Rationale: Patients with FUO (fever of unknown origin) occasionally present with an abnormal density area in the presacral region on CT scan. This report sheds light on the association between CT findings and FUO.
Patient concerns: All eight patients were admitted to our hospital between 2015 and 2020 and underwent CT examinations to determine the cause of their fever.
Diagnosis: There were no abnormal findings on CT, aside from the abnormal density area in the presacral region; therefore, they were diagnosed with a fever of unknown origin (FUO).
Interventions: Aside from the patient with a fever of 36.9°C, all patients were treated with antipyretics and/or antimicrobials and subsequently returned to normal temperature.
Outcomes: The morphological features from abnormal CT findings were divided into multi-stripe, homogeneous, and mixed patterns. The abnormal density area formed a crescent or beard-like shape on axial CT images in these patterns. The anterior (ventral) borders of the abnormal density area in the presacral region were distinct in six cases and indistinct in two cases. In addition, it was tent-shaped in three cases and bowl-shaped in five cases. The CT values of their non-fatty density area ranged from 13.8 HU to 30.3 HU.
Lessons: We report unique morphological and compositional features from CT images in the presacral region of patients with FUO. To the best of our knowledge, this is the first case report of unique CT findings in the presacral region of patients with FUO.
fever of unknown origin, presacral space, retrorectal space, presacral fascia, retroperitoneal space, computed tomography
CT: Computed tomography; FUO: Fever of unknown origin; HU: Hounsfield unit; WBC: White blood cell; CRP: C-reactive protein; CPK: Creatine phosphokinase; AST: Aspartate aminotransferase; ALT: Alanine aminotransferase; BUN: Blood urea nitrogen; CRE: Creatinine; MOD: Method of disc; ROI: Region of interest; MRI: Magnetic resonance imaging; RI: Radioisotope; PET: Positron emission tomography
CT examinations are often used as a diagnostic tool to investigate the cause of fever in daily clinical practice. CT scans can detect a range of fever-associated conditions, including pneumonia or pyelonephritis; however, they are often unable to clearly identify a causative condition. These patients are referred to as having fever of unknown origin (FUO). Patients with FUO occasionally present with an abnormal density area in the presacral region on CT scans. Although the accurate frequency is unclear, we feel that it is not rare in daily clinical practice.
The abnormal density area is currently of unknown significance. An abnormal density area in the presacral region is also observed in patients with spread of inflammation from the surrounding retroperitoneal organ, inflow of pancreatic fluid due to acute pancreatitis, blood due to rupture of an aortic aneurysm, renal injury or pelvic bone fracture, extension of neoplasm, retroperitoneal fibrosis, fasciitis, and extramedullary hematopoiesis. Although the abnormal density area in the presacral region was shown to be associated with many other abnormal findings on CT images in the aforementioned conditions in the previous literature [1-6], we could not find any reports concerning the abnormal density area in the presacral region as a unique abnormal finding on CT images in a patient with FUO.
Herein, we report and discuss the unique CT findings in the presacral region of eight patients with FUO. Additionally, using these CT images, we defined a nomenclature for these abnormal densities to aid in the classification and diagnosis of patients with FUO.
Patient cohort
This retrospective study was approved by the institutional review board of our hospital. The patient data are summarized in Table 1. All eight patients (age range, 48-94 years; 2 men, 6 women) were admitted to our hospital between 2015 and 2020 and underwent CT examinations to determine the cause of their fever. There were no abnormal findings on CT, aside from the abnormal density area in the presacral region; therefore, they were diagnosed with a fever of unknown origin (FUO). All the CT examinations were performed without contrast.
