Allium sativum, a medicinal plant is reported with antioxidant ability. However, toxicity has also been reported, which warranted this investigation into its effects on the medial prefrontal cortex of adult male Wistar rats. A toxicity test of the extract on mice was carried out, and thereafter 24 male Wistar rats were divided into 4 groups (n = 6); the control group, and 78 mg/kg, 156 mg/kg and 312 mg/kg Allium sativum extract groups. The administration was orally lasting for 14 days. On day 15, the animals were anaesthetized with 50 mg/kg ketamine hydrochloride (i.p), and perfusion-fixed. The medial prefrontal cortex was then processed for histology and some immunohistochemical studies. Results showed that LD50 of Allium sativum extract was approximately 650 mg/kg body weight. The prefrontal cortical sections showed adverse Nissl substance distribution with the presence of dark nuclei and significantly (p<0.05) higher Nissl staining in the 312 mg/kg group. Enolase-2 and GFAP were expressed more in the 312 mg/kg group, all indicating brain tissue damage processes. In conclusion, Allium sativum causes alterations in cellular integrity, and the expression of enolase-2 and GFAP in the prefrontal cortex, whose effects were dose dependent.
Allium sativum, Medial prefrontal cortex, Histology, Immunohistochemistry, Wistar rat
Herbs are considered very important in all cultures because of it medicinal roles [1]. This is further buttressed by the belief that drugs from plant extracts are generally less harmful with little or no side effects unlike the synthetic ones [2]. Studies on green tea, milk thistle, red grapes and turmeric revealed that these plants possess anti-inflammatory properties, and are also thought to be anti-carcinogenic and anti-mutagenic due to the presence of polyphenols; a naturally occurring compound found in them [3,4]. Another widely investigated herbal plant is Allium sativum; commonly known as garlic.
Allium sativum is a spicy flavoring agent widely cultivated and consumed as food in many countries and has been widely used as a popular remedy for various disorders for thousands of years [5]. In local Nigerian languages it is called ayim mbakara in Ibibio, ayuu in Ibo and ayo in Yoruba. Garlic belongs to the Allium family, which also constitute onions, shallots and leeks. Its preparations are available commercially in the form of garlic oil, garlic powder and pills, and are widely used for certain therapeutic purposes. This is based on the various therapeutic functions of the plant including its role as a protective agent against the deleterious effects of metal poisoning [4,6].
Studies have also shown that garlic serves as an anti-diabetic drug where it significantly reduces serum glucose in streptozotocin-induced diabetic rats while increasing serum insulin in non-diabetic rats [7]. It also reduces total cholesterol, triglycerides, urea and uric acid in serum [7,8]. Garlic modulate the activity of transferases and cytochrome P450 isozymes, both in vitro and in vivo [9], plays an anti-canceric role in colon and prostate cancers [10,11], as well as colorectal adenomas [12], and is also a potent analgesic and anti-nociceptive agent [13].
In the brain, studies have shown that garlic serves as a neuro-protective and neuro-rescue agent against reactive oxygen species [14], improves spatial memory [15], protects hippocampal neurons against lead-induced neural damage [16], prevents memory impairment [17], neuroprotects against traumatic brain injury [18] and also improve visual memory and attention [19]. Notably, these actions of garlic are carried out via its antioxidant, anti-apoptotic and anti-atherogenic properties [20].
Despite these numerous beneficial roles of garlic, there are reports that consumption of garlic can lead to perturbations on Sertoli cell junctions and dilatation of rough endoplasmic reticulum which can further lead to apoptosis in testicular germ cells [21]. It also causes allergic reactions, alteration of platelet function and coagulation [22], and alteration of cellular architecture of such organs as the heart, liver, kidney and stomach mucosa [23-25].
It is also reported that Allium sativum affects cognitive processes and cause cellular changes in the medial prefrontal cortex [26,27]. However, what changes could be affected? This therefore led to this study on the effects of administration of Allium sativum on the prefrontal cortex of adult Wistar rats.
