The study was designed to detect anticancer activity and proliferative effect of the Hardaliye in HT-29 and CF-1 cell lines, respectively. Based on MTT results, two dilutions namely, five (H5) and ten-fold (H10) were selected for further analyses. Apoptosis and antioxidant pathway related gene expression levels were detected using qRT-PCR and tali apoptosis assay was used to screen percentage levels of death, apoptotic and viable cells after 24h and 48h of treatment.
Results suggested that Hardaliye was effective on cancer cells and trigger apoptosis via increase of Bax (22.45 ± 3.1-fold for H5 and 6.49 ± 0.1-fold for H10) as well as SOD and CAT activity. However, Hardaliye also affected slightly healthy cells when diluted fivefold, though, did not affect when dilute tenfold. According to gene expression levels, an increase at antioxidant enzyme and apoptosis inhibitors was seen after 24 h. On the other hand, antioxidant enzymes apart from GpX of H5, decreased after 48h. Predictably, antioxidant substances of Hardaliye revealed an antioxidant activity over time.
Overall, this was the first report considering anticancer activity of Hardaliye besides its efficacy on healthy cells. In this respect, these preliminary results can indicate potential of Hardaliye as a food support in preventing colon cancer.
apoptosis, cancer prevention, cell biology, colorectal cancer, gene expression, polyphenols
Abbreviations
DR: Death receptor; Fas: Fatty Acid Synthase; XIAP: X-linked inhibitor of apoptosis; BAX: BCL-2-associated X protein; BCL-2: B-cell leukemia 2; Cyct c: Cytochrom c; IAP: Inhibitor of apoptosis protein; HSP: Heat Shock Protein; Sod: Super oxide dismutase; GpX: Glutathion Peroxidase; Casp: Caspase
Cancer therapy is a worldwide concern. Apart from chemical agent, there is research on natural products as well as food components. Accordingly, grape and its derivatives besides mustard seeds are good sources of phenolic compounds such as gallic acid, resveratrol and flavonoids, including catechin, quercetin which are beneficial for a wide variety of diseases as well as cancer. In fact, in vivo studies have shown that phenolic compounds and anthocyanidins diminish growth of colorectal cancer. Feeding mice with grape seed extract which is rich source of anthocyanidins at a dose of 200 mg/kg has been demonstrated to decrease colorectal tumor 44% in volume after 8 weeks of treatment and also induce apoptosis and cell cycle arrest of colon cancer. In addition, in rats fed with resveratrol orally at a dose of 60 mg/kg for 49 days, precancerous colonic lesions decreased [1-4].
Hardaliye is a popular non-alcoholic and traditional beverage of Thrace region produced from grape, mustard seeds, sour cherry leaves and it is fermented by lactic acid bacteria for 5-10 days. Hardaliye is well known with benefits for anemia, cardiac diseases and thought that it is also beneficial for cancer [5-7].
Defects in the apoptosis pathway cause to the development of malignity due to the lack of the programmed cell death which protects cell from effects of mutations and provides physiological growth control as well as tissue homeostasis [8]. Two major apoptosis signaling pathway exist in cells, namely: i) intrinsic which occurs in mitochondria and ii) extrinsic pathway. The latter is induced by death receptors which belong to TNF (Tumor Necrosis Factor) receptor super family such as TNF-α, DR 4, DR 5, Fas (CD 95) [9,10] and afterwards induces activity of caspase 8 and caspase 3 which are initiator and effector caspases, recpectively [11,12]. However, stimuli such as anticancer drugs trigger intrinsic pathway through differentiation of the mitochondrial permeability. Subsequently cytochrome c is released and apoptosome formation is initiated [13]. Also, the intrinsic pathway includes pro-apoptotic and anti-apoptotic proteins belonging to Bcl-2 protein family. Of this family, Bax induces apoptosis by promoting cytochrome c release while Bcl-2 inhibits [14]. Additionally, activation of caspases such as casp-3 and casp-9 is disrupted by inhibitor of apoptosis proteins (IAps) such as c-IAP1 (BIRC2), c-IAP2 (BIRC3), X-linked IAP (XIAP, BIRC4), Survivin (BIRC5) [13,15].
Antioxidant enzymes such as superoxide dismutases (SODs), glutathione peroxidases (GPXs), glutathione transferase (GST) and catalase (CAT) provide a defense against harmful effect of these molecules by converting them to non-toxic molecules. However, antioxidant substances and elements such as selenium and zinc are nonenzymatic antioxidants and they neutralize harmful effect of these free radicals [16,17]. On the other hand, a heatschock protein HSP 70, protects cells in response to adverse effects of stress conditions. However, an overexpression of Hsp 70 is seen in tumor cells and Hsp 70 inhibits apoptosis in both intrinsic and extrinsic pathways. Thereby, Hsp 70 has recently become a marker for prognosis and a pharmological target which its inhibitors have been researched [8-21].
Herein, primarily, we determined the phenolic and metal content of Hardaliye, later on, the effect of the Hardaliye on apoptosis pathway and antioxidant responsive gene expressions of colon cancer cell line (HT-29) as well as proliferative effect on mouse embryonic fibroblast (CF-1) was evaluated. This is the first report on anti-cancer activity of the Hardaliye, although a few studies are available on anticancer effect of grape seed or phenolic compounds on colon cancer or other cancer types [22]. Results of this study demonstrated that Hardaliye reduced viability and triggered apoptosis of HT-29 cancer cells. Hardaliye blocked apoptosis via apoptosis inhibitors in healthy cells and decreased most of oxidative stress related gene expressions.