Table 1. Patient characteristics (M: Male; F: Female; WBC: White blood cells; CRP: C-reactive protein; CPK: Creatine phosphokinase; HPF: High-power field)
Case No |
age |
sex |
Reason of hospitalization |
Body temperature
(℃) |
WBC/μL
(blood) |
CRP
(mg/dl) |
CPK
(IU/L) |
WBC/HPF
(urine) |
1 |
77 |
M |
rt-rotator cuff tear |
39.6 |
19900 |
6.27 |
80 |
<1 |
2 |
94 |
F |
lt-femoral trochanter fracture |
37.4 |
9000 |
4.43 |
89 |
1~4 |
3 |
78 |
F |
rt-femoral neck fracture |
39.1 |
9800 |
5.60 |
31 |
1~4 |
4 |
94 |
F |
cerebral infarction |
37.8 |
4400 |
16.06 |
44 |
50~99 |
5 |
64 |
F |
rt-putaminal hemorrhage |
37.4 |
6900 |
0.28 |
32 |
100~ |
6 |
83 |
F |
subarachnoid hemorrhage |
36.9 |
19600 |
6.99 |
50 |
<1 |
7 |
69 |
M |
fever |
38.3 |
13700 |
9.64 |
361 |
10~19 |
8 |
48 |
F |
cerebral infarction |
38.0 |
14000 |
5.02 |
92 |
10~19 |
The reasons for hospitalization were fever, cerebral infarctions (2 patients), right putaminal hemorrhage, traumatic subarachnoid hemorrhage, rotator cuff tear of the right shoulder joint, left femoral trochanter fracture, and right femoral neck fracture. In the latter three patients, findings were detected on postoperative CT scans performed 8, 4, and 13 days post-surgery. Aside from patients with cerebral infarction and right putaminal hemorrhage, the other five patients underwent multiple CT examinations. Three patients had a previous CT examination, one patient underwent a subsequent CT examination, and one patient underwent both previous and subsequent CT examinations.
All patients had a fever of 36.9 °C -39.6 °C and no abnormal findings related to fever in the physical examination when they received their CT scan. The patient with a fever of 36.9 °C had an ordinary temperature of 35 °C level before the day when he received a CT scan. Aside from the patient with a fever of 36.9°C, all patients were treated with antipyretics and/or antimicrobials and subsequently returned to normal temperature. Glycerin enema was performed on the day before CT examination in a patient with a rotator cuff tear of the right shoulder joint. None of the patients received any neuroleptic drugs. Aside from the patient with right putaminal hemorrhage and one patient with cerebral infarction, all patients exhibited elevated white blood cell (WBC) counts. Aside from the patient with right putaminal hemorrhage, all patients showed elevated C-reactive protein (CRP; 4.43~16.06 mg/dl) in their blood test. The value of creatine phosphokinase (CPK; 361 IU/L) was slightly elevated in one patient, and the reason for hospitalization was fever. All patients had normal hepatic function aside from the patient with right putaminal hemorrhage, who showed slight hepatic dysfunction with increased aspartate aminotransferase (AST; 63 IU/L) and alanine aminotransferase (ALT; 112 IU/L). Renal function was normal in all patients, aside from the patient with traumatic subarachnoid hemorrhage, who had moderate renal dysfunction with increased blood urea nitrogen (BUN; 43.8 mg/dL) and creatinine (CRE; 2.00 mg/dL). Cardiac function was normal in all patients except for one patient with cerebral infarction who had chronic cardiac dysfunction, and echocardiography revealed diffuse hypokinesis of the left ventricular wall motion and decreased ejection fraction (by MOD: method of disc) of 31.9%. However, the patient with chronic cardiac dysfunction had no pulmonary edema, ascites, or subcutaneous edema. None of the patients exhibited anasarca, pelvic bone fracture, compression gangrene around the sacrum, neoplasm or retroperitoneal fibrosis, or fasciitis. No patient had a history of radiation therapy or surgery in the pelvic region.
Computerized tomography (CT) scan
CT scans were performed using a Revolution EVO (GE Healthcare) with a 64-detector row until May 2017 and Light speed VCT (GE Healthcare) with a 64-detector row since June 2017. All images were reconstructed using a slice thickness of 2.5 mm. CT values of the abnormal density area in the presacral region were obtained by calculating a mean value based on the data obtained from several regions of interest (ROI) from sites of non-fatty density area by setting the ROI manually in order of size.
We retrospectively investigated the morphologic features and CT values from the abnormal density area in the presacral region at the level of the bilateral acetabulum of the hip joint or upper portion of the femoral head, corresponding to the fourth or fifth sacral level.
