Background: New artificial transplants for reconstruction of longer defects in the urinary tract are still needed. The amnion membrane provides a natural, water-impermeable and mechanically stable basal membrane with several incorporated cell growth promoting factors. Therefore, amnion could represent promising components for scaffolds in artificial urinary transplants. The aim of our study was to define suitable cell and tissue culture conditions for application of amnion membrane (AM) in biological constructs for reconstruction of the urinary tract.
Methods: Several cell culture conditions were examined for the proper isolation of fibroblasts and urothelial cells from porcine urinary bladder. After successful cell culture we seeded both cell types on native and de-epithelized AM. After 15 and 30 days we analyzed cell formation by HE and immunofluorescence staining.
Results: Cultivation of 1cm² tissue specimens with supplemented DMEM-10%FBS led to a positive selection of urothelial cells whereas RPMI with 10%FBS was the medium of choice for successful isolation of fibroblasts. At 15-days, decellularized AM showed insufficient cellular attachment on the major parts of the surface. In native amniotic membranes however, urothelial cells and fibroblasts developed a well differentiated multi- layer cell formation after seeding.
Conclusion: AM is a suitable membrane, giving urothelial cells and fibroblasts ideal conditions. In our experiments, native AM showed better characteristics for cell growing, compared to decellularized AM.
amnion, tissue engineering, urology, urothelial cells
AM: Amniotic membrane; AMG: Medicinal Products Act; BSA: Bovine Serum Albumin; DMEM: Dulbeccos modified Eagles medium; EDTA: Ethylenediaminetetraacetic acid; EGF: Epithelial Growth Factor; FBS: Fetal bovine serum; HE: Hematoxylin and Eosin; IF: Immunofluorescence; Pen/Strep: Penicillin/streptomycin; PBS: Phosphate buffered saline; REM: Renal epithelial medium; RPMI: Roswell park memorial institute; RT: Room Temperature.
One of the main challenges in Urology is the reconstruction of the urinary tract. Up to date, complex surgical interventions are required to reconstruct long strictures and defects in the ureter or urethra. Open resection and reanastomosis represents a successful surgical procedure, which however is limited to short-sized stricture defects [1]. Surgical treatments for longer strictures using adjoining tissue like bowel segments are still in elaboration [2].
In the past 20 years, new surgical attempts for reconstruction of the urethra (involving the use of free transplants) have been developed. Oral mucosa and epidermal skin are the most commonly used tissues for augmentation. Due to the easy application and the post-operative success rate, the transplantation of free buccal mucosa grafts into the affected area has been established over the years [3-5]. Nevertheless, complications at donor site after treatment including dysfunctions in the face mimic and food ingestion require the development of alternative strategies for the reconstruction of the urinary tract [6-9].
In the past decade, tissue engineering has risen as a new discipline in regenerative medicine. Among others, a potential application of this technique in urological issues has come into the focus. The probably most relevant prerequisites for the successful extracorporal engineering of functional tissues are - first - an appropriate seeding cell population and - second - a suitable matrix (also called scaffold) to be seeded and subsequently populated by cells.
Different matrices have been used in several animal trials with urological scopes [10-14]. As an example of natural scaffolds, venous grafts populated with urothelial cells have been used [15-17]. Although showing promising first line results, subsequent studies in minipigs uncovered a significant limitation of the application of veins as matrices due to stricture formation in long time follow up [18].
Numerous studies including animal- and patient trials have revealed amnion membrane (AM) to be a hopeful candidate for the reconstruction of several tissue entities, such as blood vessels and skin [19-21]. The application of AM also shows promising perspectives for the reconstruction of ureter defects; probably due to the AM-genuine capacity to induce self-regeneration and anti-inflammation in neighboring cells [22]. Apart of this, the amniotic epithelia and its basal lamina display a tight and (water-) impermeable architecture and thus have the capacity to separate distinct intracorporal compartments -a characteristic which predisposes this scaffold for the application in urologic organs like urethra or bladder.