Chemicals, standards and reagents
Formic acid (98-100%), methanol (Hypergrade LC MS), isopropyl alcohol (2-Propanol) and DMSO were purchased from Merck, Darmstadt, Germany. Ammonium formate (HPLC grade) was from Sigma-Aldrich, Germany. Reference standards; gallic acid, catechin, 2-5 dihydroxybenzoic acid, trans caffeic acid, syringic acid, trans sinapic acid, trans-p-coumaric acid, trans ferulic acid, resveratrol, salicylic acid were from Fluka while protocatechuic acid was purchased from HWI Analytik Gmbh, Germany. MTT (3(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazo-lium-bromide) was purchased from Biomatik Cambridge, Ontario. Phosphate buffer saline (PBS) and molecular biology grade water were from Gibco, Invitrogen, Carlsbad, CA, USA. The resistance of ultra-distilled water used for instrumental analysis was 18.2 MΩ.
Sample preparation
Commercially obtained Hardaliye sample was centrifuged at 15.000 g for 30 min and filtered with a PTFE syringe filter (0.22µm pore size). Hardaliye was diluted twofold with 50% UPW; 25% isopropanol, 25% methanol and mixed with a mixing block for 2h., then, diluted fivefold with mobile phase A. Samples were transferred to amber vials. Analysis was repeated three times and presented as mean ± SD.
Liquid chromatography and mass spectrometry conditions
Phenolic compounds were detected on Agilent 1260 infinity LC in combination with the Agilent 6460 Triple Quadrupole MS/MS System equipped with Jet Stream Electrospray ionization source (Agilent Technologies, Palo Alto, CA, USA). The analytical column was Agilent Poroshell 120 EC-C18 (4.6x50 mm, 2.7 µm particle size) and set at 25oC. Mobile Phase A consisted of UPW, 0.2% ammonium formate (v/v), 0.2% formic acid (v/v). Mobile Phase B consisted of metanol, 0.2% ammonium formate (v/v), 0.2% formic acid (v/v). Flow rate was 0.3 ml/min under ambient temperature. The injection volume was 1µl and the LC gradient conditions were as follows: 0 to 1 min, 70% A, 30% B; 3 to 7 min; 30%, 70%; 9 to10 min. 50% A, 50% B; 11 to 12 min; 70% A, 30% B. The run time was 12 min.
The optimized MS parameters of analysis were as follows: Gas temperature was set at 325oC, the nebulizer gas pressure was set at 45 psi, the nozzle voltage was set at 500V, the capillary monitoring (MRM) was performed in the positive and negative ion mode.
Data acquisition was carried out with Mass Hunter (version B.06.01) software. Collision energy, fragmentor voltage, precursor ion, product ion and polarity were presented in Table 1. Nitrogen (N2) was used as the collision gas 1.12 mTorr.
Calibration standard mixes were prepared in 50% UPW, 25% methanol, 25% isopropanol and at the calibration concentration ranging from 1 to 200 ng/mL.
Metals in hardaliye by inductively coupled plasma-mass spectrometry (ICP-MS)
Hardaliye sample filtered with 0.22µm PTFE syringe filter was diluted hundredfold with UPW and metal content was detected using ICP-MS (Agilent 7700x, Agilent Technologies, USA). Operating conditions of the ICP-MS were as follows: nebulizer gas flow 0.91 L/min, radio frequency (RF) 1200 W, lens voltage 1.6 V, cool gas 13.0 L/min, and auxiliary gas 0.70 L/min.
HT-29 and CF-1 (MEF) cell culture and the treatment of the hardaliye
Human HT-29 cell culture line and Mouse embryonic fibroblast cells (MEF) were purchased from ATCC (Manassas,VA,USA). Cells were initially cultured in 75 cm2 flasks with DMEM (Dulbecco's Modified Eagle's medium) supplemented with 10% FBS and Penicillin/Streptomycin (100 IU/100 µg/ml) (Wisent Inc., Canada) and grown at 37oC in the presence of 5% CO2 until 70-80% confluent. Cells were trypsinized at 37 ̊C, washed with PBS and were seeded in 6- well plates (~106 cell per well) for Tali apoptosis analysis as well as RNA isolation and in 96-well plates (~ 5000-10.000 cell per well) for MTT analysis. Exponentially growing cells were treated with Hardaliye which was filtered with a 0.22 µm sterile syringe filter, by serially diluting 10 to 80-fold for MTT assay, 5 and 10-fold for Tali apoptosis assay and qRT-PCR. Each experiment has been repeated independently at least three times.
MTT cell viability assay
MTT (3(4, 5-dimethylthiazol-2-yl)-2,5-diphenyltetrazo-lium-bromide) is an assay to determine viability of cells with the conversion of the water-soluble MTT to an insoluble purple formazan by respiring cells. MTT assay was carried out after 24 h and 48 h of experiment. 20 µl MTT (5mg/ml in PBS) was added and incubated at 37oC for 4h. Medium with MTT was carefully removed from all wells and the formazan crystals were dissolved with 200 µl of DMSO and incubated at 37oC for 5 min. Absorbance was recorded at 492 nm using MultiscanGo microplate reader (Thermo Scientific, USA). The viability% was calculated [23]. The value that reduces absorbance 50% compared to untreated cells was assessed as IC 50.
Annexin V and PI apoptosis analysis with Tali™ image-based cytometer
To assess apoptosis, cells were harvested with Trypsin/EDTA post-treatment 24h and 48h. Tali apoptosis detection kit (Life Technologies, USA) protocol was followed according to manufacturer's instruction. Cells were stained with annexin-V Alexa Fluor® 488 and PI (propidium iodide), incubated for 20 min and 5 min in the dark, respectively. 25 µl of stained cells were loaded into a Tali Cellular Analysis Slide. The viable, apoptotic and dead cells were analyzed using Tali image-based cytometer. Results were presented as mean ± SD. The results were statistically analyzed after arcsine square root transformation.