We used a retroactive approach to analyze CT images from patients presenting with fever of unknown origin (FUO) (Table 2). From the CT images, we examined an abnormal density area in the presacral region, as it had been previously observed in patients with FUO. The morphological features corresponding to abnormal CT findings were a crescent or beard-like shape on an axial image. These abnormal findings can be divided into three patterns: multi-stripes, homogeneous, or mixed. A multi-stripe pattern was a multiple curvilinear non-fatty and fatty density area, which ran parallel to the perirectal fascia in the presacral region (Figure 1). A homogeneous pattern consisted of a homogeneous non-fatty density area in the presacral region (Figures 2 and 3). The mixed pattern included both of these patterns (Figure 4). A multi-stripe pattern occurred in two cases, a homogeneous pattern in three cases, and a mixed pattern in three cases. The anterior (ventral) borders of the abnormal density area in the presacral region on axial CT images were distinct in six cases and indistinct in two cases. In addition, the abnormal density displayed a tent shape in three cases and a bowl shape in five cases. The CT value of their non-fatty density area ranged from 13.8 HU to 30.3 HU. There were no abnormal findings in the presacral region on any of the previous CT scans obtained 3, 4, 14, and 33 days before. One patient exhibited mild abnormal findings on a subsequent CT scan obtained 6 days later. There were mild abnormal findings on the first follow-up CT scan 3 days later, but were no longer present on a scan 19 days later.
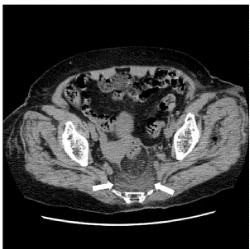
Figure 1. A CT image of a multi-stripe pattern in the abnormal density area of the presacral area. Case 2. Non-contrast axial CT at the level of the acetabulum of the hip joint shows an abnormal density area of the multi-stripe pattern in the presacral region (arrow). The ventral border was distinct and bowl-shaped
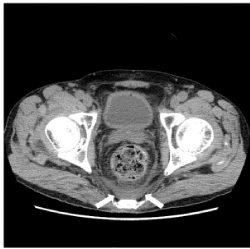
Figure 2. A CT image of a homogeneous pattern in the abnormal density area of the presacral area. Case 1. Non-contrast CT at the level of the femoral head shows an abnormal density area of homogeneous pattern in the presacral region (arrow). The ventral border was distinct and bowl-shaped
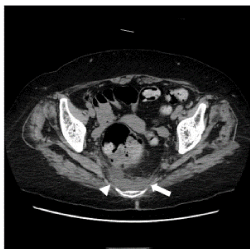
Figure 3. A CT imaging of a homogeneous pattern in the abnormal density area of the presacral area. Case 5. Non-contrast CT at the level of the acetabulum of the hip joint shows an abnormal density area with a homogeneous pattern in the presacral region (arrow). The ventral border was indistinct (arrowhead) and bowl-shaped
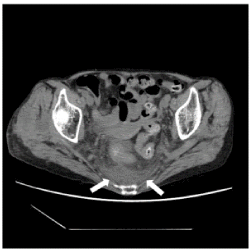
Figure 4. A CT image of a mixed pattern in the abnormal density area of the presacral area. Case 3. Non-contrast CT at the level of the acetabulum of the hip joint shows an abnormal density area of mixed pattern in the presacral region (arrow). The ventral border is distinct and tent-shaped
Table 2. CT findings (ND: CT examination was not done; - : Abnormal density area in the presacral region was not detected on CT images; +: Abnormal density area in the presacral region was detected on CT images)
Case No |
pattern |
CT value
(HU) |
Ventral border /shape |
Previous CT finding |
1st subsequent CT finding |
2nd
subsequent CT finding |
1 |
homogeneous |
30.3 |
distinct/bowl |
−(33 days before) |
ND |
ND |
2 |
multi-stripe |
13.8 |
distinct/bowl |
−(4 days before) |
+(3 days later) |
−(19 days later) |
3 |
mixed |
17.2 |
distinct/tent |
−(14 days before) |
ND |
ND |
4 |
mixed |
20.0 |
distinct/bowl |
ND |
ND |
ND |
5 |
homogeneous |
23.7 |
indistinct/bowl |
ND |
ND |
ND |
6 |
multi-stripe |
18.2 |
distinct/tent |
−(3 days before) |
ND |
ND |
7 |
homogeneous |
22.7 |
indistinct/bowl |
ND |
+(6 days later) |
ND |
8 |
mixed |
27.6 |
distinct/tent |
ND |
ND |
ND |
Using retroactive analysis of CT images, we sought to determine the cause of fever in patients with fever of unknown origin (FUO). Our previous observations revealed an abnormal density region in the presacral region of patients with FUO; however, a more in-depth analysis is required to provide a correlative conclusion. A study of cadavers and CT images revealed the anatomical structure of the pelvis [1,2]. We did not detect a structure between the perirectal space (mesorectum) around the rectum covered by the proper rectal fascia and the transversalis fascia anterior to the sacrum on normal CT images. Between the proper rectal fascia and the transversalis fascia, there are two occult spaces divided by the presacral fascia (retrorectal and presacral spaces). The retrorectal space is divided into inferior and superior compartments by Waldeyer’s fascia (rectosacral fascia), which originates from the presacral fascia at the S2-S4 level and is composed of two leaves [7-10]. The region containing the abnormal density area in the presacral region exists in the retrorectal and presacral spaces. Free retroperitoneal fluid, including blood, due to renal injury, aortic rupture, pelvic bone fracture, and pancreatic fluid due to acute pancreatitis, flows easily into the above spaces [1,2]. Inflammation in the abdominal and pelvic retroperitoneal space from conditions such as pyelonephritis and cystitis can also spread easily to these spaces [1,3,4].
