for merely 5% of the total IMT cases [4]. However, literature regarding this entity is limited. This entity is clinically very important since these lesions often mimic malignant neoplasms, such as sarcomas, lymphomas or metastases which could lead to a misdiagnosis and consequent mismanagement [2,5]. Here, we present a case of a 4 month old child from Kyrgyzstan whowas diagnosed with an abdominal tumour at 1 month of age. He underwent two successive laparotomies in local hospitals there but the tumour could not be removed and was referred to us for further management.
A 4 month old baby, resident of Kyrgyzstan came to Medanta Hospital on 30/04/21 with complaints of breathlessness since 3 months, abdominal distension and fever on and off since 1 month. Baby is 3rd issue of a non-consanguineous marriage, born on 30/12/2020, preterm, vaginal delivery at 34 weeks gestational age, weight- 2.2 kg at birth. During antenatal period of the baby, the mother had an episode of very high fever at 8 weeks of pregnancy. COVID-19 was prevalent at the time but the mother could not be tested. The baby has 2 older siblings- 8 year old female and 1.5 year old male. The mother was operated for an abdominal cyst 6 years ago.
On admission (30/04/2021) the patient had breathlessness, abdominal distension and jaundice(total billirubin-2.5 mg/dl, direct billirubin-2.0 mg/dl). The SpO2 level was 88%, Hb-11.9,TLC-20,210, platelets- 7,24,000,CRP- 339.6. Blood culture was negative for any microbial growth.The child was given oxygen by mask and stabilized. This child had undergone 2 successive laparotomies on 05/04/21 and 16/04/21 at local hospitals in Bishkek, Kyrgyzstan and two biopsies revealing the nature of a hard swelling in the abdomen as ‘hemangioma’ and ‘fibromatosis’ respectively. These procedures were unsuccessful and the patient was referred here for further management.
The baby had a paramedian 18 cm long abdominal scar extending from left hypochondrium to left iliac fossa. The abdomen was distended with visible multiple superficial veins. Upon palpation a large hard mass was felt measuring 15 cm x 12 cm with mild guarding in epigastric, umbilical quadrant and lower abdomen. The mass was hard in consistency, multilobular to touch, extending 4 cm above the umbilicus to inguinal crease line and laterally to the flank on the right side- 2cm beyond the midline and umbilicus on the left. The mass was barely mobile along any axis and the skin above it showed no signs of inflammation. Similar masses were felt above the testis in the scrotum.
Patient was diagnosed with pneumonia and was already on cephalosporin and amikacin. Covid-19 RT-PCR negative on admission. Possibility of neuro fibromatosis, fibromatosis and hemangioma were entertained.
CECT CHEST(01/05/21) showed- Patchy consolidations with atelectatic bands seen in superior and posterior segments of both lower lobes on right side.The CECT and PET-CT abdomen shows a large nodular mass at 11x9 cm in mesenteric region displacing bowel loops anteriorly and kidneys and aorta posteriorly along with multiple peritoneal and scrotal deposits, deposits in omental region, surface deposits along liver, perisplenic region, paracolic gutter, pelvis and on spermatic cords. The enhancing pattern in the CT-scan depict high fibrous content of the tumour. These lesions depict metastatic propensity of IMT in the abdomen. It further rules out complete resection as a possible remedy.CT GUIDED BIOPSY(03/05/21) showed- Linear tissue cores infiltrated by tumour cells along with numerous thin blood vessels and inflammatory cells. The tumour cells have moderately pleomorphic nuclei with moderate amphophillic cytoplasm-compatible with inflammatory myoblastic tumour.
Since the patient had features of sepsis and pneumonia and SpO2 at room air was at 88%, he was put on IV antibiotics including colistin and oxygen at high flow. Upon stabilization the patient was taken up for surgery on 10/05/21.
Along with breathlessness, abdominal distension and fever, our child also had jaundice upon arrival. A comparative chart stating its resolution is listed in table 1. In the study by Su Jin, et al. 2 out of 9 children had jaundice(1 year old male and 4 year old female) (Table 1).