Total RNA isolation and cDNA synthesis
All media was removed and 1 ml of lizis buffer was added to cells. Total RNA was isolated according to manufacturer’s instruction with PureLink® RNA Mini Kit (Life Technologies, USA). The RNA concentrations were determined by measuring UV absorbance at 260 nm with NanoQ (Pathtech, Australia). RNAs were equalized to 100 ng/µl and reverse transcription was performed using High Capacity cDNA synthesis kit (Life Technologies, USA) on Veriti 96-Well Thermal Cycler system with the following protocol: step 1, 25oC, 10 min; step 2, 37oC, 120 min; step 3, 85oC, 5 min. RNA was stored at -80oC while cDNA was stored at -20oC for subsequent steps.
Detection of apoptosis and antioxidant related gene expressions using the qRT- PCR (Quantitative real-time PCR)
Expression levels of 19 genes related to apoptosis pathway, namely; tumour suppressor gene p53, apoptosis activator, Bax, apoptosis inhibitors, c-IAP 1, c- IAP2, Survivin (bırc5), XIAP Bcl-2 and cyt c, Dr4, Dr5, Fas, Casp 2, Casp 9, Casp 3 as well as antioxidant related; Sod, Sod 2 (MnSOD), Cat, GpX, HSP 70 (Primers are listed in Table 3) were determined on ABI 7500 Fast qRT-PCR system (Life Technologies, USA) using following protocol: 20 µL reactions containing 0.2 µM each primer (24,25), 10 µL SYBR Select Master Mix (Life Technologies, USA) (2 x) and 1.5 µL template cDNA. After initial denaturation 1x 94 oC for 3 min; amplifications were performed for 40 cycles 94 oC for 45 s; 58 oC for 45 s; and 72 oC 1 min and 1x 72 oC 10 min. GADPH mRNA expressions were used as housekeeping gene for normalization. Relative fold-change of mRNA expression levels were determined by the Comparative CT method (2−ΔΔCT method) (26) and presented as mean ± SD. All reactions were set up in triplicates.
Table 3. Primer list
Primer list related to apoptosis and oxidative stress pathway |
DR4 |
F: CAGAAC GTCCTGGAGCCTGTAAC
R: ATG TCC ATT GCC TGA TTC TTT GTG |
SOD |
F:GTTCGGTGACAACACCAATG
R:GGAGTCGGTGATGTTGACCT |
DR5 |
F: GGGAAGAAGATTCTCCTGAGATGT G
R: ACATTGTCC TCAGCC CCAGGTCG |
SOD2 |
F: CGTCACCGAGGAGAAGTACC
R: CTGATTTGGACAAGCAGCAA |
Fas |
F: TCTAACTTGGGGTGGCTTTGTCTTC
R: GTGTCATACGCTTTCTTTCCAT |
CAT |
F: TACGAGCAGGCCAAGAAGTT
R: ACCTTGTACGGGCAGTTCAC |
BAX |
F: ATGGACGGGTCCGGGGAG
R: TCAGCCCATCTTCTTCCA |
GpX |
F: CCAAGCCTCATCACCTGGTCT
R: TCGATGTCAATGGTCTGGAA |
BCL-2 |
F: CAGCTGCACCTGACG
R: ATGCACCTACCCAGC |
HSP 70 |
F: CTTATCAGTGAAATTAAGCGAGAGC
R: ACAAGGATAACTTCATCAACCTTTG |
CytC |
F: TTTGGATCCAATGGGTGATGTTGAG
R: TTTGAATTCCTCATTAGTAGCTTTTTTGAG |
Survivin |
F: GACGACCCCATAGAGGAACA
R: GACAGAAAGGAAAGCGCAAC |
Caspase-2 |
F: TGGCACTGATGGCAAACTCC
R: ATCGGAGCGTGTAGGCAAAC |
c-IAP 1 |
F: GCATTTTCCCAACTGTCCAT
R: ATTCGAGCTGCATGTGTCTG |
Caspase-3 |
F: GTGGAATTGATGCGTGATGT-3’
R: ACAGGTCCATTTGTTCCAAAA-3 |
c-IAP 2 |
F: ATTTTCCACCACAGGCAAAG
R: GCATTTTCCCAACTGTCCAT |
Caspase-9 |
F: TGTCCTACTCTACTTTCCCAGGTTTT
R: GTGAGCCCACTGCTCAAAGAT |
XIAP |
F: GGGGTTCAGTTTCAAGGAC
R: TGCAACCAGAACCTCAAGTG |
p53 |
F: GCTCTGACTGTACCACCATCC-3′
R: CTCTCGGAACATCTCGAAGCG-3′ |
GAPDH |
F: CGGAGTCAACGGATTTGGTCGTAT-3
R: AGCCTTCTCCATGGTGGTGAAGAC-3 |
Statistical analyses
Differences between control and treatments were analyzed using a statistical software JMP 12.0 (SAS Institute, Cary, NC, USA) with one-way ANOVA followed by Tukey-Kramer multiple comparison test. Differences were considered significant at p < 0.05 and specified with asterix as *** p< 0.001, **p<0.01, *p<0.05.
Phenolic and metal content of hardaliye
Gallic acid and resveratrol, which are naturally occur in grape and grape seed, were the highest in Hardaliye (Table 1). Additionaly, Hardaliye contained notably amount of potassium and magnesium as well as zinc and selenium which are antioxidant metals (Table 2). This was consistent with analyses performed with grape juice (27).