In four of eight febrile patients, urine WBC counts were elevated, indicating a possible urinary tract infection; however, CT findings could not detect an infection. In addition, CT findings could not detect inflammation in other retroperitoneal organs in these specific cases.
The pelvic extraperitoneal space is in communication with extra-abdominal spaces, which can also serve as pathways for the spread of infection via the inguinal canal or the femoral vascular sheath space. Necrotizing fasciitis of the lower extremity, particularly the thigh, is a common cause of retroperitoneal fasciitis [3]. In the case of patients with left femoral trochanter fracture and right femoral neck fracture, the abnormal CT finding was seen on the 4th and 13th day after surgery, respectively. Although the surgical site was in close proximity to the presacral region, inflammation was not readily detected at either site through the sciatic or obturator foramen. Inflammatory changes in the inguinal canal and femoral vascular sheath space were also not detected on the CT images. We are unable to definitively exclude the possibility that inflammation spreads to the presacral region from undetected infections in the urinary tract or femoral bone fractures. As the abnormal CT finding was not seen on the previous CT performed 3 days before and was seen on the subsequent CT performed 6 days later in spite of the treatment with antimicrobials, it is suggested that the abnormal density area in the presacral region appears relatively rapidly and persists relatively for a long time, and it is composed of inflammatory changes.
As the CT values of the abnormal density area in the presacral region ranged from 13.8 HU to 30.3 HU, we postulate that it is composed of a mixture of water and soft tissue density materials including presacral fascia, Waldeyer’s fascia, and inflammatory cells. We hypothesized that the multi-stripe pattern is formed by the invasion of water and/or inflammatory cells along with several existing fascial and fatty planes, including the proper rectal fascia, retrorectal space, presacral fascia, Waldeyer’s fascia, presacral space, and transversalis fascia. Furthermore, water and/or inflammatory cells increased in the multi-stripe, mixed, and homogeneous patterns, respectively.
The perirectal space contains the major routes of blood supply to, and lymphatic drainage from, the rectal wall, comparable to the mesenteries of the other intestinal segments. The perirectal fatty tissue corresponds to the mesorectum, which is most developed at the dorsal side of the rectum, displaying two mesorectal “cheeks” [9]. In our three cases, the anterior (ventral) border of the abnormal density area in the presacral region on CT images was above the two “cheeks” and formed a tent shape. The retrorectal space behind the perirectal space is avascular and nerve-free. This avascular and nerve-free, slit-like space corresponds to the access route for dorsal mobilization of the rectum during total mesolectal excision [7-9], called the holy plane [8]. In contrast, the presacral fascia and presacral space behind it contain arteries, veins, lymphatics, and nerves. Due to the increased presence of these aforementioned structures, this is considered to be a dangerous site during total mesorectal excision [7-9]. It is thought that pathogens that contribute to fever are carried easily to the presacral fascia and the presacral space through arteries, veins, lymphatics, and nerves from other abdominal and pelvic retroperitoneal spaces, the buttocks, and the lower extremities [1,3]. Fascial planes may act as barriers to prevent the spread of inflammation and tumor invasion, and paradoxically act as conduits for their propagation [1]. There are likely individual variations in fascial anatomy, including incomplete fascial planes, disruption by acute suppurative infection, and rapid accumulation of fluid collection [1]. In our cases, with an indistinct ventral border for the abnormal density area in the presacral region, it is thought that inflammatory cells or fluid flowed into the perirectal space from the retrorectal space. Fluid and inflammatory cells in the presacral space are thought to enter through the presacral fascia and perirectal fascia.