Cell culture: Urothelial cells and fibroblasts were isolated from porcine bladder tissue taken from minipigs. Briefly, bladder was processed and cut into small pieces and placed in a 6-well plate. Pairs of these tissue-samples were then overlayed with one of the following culture media: Dulbecco´s Modified Eagles Medium (DMEM) plus 10% fetal bovine serum (FBS), Roswell Park Memorial Institute (RPMI) medium plus 10% FBS (GibcoTM, Carlsbad, California) or Renal Epithelial Medium (REM) supplemented with Triiodo-L-thyronine, Epithelial Growth Factor (EGF), Insulin, Hydrocortisone and Transferrin (all from PromoCell, Heidelberg, Germany). At a confluence of approximatley 70%, the primary cell cultures were trypsinated and further cultured with their corresponding media. Cells were frozen at first and second passage or subcultivated for subsequent cell-type verification by means of Immunfluorescence (IF).
De-epithelization of amnion: Human AM was kindly shared by the transplant-tissue bank of the Insitute of forensic medicine of the University Medical Center Hamburg-Eppendorf (UKE), Germany. AM was transported to the transplant-tissue bank and prepared for kryo-preservation (aiming subsequent transplantation) according to the German Medicinal Products Act and Transplantation Law (legal permission according to §20b, c and §21a AMG) applying conventional methods (Wilhelm, Duncker Bredehorn: Augenbanken, Walter de Gruyter, Berlin / NewYork 2002). AM-preparations consisted of 1.5 x 1.5 cm-squares of AM (bound on nylon-membranes) in 10-ml falcon-vials filled with cryo-media (1:1 mixtures of RPMI (+ Penicilline and Streptomycin (Pen/Strep), Amphotericin B and Gycerol]) and were stored at -80°C. According to the requirements of the responsible ethical committee, only non-transplantable AM was used for this study at informed consent of the donors.
24h before scaffold cell-seeding, half of the AM-preparations was decellularized. For that purpose, the 1.5 x 1.5 cm AM squares were pulled of the nylon-membranes, rinsed in phosphate buffered saline (PBS) and further incubated in a de-epithelization solution (0,5% Sodiumdeoxycholate-0,02% Ethylenediaminetetraacetic acid (EDTA) with Proteaseinhibitor) for 1h at 4°C. Subsequently, a 45min- Dispase II (1,2 U/ml)-step at 37°C was followed by scraping AM until cell layer was released. After a
3h-incubation of the AM pieces with a Desoxyribonuclease (DNAse) and Ribonuclease (RNAse) solution (1µl/ml and 20µl/ml, respectively), membranes were kept in PBS with Pen/Strep until further use.
Cylinder test: At time of scaffold (re-)population, the main challenge was to concentrate cell population in order to increase cell-settle and -anchorage effectivity. Since gain of cells was limited by the nature of the biopsy and the cell culture itself, we aimed to address this challenge by increasing cell concentration at the repopulation phase by the use of several structures. For this purpose, cell culture cylinder (1cm- diameter) and rings from silicon, zinc, titan or glass (Figure 1) were used. After application of these different cylinders on amniotic membranes, cell seeding and subsequent culturing for several time points were followed.
Figure 1. Application of glass rings on amniotic membranes to increase cell concentration
(Re-)population of decellularized amnion: 24h after AM de-epithelization, decellularized AM were seeded with 2,5x105 fibroblasts in RPMI 10%FBS per amnion-square and cultured for 48h. Subsequently, 5x105 urothelial cells were seeded on these AM preparations (already seeded with fibroblasts) and further cultured in DMEM 10%FBS. Unseeded AM were considered as negative controls. Medium was changed every two days. After 15 days or 1 month samples were harvested, embedded with Tissue- Tek® O.C.T.™ (Sakura®, Alphen aan den Rijn, Netherlands) and kept at -80°C. Frozen AM blocks were processed by means of a cryostat at a thickness of 7µm. The resulting specimens were air-dried on an object slide.