Table 1. Phenolic content in Hardaliye and the basic LC MS/MS parameters. Data are presented as mean ± SD, n = 3
Phenolic Compound |
Concentration (µg/L) |
R2 |
Retention Time (min) |
Transition |
Fragmentor Voltage |
Collision Energy |
Polarity |
Linear Regression Equation |
gallic acid |
6273 ± 93 |
0.9994 |
2.286 |
227.2[143.1] |
127 |
24 |
Negative |
y=60.368200*x+388.226380 |
resveratrol |
2070 ± 260 |
0.9994 |
5.966 |
169.1[125.1] |
88 |
8 |
Negative |
y=13.743032*x+14.968057 |
trans caffeic acid |
1633 ± 70 |
0.9997 |
4.951 |
291.2[139.1] |
93 |
12 |
Positive |
y=327.680539*x+1408.910941 |
catechin |
1438 ± 93 |
0.9997 |
2.519 |
193.1[134.1] |
83 |
32 |
Negative |
y= 467.977964*x+712.899032 |
salicylic acid |
1343 ± 100 |
0.9994 |
6.756 |
153.1[109.1] |
83 |
12 |
Negative |
y=228.229004*x+1137.887913 |
trans-p-coumaric acid |
961 ± 16 |
0.9996 |
5.703 |
179.2[135.1] |
93 |
12 |
Negative |
y=308.850638*x+815.804776 |
protocatechuic acid |
601 ± 25 |
0.9984 |
2.687 |
137.1[93.1] |
83 |
12 |
Negative |
y= 97.497135*x+688.355588 |
2-5 dihydroxybenzoic acid |
582 ± 23 |
0.9996 |
2.463 |
223.2[208] |
101 |
16 |
Negative |
y= 91.923631*x+1176.082779 |
syringic acid |
117 ± 13 |
0.9995 |
5.023 |
163.2[119.1] |
86 |
12 |
Negative |
y=9.810302*x-8.343955 |
trans ferulic acid |
20 ± 1 |
0.9995 |
5.772 |
153.1[108] |
91 |
20 |
Negative |
y=34.812488*x+44.103431 |
trans sinapic acid |
19 ± 6 |
0.9993 |
5.668 |
197.2[182] |
86 |
8 |
Negative |
y=25.763802*x+72.277006 |
Table 2. Metal content in Hardaliye. Data are presented as mean ± SD, n = 3
Metal |
Concentration (µg/L) |
Metal |
Concentration (µg/L) |
Metal |
Concentration (µg/L) |
K |
156656.73 ± 1794 |
Mn |
190.03 ± 5 |
Cr |
12.70 ± 0 |
Mg |
11476.99 ± 261 |
Zn |
131.41 ± 3 |
Ni |
12.6 ± 0 |
Ca |
3132.32 ± 70 |
Sr |
67.20 ± 4 |
Sb |
4.25 ± 1 |
Na |
2128.51 ± 80 |
Se |
26.58 ± 1 |
Co |
2.38 ± 0 |
Fe |
441.20 ± 14 |
Ag |
23.34 ± 2 |
Cd |
0.48 ± 0 |
Al |
234 ± 3 |
Cu |
17.76 ± 0 |
|
|
Efficacy of hardaliye on HT-29 and CF-1 cells
Cell viability and tali assay: The effect of Hardaliye was assessed after 24h and 48h of experiments. According to MTT assay, 10 and 20 fold diluted Hardaliye decreased viability of% cancer cells, namely; 58 ± 3% and 62 ± 5% after 24h while 34 ± 2% and 38 ± 5% after 48h. However, percentage of healthy cells almost did not change, on the contrary, increased 20% after diluted 20, 40 and 80 fold. Correspondingly, according to Annexin V-PI results, the effect of H10 on cancer cells was 47 ± 11% and 26 ± 2% after 24h and 48h, respectively. But, in this method, H5 40% decreased viability of healthy cell even though this rate was 25% for HT-29. In addition, apoptosis level of cancer cells was triggered by Hardaliye while it was close to 1-3% in healthy cells.
IC50 was detected as 10 fold. The other experiments were carried out with 5 and 10 fold diluted Hardaliye which are expressed as H5 and H10 (Figure 1 and 2).
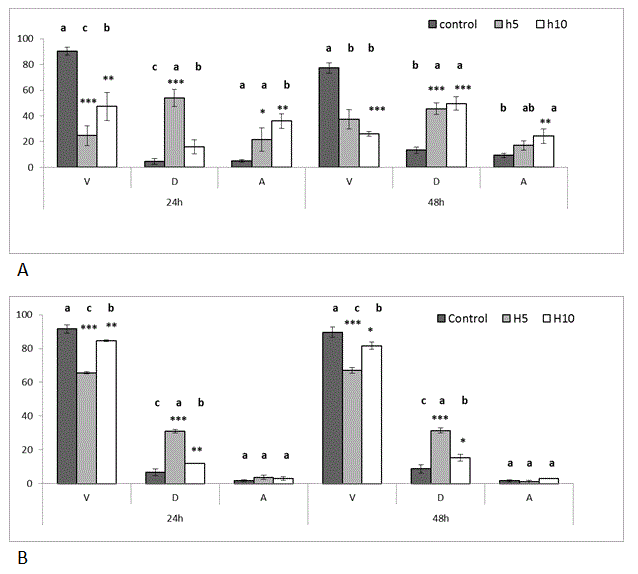
Figure 1. Effect of Hardaliye on HT-29 (a)and CF-1 (b) cell line after 24 h and 48 h of treatment (Tali Apoptosis assay). V: Viable, D: Dead, A: Apoptosis. H5 indicates Hardaliye which is diluted fivefold. H10 indicates Hardaliye which is diluted tenfold. Different letters in superscript indicate statistically significant differences between control and treatments. Data sets were normalized using arcsine square root transformation. Data are presented as mean ± SDand show differences between control and treatments, n = 3: *** p< 0.001, **p<0.01, *p<0.05.