Twenty four adult male albino Wistar rats of body weights 150 g -180 g were obtained and housed in 14 cages (40 cm × 35 cm) of 3 rats each in the Animal House facility of the Faculty of Health Sciences, University of Uyo, Uyo, Nigeria. The animals were allowed 12 hours light and 12 hours dark cycles at 27oC -30oC room temperature. They were fed standard rat pelletized diet (Grand Cereals Ltd, Nigeria) and water ad libitum. Ethical approval was obtained from the Faculty of Basic Medical Sciences Ethics Committee and handling of the animals followed the guidelines of National Institute of Health of the United States. The rats were randomly divided into groups 1-4 (n = 6). Group 1 served as the control and groups 2-4 were the test groups (Table 1).
Table 1. Groupings of the experimental animals
Groups
(n=6)
|
Dosage
|
Duration (Days) |
1 (Control) |
Distilled water (5ml/kg) |
28 |
2 |
78 mg/kg Allium sativum extract |
14
|
3 |
156 mg mg/kg Allium sativum extract |
14
|
4 |
312 mg/kg Allium sativum extract |
14
|
Preparation of the extract
Fresh garlic bulbs were obtained from a local market in Uyo, Akwa Ibom State of Nigeria, and was identified and authenticated by the Curator of the University of Uyo Herbarium. Each fresh clove was peeled, pounded with mortar and pestle and blended with a blender (Century, China). Extraction of garlic components ensued by suspending the pulp in 60% ethanol for 30 minutes after which it was evaporated in a water bath at 40oC to reduce water content without losing the needed components. This was followed by reconstitution in distilled water; after which it was preserved in the refrigerator at 4oC.
Lethal dose determination
The up and down method was used in the determination of the lethal dose (LD50). This method does not use death of the animal as end point but an estimate of LD50 is derived by an increase or decrease in dosage of the extract administered to the animal one at a time. For this research, 21 albino mice were used (3 mice per dosage). First administered dosage was 400 mg/kg, and was steadily increased by 50 mg/kg after 2 days if each mouse survived the initial dosage. The highest administered dosage was 700 mg/kg. The surviving animals were monitored for delayed death for a total of 7 days and they were observed for physical signs of toxicity such as writhing, gasping, decreased respiratory rate, body limb and death [28,29].
Administration of the garlic extract
Group 1 which was the control received 5ml of distilled water per kilogram body weight (kg b.w.) as placebo, while groups 2, 3 and 4 received 78 mg, 156 mg and 312 mg of Allium sativum extract per kg b.w., respectively for 14 days. Oral administration of the garlic extract and water was done in the mornings (8-9 am).
Termination of the experiment
On day 15, the animals were anaesthetized intraperitoneally with 50 mg of ketamine hydrochloride per kg b.w. (Rotex Medica, Germany), and immediately followed with intracardial perfusion of phosphate-buffered saline (1M, pH 7.35) via their thoraco-abdominal walls, and perfusion-fixed with 10 % neutral buffered formalin. The brains of the animals were then removed and post-fixed in 10 % neutral buffered formalin for 48 hours. The prefrontal cortex is located anteriorly in the frontal lobe and is defined as its agranular part [30]. Serial sections of the medial prefrontal cortex were processed for histology by the Cresyl fast violet method and immunolabelled with anti-enolase and glial fibrillary acidic protein.
Briefly, the tissues were routinely processed for paraffin wax embedding. 10 µm thick paraffin sections on slides were then routinely processed for histomorphology with Cresyl violet staining. Representative serial paraffin sections on slides were brought to water and antigen retrieval was performed using citrate buffer (pH 6.0) in a microwave oven for 5 minutes, followed by protein block using 3% hydrogen peroxide for 10 minutes. Sections were thereafter preincubated in 2% normal goat serum for 30 minutes and incubated for an hour at room temperature (20-23oC) each in monoclonal mouse anti-enolase-2 (Novocastra, Leica Biosystems, 22C9, 1:100) for neuron specific enolase and mouse monoclonal anti GFAP (Novocastra, Leica Biosystems, NCL-L-GFAP-GA5, 1:100) for GFAP, followed by incubation in goat anti-mouse secondary antibody (1:100) for an hour. Detection of reaction was by means of the avidin-biotin complex with diaminobenzidine as the chromogen. Sections were then counterstained with Harris haematoxylin, dehydrated, cleared and cover slipped with DPX. Processed slides were viewed under the light microscope (Olympus) and photomicrographs obtained using a computer assisted digital microscope’s camera (Amscope).