No patients had CT findings suggesting proctitis: rectal wall thickening and mesenteric fat stranding in the perirectal space; therefore, it is not thought that the abnormal density area in the presacral region is generated by the inflammatory spread of proctitis. Glycerin enema was performed the day before the CT examination in case 1. There was a case report concerning the complications following glycerin enema, which suggested malignant hyperthermia in the past literature, although there was no mention of CT findings in it [11]. Therefore, the possibility that fever and abnormal CT findings in our case were generated by bacterial translocation due to glycerin enema cannot be ruled out.
Neuroleptic malignant syndrome comprises hyperpyrexia, altered consciousness, muscular rigidity, and autonomic dysfunction. Syndrome occurs after administration of neuroleptic drugs. Associated laboratory abnormalities include leukocytosis, elevated serum CPK, and liver enzyme concentrations [12]. CPK is mainly present in skeletal muscle cells; therefore, the destruction of skeletal muscle cells due to rhabdomyolysis, myositis, or muscle injury contributes to the elevation of serum CPK levels [13,14]. In our study no patients took neuroleptic drugs and had a high level (more than at least 1000 IU/L) of serum CPK detected in rhabdomyolysis, myositis or muscle injury, therefore we considered that rhabdomyolysis, myositis or muscle injury of intra- and /or extra-pelvic muscle did not affect the formation of the abnormal density area in the presacral region.
Extension of neoplasm, retroperitoneal fibrosis, retroperitoneal fasciitis, and extramedullary hematopoiesis may show abnormal density areas in the presacral region on CT images [3-6]; however, no abnormal findings suggesting the aforementioned diseases were observed in our cases.
Radiation therapy and surgery cause therapeutic fibrosis and may show an abnormal density area in the presacral region on CT images [15,16]; however, our patients had no history of radiation therapy or surgery in the pelvic region.
To our knowledge, no studies have examined the association between fever of unknown origin (FUO) and abnormal density area in the presacral region by CT imaging. Moreover, we defined the nomenclature to represent the abnormal CT findings. For example, presacral fluid collection, presacral fasciitis, or presacral phlegmon (cellulitis) can be used to represent abnormal density areas in the presacral region. These terms apply when the condition has a distinct, identifiable origin, and we therefore are cautious when applying them to conditions of unknown origin. The term presacral edema can be used to represent both inflammatory and non-inflammatory conditions [17].
Our study revealed a relationship between the abnormal density area in the presacral region from CT images and FUO. We consider the CT finding to be an effect of the disease contributing to FUO and not the cause of FUO. Our study has several limitations. First, the number of cases with FUO and an abnormal density area in the presacral region on CT images was small. Second, examinations using other approaches such as contrast-enhanced CT, magnetic resonance imaging (MRI), radioisotope (RI), and positron emission tomography (PET) were not performed in our cases. Due to the absence of additional imaging techniques, we could not provide additional information about the abnormal density area in the presacral region [10,18]. Third, obtaining tissue from the abnormal density area in the presacral region is both ethically and technically challenging [10], because FUO can be easily treated with antipyretics and antimicrobials, and the abnormal density area in the presacral region is small and surrounded by the sacrum, rectum, and numerous vessels and nerves, which are too dangerous to perform biopsy. De Kok et al. suggested that image-guided posterior transperineal drainage is an effective, safe, and well-tolerated procedure for the treatment of presacral abscess; therefore, biopsy of the abnormal density area in the presacral region might be performed technically using an image-guided transperineal approach [19,20].
To solidify the relationship between FUO and the abnormal density area in the presacral region on CT images, a larger patient cohort needs to be sampled and additional imaging modalities are employed. Our pilot study provides a proof of concept to proceed further into this correlation and reveal more features that will aid in the treatment and diagnosis of FUO.
We report a unique morphology and composition from CT findings in the presacral region of patients with fever of unknown origin (FUO). Our study is the first to report these changes and therefore develop a nomenclature to facilitate their application in clinical diagnostics.
Written informed consent for the publication of this report and accompanying images were obtained from all patients.
The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
All relevant data are within the paper and its supporting information files.
The author would like to thank Enago (www.enago.com) for manuscript review and editing support.
