Abstract
Mouse dental papilla cells (MDPCs) can be differentiated into component cell types that have the ability to regenerate the pulpo-dentinal complex. At the cap stage of tooth development, MDPC component cells are programmed for tooth generation. Therefore, isolated and cultivated component cells can be useful in evaluating cellular, molecular, and environmental scenarios in tooth germ formation and tooth morphogenesis. Increasing the proliferation capacity of MDPCs and preserving their original phenotypic and genotypic characteristics for tooth organ regeneration are the main focus of this study. An immortalized mouse dental papilla cell line was created via the intracellular insertion of SV40 T antigens into the nucleus by lentivirus particles. The generated clonally isolated SV40 T immortalized MDPC line was then characterized and validated for transfection success and efficiency. These cells displayed a higher proliferation rate, and both genotype and phenotype characteristics were similar to those of the original primary cell line. These results were verified via the expression of a broad array of tooth-specific markers. Furthermore, the test results to show that transformed cells had preserved multi-potency were also positive. Thus, the stable immortalized MDPCs may be used to determine the mechanisms of an array of developmental phenomena, such as early dental cell proliferation, reconstitution of tooth germ, dentine mineralization and other significant growth factor signaling pathways influencing tooth morphogenesis.
Keywords
immortalized, mouse dental papilla cells, tooth development
Introduction
The tooth is regarded as an ectodermal organ because it emerges through the tooth germ under the reciprocal interaction of the epithelium-mesenchyme [1,2]. The characteristic feature of this ectodermal organ is the presence of hard tissues with soft tissue [3].The distinctive hard tissues are enamel, dentine and cementum. Homeostasis is mainly maintained by the presence of soft tissue within the hard tissue architecture, namely pulp and periodontium [3].This soft tissue component contains the blood vessels for nutrient supply and nerve fibers [3].A tooth is a three-dimensional multi-cellular structure that maintains functional corporation within the maxillofacial region [3].
Tooth loss due to oral diseases, such as dental caries, periodontal diseases, and traumatic injury, results in a loss of oral function in enunciation, mastication, and occlusion and agitated general health issues [4].To restore these lost functions, a number of attempts have been made, including artificial material, fixed dental bridges and removable dentures [5].These conventional dental therapies for tooth loss bring numerous functional difficulties. The most popular treatment option at present is dental implants that create an osseo-integrated bond with the jaw bone and occupy the space without affecting the adjacent teeth [6]. Although these mentioned therapies have been widely accepted and applied in the rehabilitation of tooth loss, they do not address the physiological functions of the tooth.
Thus, further technological improvements that are based on biological aspects of the tooth are important for the restoration of tooth both aesthetically and functionally [7].Recent advancements in regenerative therapies have influenced the replacement of tooth loss by embryonic development, stem-cell biology and tissue engineering [8,9].Concepts that can be applied in the regeneration of teeth include the restoration of the partial loss of organ function and repairing damaged tissues, including stem-cell transplantation and cytokine therapy targeted to structural and functional diseases [10].
Within dentistry at the molecular level, tooth tissue-derived stem cells and the cytokine network that regulates tooth development have been well characterized [11]. These advances can be applied to the repair of dental pulp and periodontal tissues, including alveolar bone. Organ replacement therapy, unlike stem-cell transplantation, has great potential for the replacement of dysfunctional organs via a regenerative strategy of the whole organ by reconstructing a fully functional bioengineered organ using three-dimensional cell manipulation in vitro [12].
In the dental field, tooth regenerative therapy would involve replacement with a bioengineered tooth built using stem cells that have the capacity to form a functional tooth unit that includes periodontal tissue [13,14]. It is anticipated that whole-tooth replacement therapy will be established in the near future as a novel treatment that contributes to functional recovery by meeting aesthetic and physiological requirements [13]. Many approaches to replace missing teeth have been evaluated in the past three decades, including three-dimensionally bioengineered teeth and tooth germ generation using biodegradable materials and cell aggregation methods [13].
Another approach is the transplantation of a bioengineered mature organ that will lead to immediately performance of full functions in vivo and have a remarkable impact on the survival outcomes of many diseases [8,15]. Transplanted bioengineered organs are also expected to be functional as natural tissue, viable in the long term and achieve the continuous production of various functional cells and their progenitors from stem cells as efficiently as the natural organ in vivo [16,17]. It has also been proposed that mature organs can be developed from bioengineered organ germ by faithfully reproducing in vivo developmental processes. Thus, dental treatment is expected to transplant a bioengineered tooth germ consisting of mature tooth, periodontal ligament (PDL) and alveolar bone into the tooth-loss region through bone integration, which is connected between recipient bone and bioengineered alveolar bone in a bioengineered tooth germ [18]. The transplantation of a bioengineered tooth germ has also been proposed as a viable option to repair the large resorption defects in the alveolar bone after tooth loss [18].