Cellular density was determined manually by means of ImageJ software. Briefly, images of the whole prefrontal cortex was obtained for each section and randomly mapped with the ImageJ gridlines. Counting of cell nuclei was done manually taking into consideration the nuclei on the upper and right borders of the mapped areas.
Statistical analysis
One way analysis of variance was used to analyze all the data, followed by a post hoc Tukey’s test. All analysis was done using Graphpad Prism for Windows (version 5.01, San Diego California, USA). Data at probability level P < 0.05 was regarded as significant and are presented as Mean ± Standard Error of Mean.
Lethal dose (LD50) Determination
From this investigation, using the up and down method, the LD50 of Allium sativum on mice was estimated to be 650 mg/kg (Table 2).
Table 2. Lethal dose (LD50) determination result
Average weight
(g)
|
Dosage
(mg/kg) |
Remark |
22 |
400 |
Survived |
22 |
450 |
Survived |
21 |
500 |
Survived |
22 |
550 |
Survived |
22 |
600 |
Survived |
21 |
650 |
Died |
22 |
700 |
Died |
Histology
Sections of the medial prefrontal cortex of the control group showed well-stained Nissl substance throughout the cortical layers (outer granular, outer pyramidal and inner granular layers). In comparison with the control group, the medial prefrontal cortex of the 78 mg/kg Allium sativum extract group also showed well-stained Nissl substance throughout the cortical layers. The 158 mg/kg Allium sativum group showed well-stained Nissl substance in some of the neurons throughout the cortical layers compared with the control group, while the 312 mg/kg Allium sativum had well-stained Nissl substance with small size and significantly (p < 0.05) higher population compared with the 78 mg/kg Allium sativum and the control groups. However, there was no difference in population between the 158 mg/kg Allium sativum group and the control group (Figure 1 and 2).
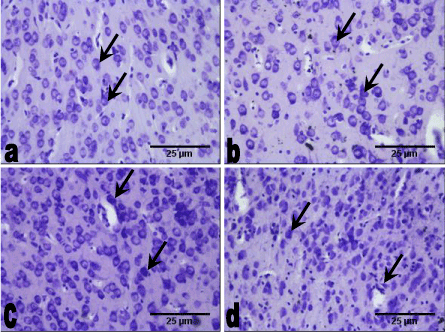
Figure 1. Sections of the medial prefrontal cortex at the end of the experimental period (Cresyl fast violet, × 200).
a. The control group animals with well-stained Nissl substance (arrow) in the cortical layers.
b. The 78 mg/kg 14 days group showing well-stained Nissl substance (arrow) throughout the cortical layers.
c. The 156 mg/kg 14 days group with well-stained Nissl substance (arrow) in throughout the cortical layers with high density.
d. The 312 mg/kg 14 days group with well-stained Nissl substance (arrow), having high density with reduce size.
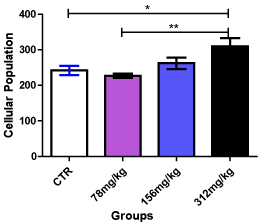
Figure 2. Cellular population in the prefrontal cortex after 14 days administration of Allium sativum
* - Significantly different from the control group at p < 0.05
** - Significantly different from the 312 mg/kg Allium sativum group at p < 0.01
Immunohistochemistry
Neuron Specific Enolase (NSE): The section of the medial prefrontal cortex of the control group animals showed the neurons with less expression of enolase-2 throughout the cortical layers. The groups that received 78 mg/kg and 156 mg/kg of Allium sativum extract also showed less expression of enolase-2 throughout the cortical layers, while 312 mg/kg group showed increased expression of the Allium sativum extract compared with the control group (Figure 3).