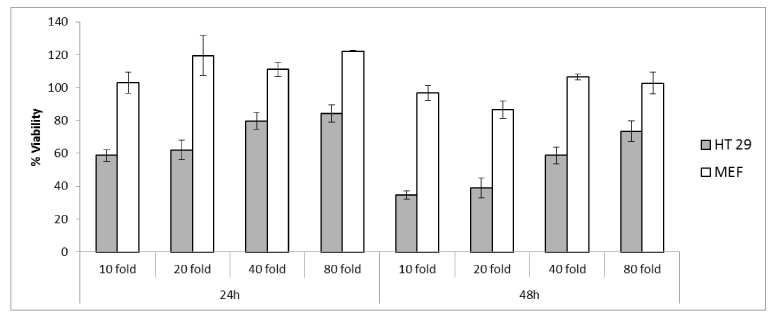
Figure 2. The viability (%) analysis with MTT assay on HT-29 and CF-1 (MEF). Data are presented as mean ± SD, n = 3.
Real time PCR and gene expression of apoptosis and antioxidant markers
The first step was to understand effect of Hardaliye on intrinsic or extrinsic apoptosis pathway markers of HT-29 cells. Following treatment with two dilutions, H10 triggered DR5 2.39 ± 0.36 fold and H5 triggered the FAS 3.39 ± 1.02- fold after 48h. However, for both dilution, Bax increased drastically (22.45 ± 3.1-fold for H5 and 6.49 ± 0.1-fold for H10 after 48h) and H10 triggered casp3 but casp9 and casp2. However, H5 triggered p53 and cyctc levels after 48h. In addition, Bcl-2 also increased but still remained lower than Bax level and other apoptosis inhibitors, namely, Survivin, XIAP, CIAP 1, CIAP 2 remained significantly low (Figure 3 and 5) as compared to control.
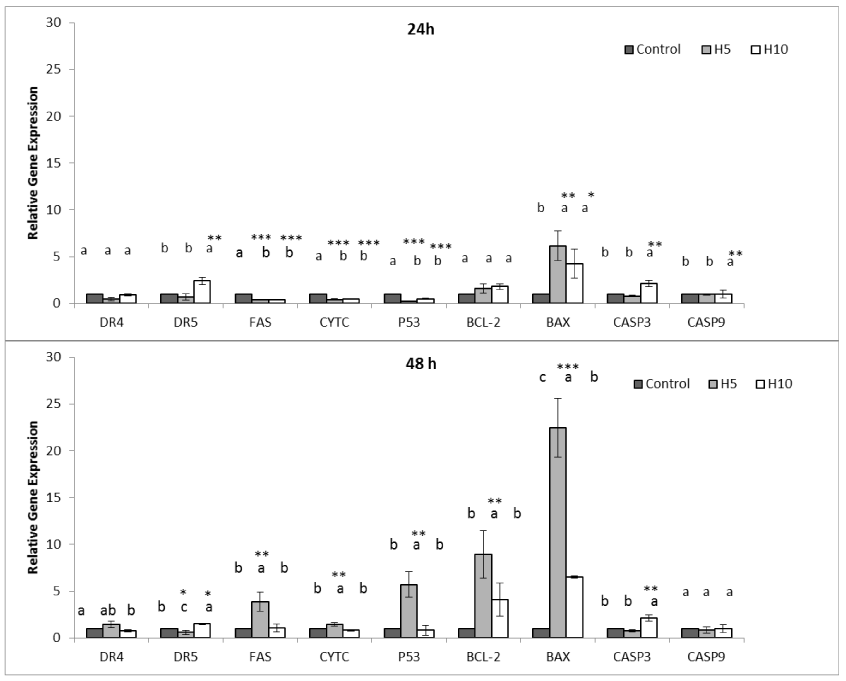
Figure 3. Effect of Hardaliye on apoptosis pathway related relative gene expressions of HT-29 cell line after 24 h and 48 h of treatment. H5 indicates Hardaliye which is diluted fivefold. H10 indicates Hardaliye which is diluted tenfold. Different letters in superscript indicate statistically significant differences. Data are presented as mean ± SD, n = 3: *** p< 0.001, **p<0.01, *p<0.05.
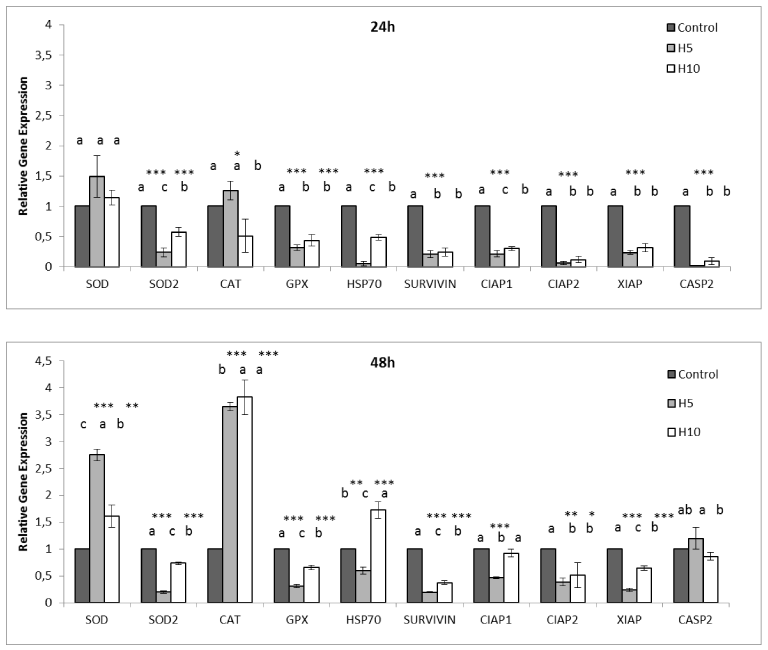
Figure 5. Effect of Hardaliye on apoptosis and antioxidant pathway related relative gene expressions of HT-29 after 24h and 48h of treatment. H5 indicates Hardaliye which is diluted fivefold. H10 indicates Hardaliye which is diluted tenfold. Different letters in superscript indicate statistically significant differences. Data are presented as mean ± SD, n = 3: *** p< 0.001, **p<0.01, *p<0.05.