The authors declare that they have no competing interest.
- Meyers MA, Charnsangavej C, Oliphant M (2011) Meyers’ dynamic radiology of the abdomen. normal and pathological anatomy. Sixth edition. New York, PA: Springer-Verlag.
- Mirilas P, Skandalakis JE (2010) Surgical anatomy of the retroperitoneal spaces part II: the architecture of the retroperitoneal space. Am Surg 76: 33-42.
- Chingkoe CM, Jahed A, Loreto MP (2015) Retroperitoneal fasciitis: spectrum of CT findings in the abdomen and pelvis. RadioGraphics 35: 1095-1107. [Crossref]
- Patel N, Maturen KE, Kaza RK (2016) Imaging of presacral masses-a multidisciplinary approach. Br J Radiol 89: 20150698. [Crossref]
- Hain KS, Pickhardt PF, Lubner MG (2013) Presacral masses: Multimodality imaging of multidisciplinary space. RadioGraphics 33: 1145-1167. [Crossref]
- Rajiah P, Shinha R, Cuevas C (2011) Imaging of uncommon retroperitoneal masses. RadioGraphics 31: 949-976.
- Zhang C, Ding ZH, Li GX (2010) Perirectal fascia and spaces: annular distribution pattern around the mesorectum. Dis Colon Rectum 53: 1315-1322.
- Jin Z, Peng JY, Zhu QC (2011) Waldeyer’s fascia: anatomical location and relationship to neighboring fasciae in retrorectal space. Surg Radiol Anat 33: 851-854. [Crossref]
- Herold A, Lehur PA, Matzel KE (2017) Coloproctology: Berlin Heidelberg: Springer-Verlag 2017.
- Minaya-Bravo AM, Merino EO, Diaz-Alonso M (2017) Tumor of retrorectal space: A rare entity scarcely known by general surgeons. J Univer Surg 5: 1-7.
- Maeda E, Mori Y, Amano E (2010) A case of the complications following glycerin enema which suggested malignant hyperthermia. Masui 59: 914-917. [Crossref]
- Wijdicks EFM (2019) Neuroleptic malignant syndrome. May 31, 2019. Available at: https://www.uptodate.com/contents/neuroleptic-malignant-syndrome.
- Miller ML (2019) Clinical manifestations and diagnosis of rhabdomyolysis. Sep 27, 2019. Available at: https://www.uptodate.com/contents/clinical-manifestations-and-diagnosis-of-rhabdomyolysis.
- Cardin SP, Martin JG, Saad-Magalhães C (2015) Clinical and laboratory description of a series of cases of acute viral myositis. J Pediatr 91: 442-447.
- Viswanathan C, Truong MT, Sagebiel TL, et al. Abdominal and pelvic complications of Nonoperative oncologic therapy. RadioGraphics 34: 941-961.
- Watanabe M, Sugimura K, Kuroda S (1995) CT assessment of postirradiation changes in the rectum and perirectal region. Clin Imaging 19: 182-187.
- Scallan J, Huxley VH, Korthuis RJ (2010) Capillary Fluid Exchange: Regulation, Function, and Pathology. San Rafael (CA): Morgan & Claypool Life Science.
- Georga S, Exadaktylou P, Petrou I (2020) Diagnostic value of 18F-FDG-PET/CT in patients with FUO. J Clin Med 9: 2112.
- Zhao N, Li Q, Cui J (2018) CT-guided special approaches of drainage for intraabdominal and pelvic abscesses: One single center’s experience and review of literature. Medicine 97: 42. [Crossref]
- De Kok BM, Marinelli AWKS, Puylaert JBCM (2019) Image-guided posterior transperineal drainage for presacral abscess: An analysis of 21 patients. Diagn Interv Imaging 100: 77-83. [Crossref]
Editorial Information
Editor-in-Chief
Yi-Hwa Liu
Yale University
Article Type
Case Series
Publication history
Received date: January 02, 2021
Accepted date: January 15, 2021
Published date: January 18, 2021
Copyright
©2021 Takeda H. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation
Hiroyuki Takeda, Eizi Hatakeyama, Shigenari Kin, Toshiya Yoshida and Shigeru Fujii (2021) Abnormal density area in the presacral region of patients with fever of unknown origin. Radiol Diagn Imaging,1: doi: 10.15761/RDI.1000172




