Abstract
HIV-associated nephropathy (HIVAN) is an aggressive kidney disease and remains an important contributor to morbidity and mortality among HIV-1-infected patients. HIV-1 mRNA has been identified in kidney epithelial cells and HIV-1 protein has been shown to be associated with epithelial cell dysregulation. HIV-1 transgenic mice exhibit characteristic morphologic features of HIVAN; nevertheless, morphologic evidence of HIV viral particles in the glomerular epithelial cells has not been reported. We present a case of HIVAN where the kidney biopsy showed accumulation of virions in visceral epithelial cells. The findings indicate that HIV can infect, survive and replicate in the glomerular epithelial cells, and in some patients, rapid replication of the virus may result in accumulation of viral particles in the epithelial cells.
Introduction
HIV-associated nephropathy (HIVAN) is an aggressive kidney disease. It remains an important contributor to morbidity and mortality among HIV-1-infected patients [1]. HIVAN is characterized by the collapse of the glomerular tufts, epithelial cell (podocyte) hypertrophy/hyperplasia and diffuse foot process effacement with concurrent tubular microcystic dilatation and tubulointerstitial nephritis [2]. These morphological changes suggest that viral infection in the kidney is directly related to renal epithelial cell injury.
Early studies indicated that HIV infection of T lymphocytes was mediated through CXCR4/CCR5 chemokine-receptors. The epithelial cells were felt to be non-susceptible to direct HIV viral infection due to lack of these chemokine-receptors in cell surfaces; therefore, renal epithelial cell injury was not considered to be due to direct viral infection [3], and rather secondary to cytokines released by activated lymphocytes and/or circulating viral proteins, such as p120, that act as a cytokine to induce cellular injury and apoptosis [4, 5].
Further studies from both human and animals suggested that renal parenchymal epithelial cells could be directly infected by HIV [6]. Infected tubular cells serve as a virus reservoir in kidney allografts in transplanted patients [7]. In-situ hybridization studies have indicated that HIV-1 viral mRNA is present in both tubular and glomerular epithelial cells [6, 8]. These findings implied that infection and active viral reproduction occur in kidney parenchymal cells. In an HIV-1 transgenic animal model, HIV protein expression induced epithelial cell dysregulation via mitotic cascade and resulted in typical HIVAN-like collapsing glomerulopathy and cystic tubulopathy [9]. However, to our knowledge, there has not been morphological evidence to support the hypothesis that HIV infection results in accumulation of viral particles in glomerular epithelial cells in both human kidney biopsies or in animal models, although viral mRNA has been identified in the renal epithelial cells.
In this report we describe kidney biopsy findings from an HIVAN patient and have provided ultrastructural evidence of accumulation HIV viral particles in the injured glomerular epithelial cells.
Clinical presentation
Patient is a thirty year old male with newly diagnosed HIVAN and acute renal failure. He presented with fatigue, acute renal failure and nephrotic range proteinuria. Laboratory examinations revealed positive serology for HIV and low CD4 count (below 50/ml). Serologic tests were negative for hepatitis panel, ANA and anti-dsDNA. His serum creatinine was elevated (2.0 mg/dl) and 24 hour urine protein was 16 grams.
Pathological findings
The renal biopsy specimen was prepared for light microscopy, immunofluorescence and electron microscopy following routinely laboratory protocols used for handling medical kidney diseases.
In the light microscopic specimen, twenty-four glomeruli were present. Ten of them were globally sclerosed. Collapse of capillary tufts associated with hypertrophy and hyperplasia of overlying epithelial cells was noted in most viable glomeruli. Usual type segmental glomerulosclerosis with solid and hyalinized capillary tufts and adhesions to Bowman’s capsule was also present. In the interstitium there was a diffuse inflammatory infiltration. Cystically dilated tubules were seen in the cortex (Figures 1A and 1B). Immunofluorescence examination was negative for all reactants IgG, IgA, IgM, C3, C1q, albumin, fibrin, kappa and lambda light chains) in glomeruli, vasculature and interstitium.
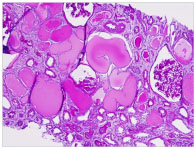
Figure 1A. Low power view of PAS stained section reviews multiple cystically dilated tubules with protein casts. Glomeruli show retraction of capillary tufts and closing of loops.
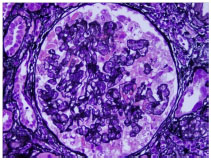
Figure 1B. Silver stain highlights collapse of capillary tufts associated with hypertrophy/ hyperplasia of overlying epithelial cells.
In the ultrastructural examination, there was diffuse effacement of foot process of visceral epithelial cell. The capillary tufts showed collapse of capillary lumina and wrinkling of glomerular basement membranes. In the overlying visceral epithelial cells, the nuclei were slightly enlarged and the cytoplasm appeared fragmented. Multiple electron dense protein droplets were seen in the cytoplasm in some podocytes (Figures 2A, 2B). Numerous viral particles were identified in a paranuclear region, in intact as well as fragmented podocytes (Figures 2C, 2D). The shape and size of viral particles were similar to previously described in HIV-infected lymphocytes and astrocytes.