As for CF-1 cells, Fas; 2.5 ± 0.46-fold, p53; 2.4 ± 0.15-fold, cytc; 2.3 ± 0.15-fold, casp 3; 2.0 ± 0.27-fold, casp2 were expressed after 24h but casp9 when treated with H5. Even though Bcl-2 did not increase, the other apoptosis inhibitors, namely Survivin, XIAP, CIAP 1, CIAP 2 drastically increased. However, following 48h, gene expressions decreased. Conversely, H10 did not trigger DR4, DR5, Fas, cytc, p53, Bcl-2 and Bax but casp 3 and casp 2 after 24h, however, Bcl-2 increased (1.7 ± 0.05-fold) after 48h. The other apoptosis inhibitors also increased while only CIAP 2 and casp 2 slightly decreased (Figure 4 and 6) as compared to respective controls.
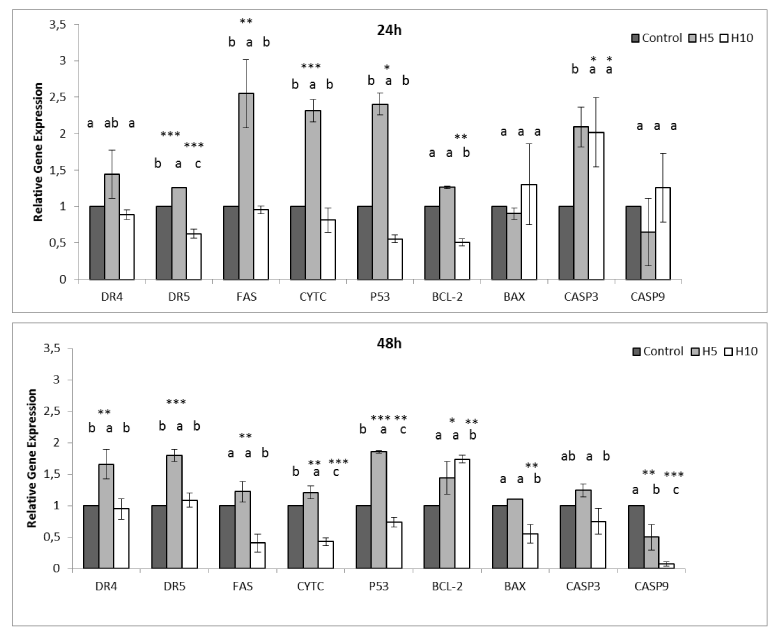
Figure 4. Effectof Hardaliye on apoptosis pathwayrelated relative gene expressions of CF-1 cell line after 24 h and 48 h of treatment. H5 indicates Hardaliye which is diluted fivefold. H10 indicates Hardaliye which is diluted tenfold. Different letters in superscript indicate statistically significant differences.Data are presented as mean ± SD, n = 3: *** p< 0.001, **p<0.01, *p<0.05.
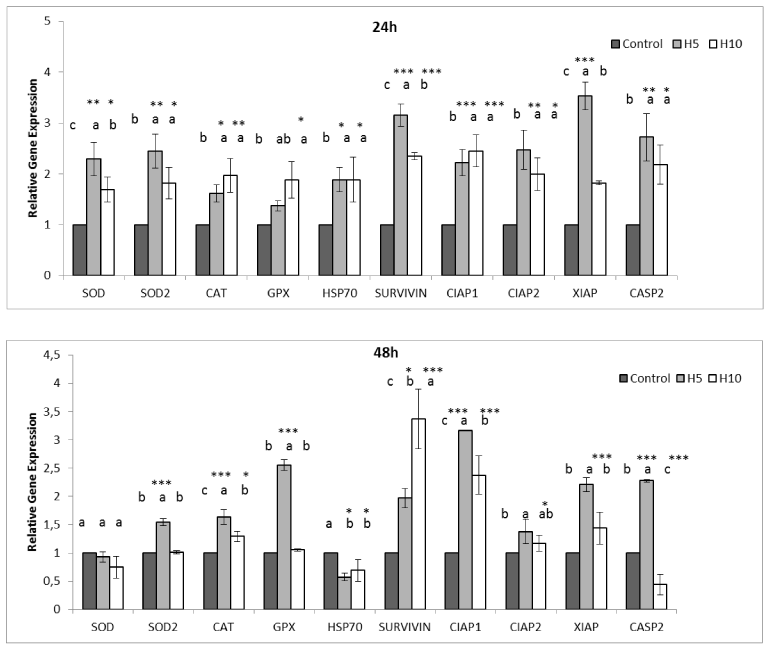
Figure 6. Effect of Hardaliye on apoptosis and antioxidant pathway related relative gene expressions of CF-1 after 24h and 48h treatment. H5 indicates Hardaliye which is diluted fivefold. H10 indicates Hardaliye which is diluted tenfold. Different letters in superscript indicate statistically significant differences. Data are presented as mean ± SD, n = 3: *** p< 0.001, **p<0.01, *p<0.05.