(Re-)population of native amnion: Native AM was seeded with fibroblasts and urothelial cells, analyzed and compared for functionality as described above.
Hematoxylin and eosin (HE) staining: Morphology and structure of the diverse amniotic samples were analyzed by HE staining of cryo-slides. Briefly, specimens were fixed with aceton and further incubated with Hematoxylin. After rinsing AM slices with water, an incubation-step with eosin followed. Finally, samples were treated with a rising alcohol-row, xylol and covered with Eukitt® mounting medium (Kindler, Freiburg, Germany).
Immunfluorescence (IF) staining: IF staining allowed the identification and verification of several cultivated cell types. For this purpose, 1·105 cells from first or second passage were cultivated with either RPMI 10%FBS or DMEM 10%FBS in 12-well plates on rounded cover slides. At a confluence of about 70%, cells were 2x washed with PBS and fixed with cold Methanol for at least 15min. Subsequently, a 1h-blocking step (1% Bovine Serum Albumin (BSA) in PBS) was followed by an overnight- incubation with a first-antibody (see below) in washing buffer solution (0,5% BSA, 0.05% Tween20 in PBS) at 4°C. After 2x washing with washing buffer (0,5% BSA,0,05% Tween20 in PBS), cover slides were incubated with secondary antibody for 1h at room temperature (RT), further undergoing a multiple washing-row. Finally, nuclei-staining with DAPI was followed by covering the cells with Vectashield® mounting medium (Vector, Burlingame, California) on an object slide.
For the recognition of urothelial cells, anti-cytokeratin 7 antibody (1:200, Abcam, Cambridge, UK) was used. Anti-vimentin antibody (1:600, Abcam, Cambridge, UK) detects fibroblasts whereas myofibroblasts double-stained for vimentin and α-actin (the latest at 1:300, Abcam, Cambridge, UK). Secondary antibodies were conjugated with either Cy2 or Cy3 (Dianova, Hamburg, Germany).
Analysis of cell growth and morphology in cell cultures using several culture media: Cultivation of porcine bladder biopsies with REM resulted in a complete lack of cell outgrowing. In biopsies cultured in DMEM 10%FBS and RPMI 10%FBS, outgrowth of diverse single cells was already observed after 4-6 days cultivation. Whereas urothelial-morphology was more prevalent in DMEM 10%FBS - feeded cultures, RPMI 10%FBS induced more heterogenic cell populations with high cell rate and homogenous fibroblast pattern (Figure 2).
Figure 2. Microscopic view of cells growing out of porcine bladder biopsies cultured in RPMI 10%FBS for about 10 days. Microscopically augmentation: x10
Cell isolation, subcultivation and characterisation of either urothelial cells or fibroblasts by the use of cell-specific culture medium: After about 10 days biopsy culture and a cell-confluence of around 70%, cells were further subcultivated for cell type identification by means of IF. Cytokeratin 7 is a protein member of the keratin family found in the transitional epithelium lining the cavities of the internal organs, above others, and thus may represent a suitable marker for urothelial cells detection. On the other hand, a standard marker used for the recognition of fibroblasts is vimentin, an intermediate filament protein of the cytoskeleton, specifically expressed in mesenchymal cells. As shown in Figure 3A, biopsies cultivated with DMEM 10%FBS forwarded selection of urothelial cells since about 90% of them were positive for cytokeratin 7. These cells presented a typical filamentous pattern with a heterogeneously distributed signal above the population. Cells from porcine bladder biopsies cultivated with RPMI 10%FBS demonstrated a clearly positive and homogeneous vimentin staining (Figure 3B), typical for fibroblasts.