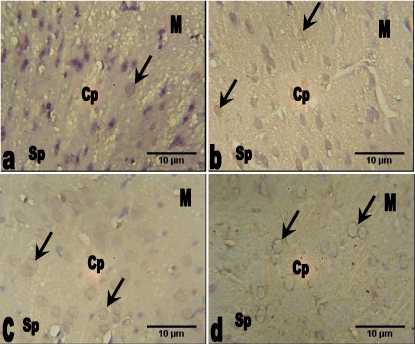
Figure 3. Photomicrograph of the sections of the prefrontal cortex of the control and groups 2, 3 and 4 animals (neuron specific enolase, NSE, Mag. ×400).
a. The medial prefrontal cortex of the control group showing the neurons with little expression of enolase-2 (arrow) throughout the cortical layers.
b. The medial prefrontal cortex of 78 mg.kg Allium sativum group showing the neurons with little expression of enolase-2 (arrow) throughout the cortical layers.
c. The medial prefrontal cortex of the 156 mg/kg Allium sativum group showing the neurons with little expression of enolase-2 (arrow) throughout the cortical layers.
d. The medial prefrontal cortex of the 312 mg.kg Allium sativum group showing the neurons with increase expression of enolase-2 (arrow) throughout the cortical layers.
Glial Fibrillary Acidic Protein (GFAP): The section of the prefrontal cortex of the control group animals showed less expression of glial fibrillary acidic protein (GFAP) throughout the cortical layers. This was also observed in the group that received 78 mg/kg Allium sativum extract compared with the control group. However groups 3 and 4 that received 156 mg/kg and 312 mg/kg Allium sativum extract showed slight increase expression of GFAP especially in the astrocytic processes throughout the cortical layers compared with the control group (Figure 4).
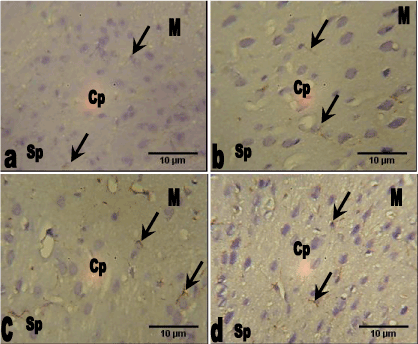
Figure 4. Photomicrograph of the sections of the medial prefrontal cortex of the control and groups 2, 3 and 4 animals (GFAP, Mag. ×400).
a. The section of the medial prefrontal cortex of the control group animals showing little expression of glial fibrillary acidic protein (GFAP) (arrow) throughout the cortical layers.
b. The medial prefrontal cortex of the 78 mg/kg Allium sativum animals showing little expression of GFAP (arrow) throughout the cortical layers.
c. The medial prefrontal cortex of the 156 mg/kg Allium sativum animals showing slight increase expression of GFAP (arrow) throughout the cortical layers.
d. The medial prefrontal cortex of the 312 mg/kg Allium sativum animals showing slight increase expression of GFAP (arrow) especially in the astrocytic processes throughout the cortical layers.
The effects of sub-chronic administration of Allium sativum extract on the medial prefrontal cortex of adult male Wistar rats were investigated in this study. The study focused on the histology arrangement of Nissl and some structural proteins in the outer granular, outer pyramidal and inner granular layers of the medial prefrontal cortex.
The acute toxicity of Allium sativum result showed that Allium sativum had an oral median lethal dosage of 650 mg/kg in mice, an indication that Allium sativum is very toxic to the body if taken at this dose. This report differs from previous studies [31-33], probably due to the Allium specie type or its constituents, or the animal species or route of administration. This is because the route of administration, the specie type of the plant and the specie of the animal is known to differ in toxicity exposure [34]. This toxic property corroborates already reported toxic effects of Allium sativum in different tissues of the body [21,23-27].
The Nissl substance results revealed no difference in staining intensity in the test groups compared with the control. There was a higher cellular population in the 312 mg/kg Allium sativum group. As there was no difference in Nissl substance, this indicates that the given doses of Allium sativum may not affect Nissl distribution. Nissl substances are rough endoplasmic reticulums that stain due to the presence of the anionic macromolecules, DNA and ribosomal RNA, thus, serves as site for protein synthesis [35]. Hence, the general protein synthesizing ability of these cells may not have been affected.
Nissl technique stains the entire population of neurons and glial cell types in the same section [36-38]. Thus, a higher cellular population is indicative of proliferation of these cells, which may be physiological or pathological [39]. This increase may be due to gliosis as the prefrontal cortex is not known to undergo adult neurogenesis. Gliosis usually results as a protective mechanism for the neural tissues [40], and this may have been the case in the present study. The prefrontal cortex of the 78 mg/kg Allium group did not show a difference with the control, an indication that this dosage and duration did not adversely affect Nissl distribution.