The second step was to observe gene expression levels of ROS (reactive oxygen species) neutralizers. H5 triggered none of expression of these enzymes after 24h but a drastic increase of SOD and CAT was observed after 48h but a mitochondrial Mn SOD2. However, as for CF-1 cells, although expression of antioxidant enzymes seems to increase after 24h, all decreased after 48 h but GpX expression of H5 treated cells (Figure 6). In addition, Hsp 70 level of H5 treated HT-29 cells remained low while H10 triggered it after 48h. CF-1 cells showed a opposite reaction and Hsp 70 decreased after 48h which indicates probable diminishing of stress conditions in cells (Figure 6).
Anticancer activities of natural remedies, such as, plants, minerals and animals are of great interest since ancient times as an alternative cure. In fact, many antioxidant compounds derived from grape such as resveratrol have been screened for anticancer activity. Apart from these, this is the first report on anticancer activity of Hardaliye which is a traditional and lactic acid fermented beverage produced with mustard seed, cherry leaves and grape.
Cell proliferation results of Tali assays indicated that both H5 and H10 drastically decreased cell number. However, interestingly, H10 triggered apoptosis more than H5 which seems H5 can reveal an acute cytotoxicity. On the other hand, CF-1 cells were also affected slightly from H5 treatment but apoptosis levels were very low and equal to control. According to MTT assay, viability of HT-29 cells decreased even at 80-fold dilution. CF-1 cells were not affected by treatment after 24h and even Hardaliye stimulated proliferation. However, cell proliferation slightly decreased to a rate about 80-90% when treated with ten-fold and twenty-fold dilution after 48h.
LC MS/MS analysis showed that Hardaliye mostly contained gallic acid and resveratrol. The rate of these polyphenols were consistent with studies conducted with grape juice or wine [28]. Gallic acid and resveratrol are polyphenols found in grape, tea, wine and berries. Their anti-aging, anticancer and anti-inflammatory effects have been demonstrated in vast variety of studies [29,30]. Digalloylresveratrol, a synthetic ester of gallic acid and resveratrol has been demonstrated that dose-dependently induces apoptosis and inhibites the transition from S to G2/M phase of the HT-29 colon cancer cells [28]. Gallic acid has also induced apoptosis of colon adenocarcinoma cells [31]. On the other hand, gallic acid has also dose dependent pro-oxidant activity together with metals [32]. In accordance with this, in our results, H5 triggered expressions of SOD slightly more than H10 in healthy cells while the others were statistically equal. However, an antioxidant effect revealed after 48h and expression levels decreased as expected but GpX expression of H5 still remained high, probably due to this above mentioned activity. However, HT-29 cells exhibit an expected contrary activity, SOD and especially CAT expression drastically increased thereby induced ROS-mediated apoptosis of cancer cells. Interestingly, H5 drastically triggered BAX activity and also p53, ctyc and Fas. On the other hand, H10 triggered DR5, BAX and casp3 which seems to cause apoptosis of HT-29 cells via p53 independent way.
2021 Copyright OAT. All rights reserv
Hsp 70 plays role in folding of misfolded proteins properly and protection of cells under stress condition. Its inhibition is a key factor to diminishment of cancer cells [18,20,21]. In this study, Hardaliye decreased HSP 70 level after 24h. As for 48h, HSP 70 level of H5 treatment remained still lower than control while H10 increased. However, this latter did not trigger apoptosis inhibitors such as Survivin.
Hardaliye contained mostly potassium and magnesium as well as some other antioxidant metals such as zinc and selenium. As mentioned above, some transition metals have also pro-oxidant capacity besides their antioxidant roles. The metals detected inside of Hardaliye either forced apoptosis effect together with polyphenols or exhibit pro-oxidant as mentioned before.
In the light of all the facts mentioned above, Hardaliye can be a good food support to prevent colon cancer. Furthermore, Hardaliye contains also a few kind of lactic acid bacteria and it has been demonstrated that lactic acid bacteria have anticancer activity on colon cancer [33]. However, even though this study tried to demonstrate and illuminate direct effect of Hardaliye on cells, bioavailability studies thereby observing absorption levels of these anticancer substances will help to clarify this research.
This project was supported by Research Fund of the Trakya University. Project Number: 2015-10.
- Kaur M, Singh RP, Gu M, Agarwal R, Agarwal C (2006) Grape seed extract inhibits in vitro and in vivo growth of human colorectal carcinoma cells. Clin Cancer Res 12: 6194-6202. [Crossref]
- Morré DM, Morré DJ (2006) Anticancer activity of grape and grape skin extracts alone and combined with green tea infusions. Cancer letters 238: 202-209. [Crossref]
- Kaur M, Mandair R, Agarwal R, Agarwal C (2008) Grape seed extract induces cell cycle arrest and apoptosis in human colon carcinoma cells. Nutr Cancer 60 Suppl 1: 2-11. [Crossref]
- Alfaras I, Juan ME, Planas JM (2010) trans-Resveratrol reduces precancerous colonic lesions in dimethylhydrazine-treated rats. J Agric Food Chem 58: 8104-8110. [Crossref]
- Aydoğdu H, Yıldırım, Ş, Halkman AK, Durgun T (2014) A study on production and quality criteria of hardaliye; a traditional drink from Thrace region of Turkey. Gıda 39: 139-145.