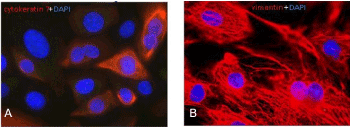
Figure 3. Detection of cytokeratin 7 and vimentin in primary porcine bladder cells. Second passage cells were cultivated for 6 days and consequently analyzed for cytokeratin 7 (red, A) and vimentin (red, B). DAPI (blue) staining was used in order to counterstain the nuclei. Microscopically augmentation: x63
Application of glass cylinders ensures cell viability compared to other constructs in cylinder test: Ring-formed constructs from silicon, zinc and titan were initially used but rapidly showed relevant disadvantages concerning manipulation of the rings when applicating on the amniotic membrane. After a short period of time, high cell mortality was observed. The use of glass cylinders offered an optimal leak-tightness and easy manipulation as well as they did not seem to influence cell culture conditions.
Native amnion shows better predisposition to multilayer-cell formation compared to decellularized amnion: Native and decellularized AM samples were seeded first with fibroblasts and subsequently with urothelial cells. At the 30-day time-point, decellularized AM showed insufficient cellular attachment, whereas native AM developed a multi-layer cell growth (Figure 4).
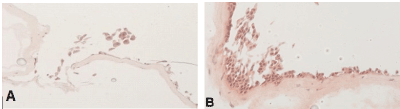
Figure 4. Decellularized AM (A) and native AM (B) were seeded with fibroblasts and urothelial cells and analyzed by HE staining after 30 days cultivation. Microscopically augmentation, x20
It is our primary aim to develop an artificial transplant with urothelial- like characteristics. This graft should be applicable in every location of the urinary tract and usable as a free transplant. Finding the perfect scaffold, different artificial scaffolds and autologous matrices - which are predisposed to avoid cellular immune response and inflammatory reaction - were investigated. The current trend is to combine stable membranes with structures, which enable ideal conditions for cell growth and adhesion. One of these potential structures is human AM. Because of its low immunogenicity and anti- inflammatory properties, it has been used in dermatology, surgery, orthopedics, dental surgery and ophthalmology for tissue substitution [23]. We followed the idea to use AM, which provides suitable conditions to urothelial cells and fibroblasts for settlement and cell growth to speed up building cell layers and cell connection. Additionally, amnion shows appropriate mechanical properties and viscoelasticity as demonstrated by Kikuchi et al. [24]. These characteristics qualify amnion being used in the urinary tract, as they are indispensable in the scenario of a moving ureter or in a dynamic unit like the urinary bladder. The possibility to fold the amnion membrane to generate three-dimensional structures or multilayer membranes seems to be a further advantage using amnion in the urinary tract. Amnion is also extensively used as “living patches” in wet environments as for cornea treatment [25]. Wet conditions are also present in the urinary tract.
We used urothelial cells due of their crucial role as elements of the biological, biochemical and biomechanical border between the bodies´ interior and the urea-containing compartment. The idea to additionally apply fibroblasts in our setting originates in their need for reconstruction and maintenance of an appropriate extracellular matrix. An integral part of this study was to test, whether decellularized amnion membrane has an effect on its colonization by fibroblast and urothelial cells and their cell growth.
Urothelial cells and fibroblasts growing on AM showed regular cell growth and adhesion in our experiments. Our approach to use a collective culture with fibroblasts and urothelial cells followed the idea to simulate original conditions as they exist in the genuine urothelial tract. The common support of different cell types in human tissue seems to be the key to produce conditions for ideal cell growth and cell connection.
To carry on this idea, we were thinking of the concept to decellularise the AM. Leaving the natural located cells in its place to achieve accurately conditions seemed to be beneficial. Therefore, we differentiated between decellularized and native AM. These naïve cells were supposed to create a natural environment for cell growth and proliferation. Although the technique of decellularization becomes more and more applicable [26], non-decellularized membranes did better in our experiments. The native cells seemed to support the urothelial cells and fibroblast which were imported to this tissue setting. Obviously, the surface and the produced messengers can support a quick settlement of transplanted cells.