The 312 mg/kg Allium sativum group showed an increase in the expression of enolase-2, while the rest of the test groups showed less expression of the protein compared with the control group. Neuron-specific enolase plays a role in the survival of neurons and can provide neuroprotective effects via binding to neurons in a calcium-dependent manner [41]. Increased NSE expression indicates neuronal damage [42], which ultimately may lead to neuronal cell death. The increase in the expression of enolase-2 in the 312 mg/kg Allium group is an indication of the damage to the neurons of the prefrontal cortex of this group. This result supports the dark nuclei observed in the Nissl stain study of this same group. The prefrontal cortex of the 78 mg/kg Allium group did not show apparent difference with the control, an indication that this dosage and duration did not adversely affect enolase-2 expression.
Allium sativum extract caused alterations in the expression of glial fibrillary acidic protein (GFAP). GFAP expression was observed in the prefrontal cortex of the test groups, which was higher in the 156 mg/kg and 312 mg/kg Allium sativum groups compared with the control group. Increased expression of GFAP indicates astroglial activation and gliosis, often resulting from neurodegeneration [43], due to the astrocytes need to mop-up the resultant debris formed [44]. This may be a reason for the increased expression of GFAP in these test groups. Although the expression of GFAP in the present study appear protective, improper GFAP regulation has been linked to various disorders and injuries which can cause glial cells to react in detrimental ways; for example, glial scarring which is partially caused by up-regulation of GFAP [45,46], further providing an enabling environment for neurodegeneration. The prefrontal cortex of the 78 mg/kg Allium group did not show apparent difference with the control, an indication that this dosage and duration did not adversely affect GFAP expression.
The prefrontal cortex of the brain is involved in cognitive processes, and damage or improper regulation of its cellular and structural proteins may adversely affect the prefrontal cortical functions [47,48]. As Allium sativum extract, especially the high dose group showed some neurodegenerative processes, it may therefore alter this function, which may trigger other consequences.
The administration of ethanolic extract of Allium sativum did not influence Nissl distribution, but cause cellular proliferation and increased NSE and GFAP expressions of prefrontal cortex in the high dosage groups, which may indicate neurodegenerative processes. Although the specific neurons affected cannot be elucidated, further research is necessary.
The staff of the animal house facility of the Faculty of Basic Medical Sciences are hereby acknowledged for their assistance throughout the course of the experiment.
Authors funded the research, and hereby declare no conflict of interest.
- Wargovich MJ, Woods C, Hollis DM, Zander ME (2001) Herbals, cancer prevention and health. J Nutr 131: 3034S-6S. [Crossref]
- Pari L, Umamaheswari J (2000) Antihyperglycaemic activity of Musa sapientum flowers: effect on lipid peroxidation in alloxan diabetic rats. Phytother Res 14:136-138. [Crossref]
- Manna SK, Mukhopadhyay A, Van NT, Aggarival BB (1999) Silymarin suppresses tnf-induced activation of nf-a b:c-jun n-terminal kinase and apoptosis. J Immunol 163: 6800-6809. [Crossref]
- Weisburger JH, Rivernson A, Aliaga C, Reinhardt J, Kelloff GJ, et al. (2013) Amelioration of aluminium induced toxicity by Allium sativum. Sci Res Essays 8: 168-177.
- Zargari A (1997) Medicinal Plant. Tehran: Tehran University Press, Iran 1.