Table 1. Relative bilirubin values
Liver Function Test |
30/04/21 |
14/05/21 |
Total Billirubin |
2.5 |
0.7 |
Direct Billirubin |
2.0 |
0.5 |
Indirect Billirubin |
0.5 |
0.2 |
On 10.05.21 Exploratory laparotomy with excision of intra abdominal /retroperitoneal tumour with lymph node dissection, excision of tumour in the supra umbilical area, resection of colon along with tumour and colostomy was done. Upon opening up the abdomen, multiple satellite lesions were confirmed. The largest mass measuring 12x15 cm was adherent to mesentry and large bowel, 6 cm of which had to be resected as well. 90% of the tumour was excised. PRBC and FFP was administered during the procedure. The baby remained haemodynamically stable during the surgery.
Post operatively, the patient was put on mechanical ventilator for 24 hours and thereafter extubated and then put on high flow nasal cannula at 2L of oxygen. Colostomy bag was put in place on post-operative day 01. By 3rd post-operative day the colostomy started working. Patient had multiple apnoeic attacks- ? seizures on 4th post-operative day and hence was put on IV Levipil and Phenytoin. No evidence of acute infarct or hemorrhage upon CT Brain. Blood transfusion was done twice to counter low haemoglobin levels. Dextrose feed through NG tube were started on 5th post-operative day. Breast feed was started on 6th post-operative day and by 7th day, child showed signs of abdominal wound dehiscence and soakage of dressing material with serous fluid discharge and taken up for secondary suturing of the abdominal wound. Patient was extubated post procedure and put at room air. Oral feeds started successfully on the 8th day and gradually build up. The baby had repeated episodes of hypoglycemia which responded to oral and IV dextrose. Weight of the baby at admission was 7.5 kgs and wt. on 25/05/21 was 5.5 kgs
Histopathology reported a well circumscribed tumour attached to the mesentry of the colon. Tumour was composed of myofibroblastic and fibroblastic spindle cells admixed with mild inflammatory infiltrate composed of lymphocytes, plasma cells, eosinophills and histiocytes. Background showed abundant blood vessels. The spindle cells are arranged in fascicles and storiform pattern. They show moderate pleomorphism. However, mitotic activity is very sparse(0-1/hpf). One resection margin of colon was free however, the other margin was involved. On IHC, the spindle cells showed diffuse immunoreactivity for ALK1 and focal immunoreactivity for smooth muscle actin(SMA) and Desmin. There was no immunoreactivity for CD117, CD34 and S100.
The most current name i.e. IMT was originally given by Umiker and Iverson in 1954 [5]. Although IMT is a benign disorder, in a paediatric abdomen it can mimic a malignancy, both clinically and radiologically [5]. First described in 1937, it has had many names such as inflammatory pseudotumour, plasma cell granuloma, inflammatory fibrosarcoma, etc [2].
IMTs are usually seen in children and adolescents. In infants this tumour is a rare and isolated occurrence [6]. The presenting feature of this entity depends upon the location and size of the tumour [2]. The commonest site for IMT is lungs and the second commonest is Abdomen [2,3].
In lungs it may be asymptomatic or present with dry cough, haemoptysis, shortness of breath, chest pain and clubbing [2]. While an intra abdominal tumour often presents with fever, abdominal distension, anorexia, wt. loss and pain [2,7]. 15-40% of the cases are asymptomatic and discovered during routine physical exam or investigations for other conditions [7]. Rare complications include intestinal obstruction and rectal bleeding [2,8].
Ibrahim, et al. reported 2 out of 7 children having mesenteric tumours(9 year and 12 year old males) while 1 out of 7 had descending colon associated with the tumour(11 year old male). Our case had the tumour arising out of the mesentery and descending colon on the left side and is the 2ndsuch case in a 4 month old infant in the literature. Another 4 months old baby with a mesenteric IMT was reported in the Japan study [9,10].
Histopathologically, IMT is a benign solid tumour composed mainly of spindle shaped cells, and has a chronic inflammatory component consisting of plasma cells, lymphocytes and occasional histiocytes [2]. Laboratory findings in IMT usually show hypochromic microcytic anemia, increased immunoglobulins, elevated ESR and elevated Thrombocyte counts that regress post-surgery, as documented in our case as well (Table 2) [2,3,7]. Persistence or recurrence of these inflammatory signs post-surgery point towards recurrence of the IMT or a residual tumour. Our patient was a known anemic, was transfused with PRBC twice in Kyrgyzstan on 05/04 and 16/04/21 and came with Hb of 11.9, had thrombocytosis and leucocytosis.There is no record of haemoglobin levels during his hospital stay in Kyrgyzstan.