- Altay F, Karbancıoglu-Güler F, Daskaya-Dikmen C, Heperkan D (2013) A review on traditional Turkish fermented non-alcoholic beverages: microbiota, fermentation process and quality characteristics. Int J Food Microbiol 167: 44-56. [Crossref]
- Arici M, Coskun F (2001) Hardaliye: fermented grape juice as a traditional Turkish beverage. Food microbiol 18: 417-421.
- Fulda S, Debatin KM (2006) Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene 25: 4798-4811. [Crossref]
- Daniel PT, Wieder T, Sturm I, Schulze-Osthoff K (2001) The kiss of death: promises and failures of death receptors and ligands in cancer therapy. Leukemia 15: 1022. [Crossref]
- Zhang, M., Harashima, N., Moritani, T., Huang, W. and Harada, M., 2015. The roles of ROS and caspases in TRAIL-induced apoptosis and necroptosis in human pancreatic cancer cells. PloS one 10: p.e0127386.
- Hassanpour H, Teshfam M, Momtaz H, Zarei H, Bahadoran S (2014) Caspase-,-2, and-3 gene expression is enhanced in the heart and lung of chickens with pulmonary hypertension (ascites). Turkish J Veter Animal Sci 38: 133-137.
- Chandra D, Liu JW, Tang DG (2002) Early mitochondrial activation and cytochrome c up-regulation during apoptosis. J Biol Chem 277: 50842-50854. [Crossref]
- Wong RS (2011) Apoptosis in cancer: from pathogenesis to treatment. J Exp Clin Cancer Res 30: 87. [Crossref]
- Kulbacka J, Saczko J, Chwilkowska A, Choromańska A, Skołucka N (2012) Apoptosis, free radicals and antioxidant defense in antitumor therapy. Antioxidant enzyme 265-302.
- Wang Z, Sun Y (2010) Targeting p53 for novel anticancer therapy. Transl oncol 3: 1-12. [Crossref]
- Hwang YJ, Lee EJ, Kim HR, Hwang KA (2013) In vitro antioxidant and anticancer effects of solvent fractions from Prunella vulgaris var. lilacina. BMC Complement Altern Med 13: 310. [Crossref]
- Tinggi U (2008) Selenium: its role as antioxidant in human health. Environ Health Prev Med 13:102-108.
- Hatfield MP, Lovas S (2012) Role of Hsp70 in cancer growth and survival. Protein Pept Lett 19: 616-624. [Crossref]
- Thanan R, Oikawa S, Hiraku Y, Ohnishi S, Ma N, et al. (2014) Oxidative stress and its significant roles in neurodegenerative diseases and cancer. Int J mol sci 16: 193-217. [Crossref]
- Goloudina AR, Demidov ON, Garrido C (2012) Inhibition of HSP70: a challenging anti-cancer strategy. Cancer Lett 325: 117-124. [Crossref]
- Murphy ME (2013) The HSP70 family and cancer. Carcinogenesis 34: 1181-1188. [Crossref]
- Agarwal C, Singh RP, Agarwal R (2002) Grape seed extract induces apoptotic death of human prostate carcinoma DU145 cells via caspases activation accompanied by dissipation of mitochondrial membrane potential and cytochrome c release. Carcinogenesis 23: 1869-1876. [Crossref]
- Abaza MS, Orabi KY, Al-Quattan E, Al-Attiyah RJ (2015) Growth inhibitory and chemo-sensitization effects of naringenin, a natural flavanone purified from Thymus vulgaris, on human breast and colorectal cancer. Cancer Cell Int 15: 46. [Crossref]
- Park SE, Yoo HS, Jin CY, Hong SH, Lee YW, et al. (2009) Induction of apoptosis and inhibition of telomerase activity in human lung carcinoma cells by the water extract of Cordyceps militaris. Food Chem Toxicol 47: 1667-1675. [Crossref]
- Ho YT, Lu CC, Yang JS, Chiang JH, Li TC, et al. (2009) Berberine induced apoptosis via promoting the expression of caspase-8,-9 and-3, apoptosis-inducing factor and endonuclease G in SCC-4 human tongue squamous carcinoma cancer cells. Anticancer res 29: 4063-4070. [Crossref]
- Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25: 402-408. [Crossref]
- Miele A, Rizzon LA, Queiroz S, Gianello C (2015) Physicochemical composition, minerals, and pesticide residues in organic grape juices. Food Sci Technol (Campinas) 35: 120-126.
- Kala R, Tollefsbol TO, Li Y (2015) Potential of resveratrol in inhibiting cancer and slowing aging. J Nutri Food Sci (S15) 1.
- Bernhaus A, Fritzer-Szekeres M, Grusch M, Saiko P, Krupitza G, et al. (2009) Digalloylresveratrol, a new phenolic acid derivative induces apoptosis and cell cycle arrest in human HT-29 colon cancer cells. Cancer letters 274: 299-304. [Crossref]
- Udenigwe CC, Ramprasath VR, Aluko RE, Jones PJ (2008) Potential of resveratrol in anticancer and anti-inflammatory therapy. Nutr Rev 66: 445-454. [Crossref]
- Yoshioka K, Kataoka T, Hayashi T, Hasegawa M, Ishi Y, et al. (2000) Induction of apoptosis by gallic acid in human stomach cancer KATO III and colon adenocarcinoma COLO 205 cell lines. Oncol rep 7: 1221-1224. [Crossref]
- Verma S, Singh A, Mishra A (2013) Gallic acid: molecular rival of cancer. Environ Toxicol Pharmacol 35: 473-485. [Crossref]
- Zhong L, Zhang X, Covasa M (2014) Emerging roles of lactic acid bacteria in protection against colorectal cancer. World J Gastroenterol 20: 7878-7886. [Crossref]