- Sharma V, Sharma A,2021 Copyright OAT. All rights reservdministration of Allium sativum extracts on lead nitrate induced toxicity in male mice. Food Chem Toxicol 48: 928-936. [Crossref]
- Eidi A, Eidi M, Esmaeili E (2006) Antidiabetic effect of garlic (allium sativum l.) in normal and streptozotocin-induced diabetic rats. Phytomedicine 13: 624-629. [Crossref]
- Warshafsky S, Kamer RS, Sivak SL (1993) Effect of garlic on total serum cholesterol. A meta-analysis. Ann Intern Med 119: 599-605. [Crossref]
- Ameen M, Musthapa MS, Abidi P, Ahmad I, Rahman Q (2003) Garlic attenuates chrysotile-mediated pulmonary toxicity in rats by altering the phase 1 and phase 11 drug metabolizing enzyme system. Biochem Mol Toxicol 17: 366-371. [Crossref]
- Dong X, Karen L, Young-Ae K, Yan Z, Eun-Ryeong H, et al. (2006) Diallyl trisulfide suppresses growth of PC-3 human prostate cancer xenograft in vivo in association with BAX and BAK induction. Clin Cancer Res 12: 6836. [Crossref]
- Takefumi K, Keiji H, Hideki I, Nariaki M, Shin-ichiro S, et al. (2006) Aged garlic extract has chemopreventative effects on 1,2-dimethylhydrazine-induced colon tumors in rats. J Nutr 136: 847S-851S. [Crossref]
- Shinji T, Ken H, Masaharu Y, Goro K, Kazuya K, et al. (2006) Aged garlic extract has potential suppressive effect on colorectal adenomas in humans. J Nutr 136: 821S-826S. [Crossref]
- Jayanthi MK, Jyoti MB (2012) Experimental animal studies on analgesic and anti-nociceptive activity of Allium sativum (Garlic) powder. Potent analgesic and anti-nociceptive agent. IJRRMS 2.
- Ray B, Chauhan NB, Lahiri DK (2011) Oxidative insults to neurons and synapse are prevented by aged garlic extract and S-allyl-L-cysteine treatment in the neuronal culture and APP-Tg mouse model. J Neurochem 117: 388-402. [Crossref]
- Moriguchi T, Saito H, Nishiyama N (1996) Aged garlic extract prolongs longevity and improves spatial memory deficit in senescence-accelerated mouse. Biol Pharm Bull 19 :305-307. [Crossref]
- Sadeghi A, Ebrahimzadeh Bideskan A, Alipour F, Fazel A, Haghir H (2013) The Effect of Ascorbic Acid and Garlic Administration on Lead-Induced Neural Damage in Rat Offspring's Hippocampus. Iran J Basic Med Sci 16: 157-164. [Crossref]
- Mukherjee D, Banerjee S (2013) Learning and memory promoting effects of crude garlic extract. Indian J Exp Biol 51: 1094-1100. [Crossref]
- Chen W, Qi J, Feng F, Wang MD, Bao G, et al. (2014) Neuroprotective effect of allicin against traumatic brain injury via Akt/endothelial nitric oxide synthase pathway-mediated anti-inflammatory and anti-oxidative activities. Neurochem Int 68: 28-37. [Crossref]
- Tasnim S, Haque PS, Bari MS, Hossain MM, Islam SMA, et al. (2015) Allium sativum L. improves visual memory and attention in healthy human volunteers. Evid Based Complement Alternat Med 2015. [Crossref]
- Mathew B, Biju R (2008) Neuroprotective effects of garlic a review. Libyan J Med 3: 23-33. [Crossref]
- Hammami I, Nahdi A, Atig F, El May A, El May MV (2016) Garlic (Allium sativum) feeding impairs Sertoli cell junctional proteins in male Wistar rat testis: microscopy study. Andrologia 48: 1281-1288. [Crossref]
- Borrelli F, Capasso R, Izzo AA (2007) Garlic (Allium sativum L.): adverse effects and drug interactions in humans. Mol Nutr Food Res 51: 1386-1397. [Crossref]
- Hoshino T, Kashimoto N, Kasuga S (2001) Effects of garlic preparations on the gastrointestinal mucosa. J Nutr 131: 1109S-13S. [Crossref]
- Banerjee SK, Maulik M, Mancahanda SC, Dinda AK, Gupta SK, et al. (2002) Dose-dependent induction of endogenous antioxidants in rat heart by chronic administration of garlic. Life Sci 70: 1509-1518. [Crossref]
- Ambati S. (2013) Garlic derivatives: Role in obesity and related disorders. Biotech 2: 1.
- Muonagolu NJ, Ekong MB (2016) Allium sativum alters the cyto-architecture of the Medial prefrontal cortex and neurobehaviour of adult Wistar rats. Nig J Neurosci 7: 53-58.
- Muonagolu NJ, Ekong MB, Edagha IA (2017) Chronic Allium sativum administration alters spontaneous alternation and cyto-architecture of medial prefrontal cortex of adult Wistar rats. Internet J Med Update 12: 9-14.
- Deora PS, Mishra CK, Mavani P, Asha R, Shrivastava B (2010) Effective alternative methods of ld50 help to save number of experimental animals. J Chem Pharmaceut Res 2: 450- 453.