The Hb dropped and required blood transfusion twice (Table 2).
Table 2. Comparative CBC values
Parameters |
30/04/2021 |
14/05/2021 |
19/05/2021 |
Haemoglobin |
11.9 |
8.8 |
9 |
WBC |
20.21 |
7.44 |
11.17 |
Platellet count |
724 |
262 |
329 |
RBC count |
4.33 |
3.06 |
3.17 |
The etiology is not clear but ALK gene rearrangements are known to play a significant role along with multiple other genes forming fusion proteins that activate tyrosine kinase having oncogenic potential [3]. 50% of the IMT are ALK positive [3,10]. It is also known to be associated with infections (such as organizing pneumonia, Mycobacterium avium intracellulare, Cornybacterium equi, Campylobacter jejuni, Bacillus Sphaericus, Coxiella burnetti, Echerichia coli,Ebstein-Barr virus etc), previous abdominal surgery, trauma, ventriculoperitoneal shunt, radiotherapy, steroid usage, and some genetic factors [2]. Ebstein-Barr virus and Herpes virus (HHV-8) are known to play significant role in ALK negative IMTs but not other wise [9]. This was confirmed by a negative EBV PCR test in our child.
The fever can be attributed to the inflammatory reaction and the abdominal distension to the large tumour. The breathlessness occurred due to a pneumonia which resulted due to the intra abdominal tumour causing the diaphragm to be raised. The inflammatory features suggest some cytokines such as interleukin (IL-6) maybe involved in the pathogenesis of IMT, while virus infections may also be the cause of inflammation in IMT [9].
Neither Needle biopsy nor Frozen sections are fully reliable in diagnosis of IMT [2]. Myofibroblasts and inflammatory cell infiltrate are the commonest finding but overall microscopic arrangement of these cells makes it difficult to distinguish from other soft tissue tumours like leiomyosarcoma, fibrosarcoma, rhabdomyosarcoma and malignant fibrous histiocytoma [2,5]. The histopathological diagnosis is helped by absence of anaplasia, intermixture of lymphocytes and plasma cells among spindle cells, and paucity of mitotic figures [2].
15 to 37% of IMT cases arising in mesentry or retroperitoneum show local recurrence, and most cases recur within 1 year after the initial surgery [2]. Rare distant metastases has also been proposed to occur because of the multifocal characteristic of the lesion rather than metastatic spread [2]. There has been multiple cases reporting the tendancy of this entity to invade local tissues like liver, large bowel and portahepatic system [5]. In our case, the tumour had invaded the large intestine necessitating the colostomy procedure. Recent reports concerning the malignant transformation of IMT and lymphoreticular malignancy arising in the residual IMT necessitate deeper analysis of this entity [2]. In fact there is no current, tested or widely studied treatment outline in literature for medical professionals to follow. Complete surgical resection with clear surgical margins remains the standard treatment of choice failing to which chances of recurrence are high [8]. The common finding of unresectable masses explains the propensity to recur and justifies the importance of either complete resection or combination of 2 or more modalities otherwise. While chemotherapy, radiotherapy and immunomodulation have questionable consistency when it comes to IMTs, newer treatment modalities are being explored such as targeted therapies like Crizotinib, regression with NSAIDS like Infliximab, diclofenac and Laser excision of mass [4,11]. Usually a combination of the above is followed for unresectable cases invading vital structures or recurrences [4,8].
In our case Crizotinib could not be used since there is no study of safety and efficacy of crizotinib in 4 month old infant. All the paediatric studies on crizotinib’s safety and efficacy in the current literature has been conducted on patients 12 months and older with only 4 reported cases of successful treatment of infantile IMTs [6]. The role of radiotherapy in eliminating unresectable IMTs has been recorded in few cases but none in paediatric age group [3].
Development of targeted therapies like Crizotinib can revolutionize the treatment of rare cancers but the availability and affordability of such medicines for middle income and underdeveloped countries remains a burning issue of capitalism and ethics.
Future plan is to observe the baby at least 12 – 24 weeks and repeat radiological investigations and biopsy. If the weight of the child could remain stable we will excise the residual tumour and close the stoma (Figure 1-4).