The combination of membranes giving ideal cell growth conditions and stabile constructs might be the future solution to build a transplant working in every location of the urinary tract. The application of amnion in different surgical disciplines emphasizes this idea, as it has been used for vaginal reconstructive surgery, repair of abdominal hernia, pericardium closure and prevention of surgical adhesions, for example [27]. The possibility of free combination of amnion with different epithelial cells like buccal mucosa [28] gives more space for future projects. Especially urology gives a big field of various applications for amnion-constructs, like using special designed sandwich-constructs with frozen amnion for bladder augmentation [29]. The most suitable location, testing new artificial transplants is the urethra. It`s bigger internal diameter and minor movement compared to the ureter will simplify wound healing and outcome success. Using amnion transplants in the urethra in animal trials is the next step to achieve further knowledge and first analyses present promising results [30]. Further studies have to confirm those findings and have to be followed by long-term follow-ups and larger testing populations.
Amniotic membrane displays suitable characteristics for the use in tissue engineering purposes. The scaffold presents ideal conditions for the growth induction and settle of both urothelial cells and fibroblasts, thus being appropriate for the reconstruction of lesions of the urinary tract. In our experiments, native AM showed better characteristics for cell growing, compared to decellularized AM. Further preclinical and clinical trials have to validate our findings of the qualification of amniotic membrane as free transplants for clinical application.
Conceptualization and Design OE, MF, LPR, AS; formal analysis, LPR, OH, OE, MR; investigation, LPR, OH, LS; resources, OH, LPR; writing—original draft preparation, all authors; writing-review and editing, all authors; project administration, MR, AS, MF. All authors have read and agreed to the published version of the manuscript.”
This research received no external funding.
The authors are grateful for Karin Beutel and Petra Dase from the research lab for developing innovative techniques and improvement of cell culture protocols.
The authors declare no conflict of interest.
The present study was approved by the Institutional Ethics Committee of our institution.
- Engel O, Soave A, Rink M, Fisch M (2016) Reconstructive Management with Urethroplasty. European. Urology Supplements 15: 13-16. [Crossref]
- Levy AC, Vanni AJ (2018) Refractory Urethral Stricture Management: Indications for Alternative Grafts and Flaps. Curr Urol Rep 19: 20. [Crossref]
- Palminteri, Manzoni E, Berdondini G, Di Fiore E, Testa F, et al. (2008) Combined dorsal plus ventral double buccal mucosa graft in bulbar urethral reconstruction. Eur Urol 53: 81-89. [Crossref]
- Xu YM, Feng C, Sa YL, Fu Q, Zhang J, et al. (2014) Outcome of 1-stage urethroplasty using oral mucosal grafts for the treatment of urethral strictures associated with genital lichen sclerosus. Urology 83: 232-236.
- Browne BM, Vanni AJ (2017) Use of alternative techniques and grafts in urethroplasty. Urol Clin North Am 44: 127-140.
- De Kemp V, De G, Fledderus P, Ruud Bosch JO, De Kort JL, et al. (2015) Tissue engineering for human urethral reconstruction: systematic review of recent literature. PLoS One 10: e0118653. [Crossref]
- Barbagli G, Sansalone S, Romano G, Lazzeri M (2011) Controversies in urethral reconstruction. Minerva Urol Nefrol 63: 251-260.
- Tanagho EA (1975) A case against incorporation of bowel segments into the closed urinary system. J Urol 113: 796-802.
- Chung BI, Hamawy KJ, Zinman LN, Libertino JA (2006) The use of bowel for ureteral replacement for complex ureteral reconstruction: long-term results. J Urol 175: 179-183. [Crossref]
- Rotariu, Yohannes P, Alexianu P, Gershbaum M, Pinkashov D, et al. (2002) Reconstruction of rabbit urethra with surgisis small intestinal submucosa. J Endourol 16: 617-620.
- Caione P, Capozza N, Zavaglia D, Palombaro G, Boldrini R, et al. (2006) In vivo bladder regeneration using small intestinal submucosa: experimental study. Pediatr Surg Int 22: 593-599. [Crossref]
- Rossetto JV, da Mota SLS, Rocha NS, Miot HA, Grandi F, et al. (2013) Grafts of porcine small intestinal submucosa seeded with cultured homologous smooth muscle cells for bladder repair in dogs. Acta Vet Scand 55: 39. [Crossref]
- Kropp BP, Cheng EY, Lin HK, Zhang Y (2017) Reliable and reproducible bladder regeneration using unseeded distal small intestinal submucosa. J Urol 172: 1710-1713.