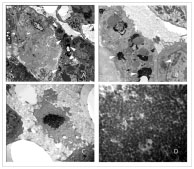
Figure 2A. Transmission electron microscopic photos shows segmentally closed capillary loops and overlying hypertrophic epithelial cells. Electron dense viral particles are seen in the cytoplasm of epithelial cells (arrows). 2B. Proliferative epithelial cells contain protein droplets and aggregated viral particles (arrows).
2C. The epithelial cells show fragmented cytoplasm that contains aggregated viral particles.
2D. High power view of packed viral particles.
Discussion
In the kidney, HIV infection disrupts multiple cellular pathways in both glomerular and tubular epithelial cells resulting in the classical pathological changes characterized as collapsing glomerulopathy and tubular microcystic changes [2].
Results from human and animal studies indicated that HIV-1 virus may reproduce and persistently remain in renal glomerular epithelial cells [8]. These epithelial cells may even serve as viral reservoir in transplanted kidneys [7]. In transgenic animal model, HIV-1 gene products were responsible for epithelial cell dysregulation that resulted in typical collapsing glomerulopathy and cystic tubulopathy [8, 9]. Because renal epithelial cells do not express the primary HIV receptor or main coreceptors, the mechanism by which HIV-1 enters into the cell remains unclear. Indirectly entering epithelial cells through interaction with infected T lymphocytes or dendritic cells is possible.
Although studies have proven that HIV-1 mRNA is present in glomerular epithelial cells and viral proteins are critical for dysregulation of epithelial cells, there is no documented morphological evidence showing presence and accumulation of viral particles in the glomerular epithelial cells. It is possible that even a low level of viral load and viral protein will induce epithelial dysregulation and significant cellular injury; therefore, exam prior to forming a large amount of viral particles that could be recognized by electron microscopy, epithelial cells could be severely injured.
It has been proven that genetic factors play a significant role in the development of collapsing glomerulopathy [10]. HIVAN shares a common pathogenic mechanism with idiopathic collapsing glomerulopathy [8, 11,12]. Certain HIVAN patients may be more genetically susceptible to viral infection and rapid viral replication that lead to accumulation of recognizable viral particles in epithelial cells, as we have described in this case.
In summary, we have presented an unusual case of HIVAN. Kidney biopsy from this patient showed accumulation of HIV viral particles in the injured glomerular epithelial cells. This finding indicates that HIV can infect, survive and rapidly replicate in the glomerular epithelial cells. Productive infection and accumulation of HIV viral particles in the epithelial cells is a rare finding. Genetic predisposition for viral infection and rapid replication may promote accumulation of virus in these cells.
References
- Banu SG, Banu SS, Saleh FM (2013) HIV-associated nephropathy (HIVAN): a short review of different authors. Mymensingh Med J 22: 613-617. [Crossref]
- Bourgoignie JJ, Pardo V (1991) The nephropathology in human immunodeficiency virus (HIV-1) infection. Kidney Int Suppl 35: S19-23. [Crossref]
- Bleul CC, Wu L, Hoxie JA, Springer TA, Mackay CR (1997) The HIV coreceptors CXCR4 and CCR5 are differentially expressed and regulated on human T lymphocytes. Proc Natl Acad Sci USA 94 :1925-30. [Crossref]
- Barbiano di Belgioj2021 Copyright OAT. All rights reservni C, Bertoli S, et al. (1990) Absence of HIV antigens in renal tissue from patients with HIV-associated nephropathy. Nephrol Dial Transplant 5: 489-92.
- Bruggeman LA, Thomson MM, Nelson PJ, Kopp JB, Rappaport J, et al. (1994) Patterns of HIV-1 mRNA expression in transgenic mice are tissue-dependent. Virology 202: 940–948. [Crossref]
- Kapasi AA, Patel G, Franki N, Singhal PC (2002) HIV-1 gp120-induced tubular epithelial cell apoptosis is medicated through p38-MARK phosphorylation. Mol Med 8: 676-85. [Crossref]
- Bruggeman LA, Ross MD, Tanji N, Cara A, Dikman S, et al. (2000) Renal epithelium is a previously unrecognized site of HIV-1 infection. J Am Soc Nephrol 11: 2079-208. [Crossref]
- Canaud G. Dejucq-Rainsford N, Avettand-Fenoel V, Viard JP, Anglicheau D, Bienaimé F, et al. (2014) The kidney as a reservoir for HIV-1 after renal transplantation. J Am Soc Nephrol 25: 407-19. [Crossref]
- Marras D, Bruggeman LA, Gao F, Tanji N, Mansukhani MM, et al. (2002) Replication and compartmentalization of HIV-1 in kidney epithelium of patients with HIV-associated nephropathy. Nat Med 8: 522-526. [Crossref]
- Albaqumi M, Barison L (2008) Current views on collapsing glomerulopathy. J Am Soc Nephrol 19: 1276-81.
- Cantor ES, Kimmel PL, Bosch JP (1991) Effect of race on expression of acquired immunodeficiency syndrome-associated nephropathy. Arch Intern Med 151:125-128. [Crossref]
- Kaufman L, Collins SE, Klotman PE (2010) The pathogenesis of HIV-associated nephropathy. Adv Chronic Kidney Dis 17: 36-43. [Crossref]