- Ekong MB, Peter AI, Ekanem TB, Osim EE (2015) Determination of elemental composition and median lethal dose of calabash chalk. Int J Biol Med Res 6: 4902-4906.
- Van De Werd HJJM, Rajkowska G, Evers P, Uylings HBM (2010) Cytoarchitectonic and chemoarchitectonic characterization of the prefrontal cortical areas in the mouse. Brain Struct Funct 214: 339-353. [Crossref]
- Nakagawa S, Masamoto K, Sumiyoshi H, Harada H (1984) Acute toxicity test of garlic extract. J Toxicol Sci 9: 57-60. [Crossref]
- Joseph PK, Rao KR, Sundaresh CS (1989) Toxic effects of garlic extract and garlic oil in rats. Indian J Exp Biol 27: 977-979. [Crossref]
- Mikail HG (2010) Phytochemical screening, elemental analysis and acute toxicity of aqueous extract of Allium sativum L. bulbs in experimental rabbits. J Med Plant Res 4: 322-326.
- Petersen KP (1980) Effect of age and route of administration on LD50 of lithium chloride in the rat. Acta Pharmacol Toxicol (Copenh) 47: 351-354. [Crossref]
- Byrne JH, Heidelberger R, Waxham MN (eds) (2014) From Molecules to Networks: An Introduction to Cellular and Molecular Neuroscience. 3rd (edn) (revised) San Diego: Academic Press, USA.
- Christensen JR, Larsen KB, Lisanby SH, Scalia J, Arango V, et al. (2007) Neocortical and hippocampal neuron and glial cell numbers in the rhesus monkey. Anat Rec (Hoboken) 290: 330-340. [Crossref]
- Miller DJ, Balaram P, Young NA, Kaas JH (2014) Three counting methods agree on cell and neuron number in chimpanzee primary visual cortex. Front Neuroanat 8: 36. [Crossref]
- García-Cabezas MÁ, John YJ, Barbas H, Zikopoulos B (2016) Distinction of Neurons, Glia and Endothelial Cells in the Cerebral Cortex: An Algorithm Based on Cytological Features. Front Neuroanat 10: 107. [Crossref]
- Rubin E, Reisner HM (2009) Essentials of Rubin's Pathology. 5th (edn) Philadelphia: Lippincott Williams and Wilkins, USA.
- Pekny M, Nilsson M (2005) Astrocyte activation and reactive gliosis. Glia 50: 427-434. [Crossref]
- Cooper EH (1994) Neuron-specific enolase. Int J Biol Markers 9: 205-210. [Crossref]
- Krohn M, Dreßler J, Bauer M, Schober K, Franke H et al. (2015) Immunohistochemical investigation of S100 and NSE in cases of traumatic brain injury and its application for survival time determination. J Neurotrauma 32: 430-440. [Crossref]
- Brahmachari S, Fung YK, Pahan K (2006) Induction of glial fibrillary acidic protein expression in astrocytes by nitric oxide. J Neurosci 26: 4930-4939. [Crossref]
- Mázló M, Gasz B, Szigeti A, Zsombok A, Gallyas F (2014) Debris of “dark” (compacted) neurons are removed from an otherwise undamaged environment mainly by astrocytes via blood vessels. J Neurocytol 33: 557-567. [Crossref]
- Block E, Ahmad S, Jain MK, Crecely RW, Apitz-Castro R, et al. (1984) (E, Z)-Ajoene: A potent antithrombic agent from garlic. J Am Chem Soc 106: 8295-8296.
- Smith ME, Eng LF (1987) Glial fibrillary acidic protein in chronic relapsing experimental allergic encephalomyelitis in SJL/J mice. J Neurosci Res 18: 203- 208. [Crossref]
- Siddiqui SV, Chatterjee U, Kumar D, Siddiqui A, Goyal N (2008) Neuropsychology of prefrontal cortex. Indian J Psychiatry 50: 202-208. [Crossref]
- Puig MV, Jonas R, Schmidt R, Freund N (2014) Dopamine modulation of learning and memory in the prefrontal cortex: insights from studies in primates, rodents and birds. Front Neural Circuits 8: 93. [Crossref]




