Binding of drugs with human serum albumin (HSA) for transport is a known phenomenon [1]. However, the clinical relevance of study of such phenomenon is recently reviewed. Apart from importance of HSA binding for free plasma concentration of a particular drug therapeutic application of drug HSA interaction is currently developed. Extracorporeal albumin dialysis of endogenous toxins that are produced in hepatic failure is successfully done. Drug overdose particularly that have a narrow therapeutic index is a candidate for therapeutic application of such albumin dependent dialysis and removal of the overdosed drugs [2].
It is suggested that it is not clinically much relevant to consider the HSA binding with drugs that exhibit a wide therapeutic index and algorithms are developed for drug developers in that regard [3]. In some situations, like for therapeutic drug monitoring protein binding studies may be necessary with drugs [4]. However, it is believed by all authorities that in case of narrow therapeutic index drugs knowledge of HSA binding with the drug is vitally important. Generally, for such class of drug the details of plasma protein binding are available in the literature. We have noted one exception in this regard that we wish to highlight in this communication.
The drug, Orceprenaline is a moderately selective β2 – adrenergic receptor agoinst and is also known as Metaproterenol. The structure of the drug is depicted in (Figure 1) which is designed by using the tool PubChem Sketcher V2.4 and this drug belongs to class of organic compounds known as Resorcinols. This drug contains a benzene ring having hydroxyl group at position 1 and 3.
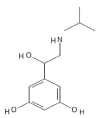
Figure 1. Structure of Orciprenaline
It is a synthetic amine and structurally similar to Isoproterenol. It is used as bronchodilator and in treatment of bronchospasms, asthma, chronic bronchitis and emphysema [5]. The action of this drug is mediated by cAMP and it is believed to work by activating adenylatecyclase. Increased cAMP levels in cytoplasm leads to relaxation of bronchial smooth muscle. It also stimulates G protein coupled receptor in smooth muscle of lungs, uterus and vasculature and has no effect on alpha- adrenergic receptors.
The absorption of Orciprenaline takes place from Gastrointestinal tract [6]. Till date no information is present related to formation of glucuronide conjugates of this drug. It is not metabolised by catechol- O- methyltransferase. The clearance of this drug is through urinary excretion mainly as metabolite. Given in a sustained released formulation the drug condenses with endogenous formalin and a part of it is excreted unchanged [7]. The side effects of this drug include mainly problems of cardiac arrhythmias [8]. Due to lack of protein (HSA) binding information, it is unclear how the drug gets transported at the site of action.
Orciprenaline has been known to have narrow therapeutic index and thus, complete information regarding its interaction with proteins (or albumin binding) is clinically relevant [9]. This drug is available in the form of tablet as Orciprenaline sulphate and freely available in the market in various nations (Figure 2). However, it has been considered for banning due to its abuse potential [10]. The drug binding with human serum albumin is important as it help to know about the pharmacokinetic and pharmacodynamics of the drug. To best of our knowledge it’s protein binding is only reported in one study [11]. However, no protein binding or specifically albumin binding is reported in Drug Bank till date [12]. Thus, it is need of an hour to study interaction of HSA with orciprenaline and report it to public databases.
Figure 2. Orciprenaline Sulphate tablets manufactured by Zydus Healthcare Ltd.obtained from a registered drug shop at Chandigarh, India
References
- Beardshaw J, MacLean L, Chan-Yeung M (1974) Comparison of the bronchodilator and cardiac effects of hydroxyphenylorciprenaline and orciprenaline. Chest 65: 507-11. [Crossref]
- Bosak A, Gazi Smilovi I, Sinko G, Vinkovic V, Kovarik Z. (2012) Metaproterenol, isoproterenol, and their bisdimethylcarbamate derivatives as human cholinesterase inhibitors. Journal of medicinal chemistry 55: 6716-23. [Crossref]
- DRUGBANK [Internet] (2005) Canada: DRUGBANK; [cited on 2018 Mar 3]. Available from: https://www.drugbank.ca.
- FDA List [Internet] (1988) Pennsylvania Generic Substitution Law; [cited on 2018 Mar 3]. Available from: https://apps.health.pa.gov/pdf/ddc/newNTI2015.pdf .
- Fitch KD (1986) The use of anti-asthmatic drugs. Sports Medicine 3:136-50.
- Gilfrich HJ, Ober KF, Forster HJ, Rominger KL (1979) Plasma levels, renal excretion and metabolism of orciprenaline after administration in sustained-release form (author's transl). Arzneimittel-Forschung 29: 967-70. [Crossref]
- Hatch F, McKellop K, Hansen G, MacGregor T (1986) Relative bioavailability of metaproterenol in humans utilizing a single dose, stable isotope approach. Journal of pharmaceutical sciences 75: 886-90. [Crossref]
- Kumar D, Banerjee D (2017) Methods of albumin estimation in clinical biochemistry: Past, present, and future. ClinicaChimicaActa 469: 150-60. [Crossref]
- McElnay JC, D’Arcy PF (1983) Protein binding displacement interactions and their clinical importance. Drugs 25(5): 495-513. [Crossref]
- PubChem [Internet] (2005) Bethesda, USA: PubChem; [cited 2018 Mar 5] Available from: https://pubchem.ncbi.nlm.nih.gov
- Rolan PE (1994) Plasma protein binding displacement interactions—why are they still regarded as clinically important? British journal of clinical pharmacology 37: 125-8. [Crossref]
- Yamasaki K, Chuang VT, Maruyama T, Otagiri M (2013) Albumin–drug interaction and its clinical implication. BiochimicaetBiophysicaActa (BBA)-General Subjects 1830: 5435-43. [Crossref]