- Palminteri E, Berdondini E, Fusco F, De Nunzio C, Salonia A, et al. (2012) Long-term results of small intestinal submucosa graft in bulbar urethral reconstruction. Urology 79: 695-701. [Crossref]
- Palminteri, Berdondini E, Fusco F, Nunzio CD, Salonia A, et al. (2000) Selective culture conditions for different types of primary human bladder cells. World J Urol 18: 371-375.
- Juarez B, Volkmer M, Gschwend BG, Hautmann JE, Bartsch RE, et al. (2007) Tissue engineered venous matrices for potential applications in the urogenital tract. Tissue Eng 13: 2475-2482.
- Kjaer TB, Nilsson T, Madsen PO (1976) Total replacement of part of the canine urethra with lyophilized vein homografts. Invest Urol 14: 159-161.
- Engel O, Petriconi RD, Volkmer BG, Gust KM, Mani J, et al. (2014) The feasibility of ureteral tissue engineering using autologous veins: an orthotopic animal model with long term results. J Negat Results Biomed 13: 17. [Crossref]
- Amensag S, McFetridge PS (2012) Rolling the human amnion to engineer laminated vascular tissues. Tissue Eng Part C Methods 18: 903-912.
- Yang L, Shirakata Y, Shudou M, Dai X, Tokumaru S, et al. (2006) New skin-equivalent model from de-epithelialized amnion membrane. Cell Tissue Res 326: 69-77. [Crossref]
- Yang L, Shirakata Y, Tokumaru S, Xiuju D, Tohyama M, et al. (2009) Living skin equivalents constructed using human amnions as a matrix. J Dermatol Sci 56: 188-195. [Crossref]
- Koziak A, Salagierski M, Marcheluk A, Szcześniewski R, Sosnowski M, et al. (2007) Early experience in reconstruction of long ureteral strictures with allogenic amniotic membrane. Int J Urol 14: 607-610.
- Mohan R, Bajaj A, Gundappa M (2017) Human Amnion Membrane: Potential Applications in Oral and Periodontal Field. J Int Soc Prev Community Dent 7: 15-21.
- Kikuchi, Feng M, Kosawada Z, Sato T, Nakamura D, et al. (2016) Stress relaxation and stress-strain characteristics of porcine amniotic membrane. Biomed Mater Eng 27: 603-611.
- Fan J, Wang M, Zhong F (2016) Improvement of Amniotic Membrane Method for the Treatment of Corneal Perforation. Biomed Res Int 1693815.
- Francisco JC, Cunha RC, Cardoso MA, Simeoni RB, Mogharbel BF, et al. (2016) Decellularized Amniotic Membrane Scaffold as a Pericardial Substitute: An In Vivo Study. Transplant Proc 48: 2845-2849. [Crossref]
- Favaron PO, Carvalho RC, Borghesi J, Anunciação ARA, Miglino MA, et al. (2015) The Amniotic Membrane: Development and Potential Applications - A Review. Reprod Domest Anim 50: 881-892. [Crossref]
- Qi F, Yoshida T, Koike T, Aizawa H, Shimane T, et al. (2016) Construction and characterization of human oral mucosa equivalent using hyper-dry amniotic membrane as a matrix. Arch Oral Biol 65: 26-34. [Crossref]
- Adamowicz J, Pokrywczyńska M, Tworkiewicz J, Kowalczyk T, Breda SV, et al. (2016) New Amniotic Membrane Based Biocomposite for Future Application in Reconstructive Urology. PLoS One 11: e0146012. [Crossref]
- Wang F, Liu T, Yang L, Zhang G, Liu H, et al. (2014) Urethral reconstruction with tissue-engineered human amniotic scaffold in rabbit urethral injury models. Med Sci Monit 20: 2430-2438. [Crossref]