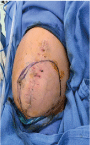
Figure 1. Pre OP image left paramediain incisionwith the tumour marked on the skin
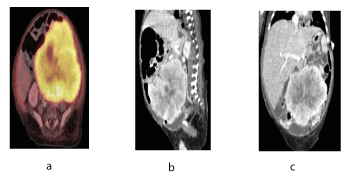
Figure 2. CT a. Transverse view b. Sagittal view c. Tumour
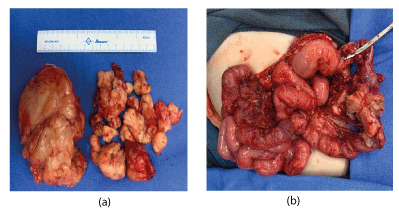
Figure 3. (a) Intra Abdominal Tumour (b) with colonic perforation
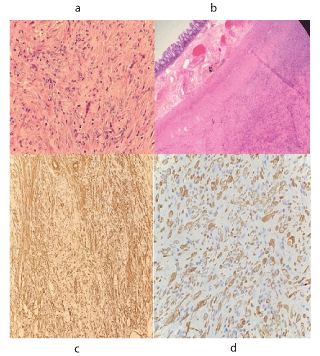
Figure 4. Histopathology of tumour
(a) Tumour attached to colon wall (HE x 40x)
(b) Plump epitheloid tumour cells admixed with sparse inflammation (HE x 400x)
(c) IHC for Smooth Muscle actin
(d) IHC for ALK
We wish to thank Mrs. Jyoti Badola, our team co-ordinator for her help with documenting and publishing this case study.
- https://www.cancer.gov/pediatric-adult-rare-tumor/rare-tumors/rare-soft-tissue-tumors/inflammatory-myofibroblastic-tumor
- Karnak I, Senocak ME, Ciftci AO, Caglar M, Bingöl-Kologlu M, et al. (2001) Inflammatory Myofibroblastic Tumor in Children: Diagnosis and Treatment. J Pediatr Surg 36: 908-912. [Crossref]
- Khalil S, Ghafoor T, Raja KFA (2000) Inflammatory Myofibroblastic Tumor: A Rare Presentation and an Effective Treatment with Crizotinib. Case Reports in Oncological Medicine.
- Tao Y, Wang Z, Han J, Wei P (2012) Inflammatory Myofibroblastic Tumor successfully treated with chemotherapy and nonsteroidals: a case report. World J Gastroenterol 18: 7100-7103. [Crossref]
- Kim SJ, Kim WS, Cheon J, Shin S, Youn BJ, et al. (2009) Inflammatory Myofibroblastic Tumors of the Abdomen as Mimickers of Malignancy: Imaging Features in Nine Children. AJR Am J Roentgenol 193: 1419-1424. [Crossref]
- Oscar Lopez-Nunez, Ivy John, Ryane N. Panasiti, et.al. Infantile inflammatory myofibroblastic tumors: clinicopathological and molecular characterization of 12 cases. Mod Pathol 33: 576-590 [Crossref]
- Çorapçioglu F, Kargi A, Olgun N, Özer E, Olguner M, et al. (2005) Inflammatory myofibroblastic tumor of the ileocecal mesentery mimicking abdominal lymphoma in childhood: report of two cases. Surg Today 35: 687–691. [Crossref]
- Soyer T, Talim B, Karnak I, Ekinci S, Andiran F, et al. (2017) Surgical Treatment of Childhood Inflammatory Myofibroblastic Tumors. Eur J Pediatr Surg 27: 319-323. [Crossref]
- Yamamoto H, Kohashi K, Oda Y, Tamiya S (2006) Absence of human herpesvirus-8 and Epstein–Barr virus in inflammatory myofibroblastic tumor with anaplastic large cell lymphoma kinase fusion gene. Pathology international 56: 584-590.
- Mossé YP, Voss SD, Lim MS, Rolland D, Minard CG, et al. (2017) Targeting ALK With Crizotinib in Pediatric Anaplastic Large Cell Lymphoma and Inflammatory Myofibroblastic Tumor: A Children’s Oncology Group Study. J Clin Oncol 35: 3215-3221. [Crossref]
- Vijayasekaran D, Thirunavukkarasu S, Ramesh S (2018) Laser Treatment of a Tracheal Inflammatory Myofibroblastic Tumor. Indian Pediatr 55: 907-908. [Crossref]