However, obtaining an adequate number of tooth germs for transplantation is difficult. Thus, it is necessary to find ways to multiply a number of tooth germs from a single tooth germ that can be used conveniently. Hence, it is necessary to isolate progenitor cells that have the ability to transform into different cell types in a developing tooth. Molecular and genetic studies conducted in tooth germ have identified a specific stage of the tooth germ cell that constitutes dental papilla with the ability to form dental pulp, dentine, PDL, cementum and alveolar bone. In vivo studies conducted on murine models further demonstrated that these cells can form a fully functional tooth germ when recombined with embryonic epithelium. Additionally the number of dental papilla mesenchymal cells obtained from each tooth germ is limited in number. Hence, these cells should undergo enhancing proliferation in vitro to increase the number of cells in order to make multiple tooth germs. Thus, in this study, we immortalized the murine dental papilla cells at E 14.5 using the SV 40 T antigen.
The SV40 large T antigen (Simian Vacuolating Virus 40 TAg) is a hexamer protein that is a proto-oncogene derived from polyomavirus SV40, which is capable of transforming a variety of cell types. The transforming activity of TAg is due in large part to its perturbation of the retinoblastoma (pRB) and p53 tumor suppressor proteins. In addition, TAg binds to several other cellular factors, including the transcriptional co-activators p300 and CBP, which may contribute to its transformation function. TAg is a product of an early gene that is transcribed during viral infection by SV40 and is involved in viral genome replication and the regulation of the host cell cycle. SV40 is a double-stranded, circular DNA virus belonging to the Polyomaviridae (earlier Papovavirus) family, Orthopolyomavirus genus. Polyomaviruses infect a wide variety of vertebrates and cause solid tumors at multiple sites. SV40 was isolated by Sweet and Maurice Hilleman in 1960 in primary monkey kidney cell cultures that were used to grow Sabin OPV.
Thus, we have evaluated the immortalized cells regarding their transcription efficacy through gene and protein analysis. Furthermore, their multilineage differentiation was assessed by different lineages, such as adipogenic, chondrogenic and osteogenic induction.The genotypic and phenotypic characteristics of the immortalized cells were compared with the primary cells, and the exceptional colonies were selected. These selected colonies were passaged for the experiment in the reconstitution of the tooth germ. Here, we aimed to establish an immortalized mouse dental papilla cell line and observed its phenotypical and genotypic characteristics. We further reconstituted tooth germ using primary mouse dental epithelial cells and immortalized dental papilla cells. The reconstituted tooth germs were implanted in the kidney capsule of mice and used to observe tooth formation.
Materials &methods
Ethics statement
All of the animals and experimental protocols were approved by the University of Hong Kong Animal Care and Use Committee (CULART:3239-14) and the Intramural Animal Use and Care Committee, School of Dentistry, Yonsei University. All surgery was performed under sodium pentobarbital anesthesia, and all efforts were made to minimize the suffering of the animals involved in the study.
Single cells
Molar tooth germs were dissected from the mandibles of ED14.5 mice. The isolation of tissues and single-cell preparations from the mesenchyme has been described previously [19]. Once the epithelium and mesenchymal tissues were separated, the mesenchymal tissues were subjected to collagenase digestion for 10 minutes. Dissociated mesenchymal cells were precipitated by centrifugation in a siliconized microtube, and the supernatant was completely removed. Cells were cultured in DMEM medium that was supplemented with 10% FBS and 1% P/S. Once the cells were confluent, they were seeded in 24-well plates for transfection.
Establishment of immortalized MDPC cells
The primary cells of passage 2 were infected by lentivirus particles with SV40 T-Ag according to the manufacturer’s instructions. Once infected, the cells were plated at a low density to obtain clones from single cells. Three clones were isolated from the dental papilla mesenchymal cells for further growth and testing. After 40 passages over a period of four months, the selected clones were developed into cell lines. One of the cell lines was randomly selected for further analysis.
Western blotting
Cultured cells were lysed in RIPA buffer. The total proteins in the supernatant were measured with a BCA Protein Assay Kit (Pierce Biotechnology, Rockford, IL). An equal amount of proteins was fractionated with a 10% polyacrylamide gel, transferred to PVDF membranes (GE Healthcare), and blotted as previously indicated.
Colony formation assay
The soft agar assay was used for evaluating colony formation.This was performed by seeding MDPCs and iMDPCs that were cultured at 300 cells/well of a six-well culture plate in agarose gel and followed for 2 weeks. In order to maintain the viability, the medium was placed on the top of the agarose gel. The medium was replaced every 3 days. After 2 weeks, the cells were fixed using 100% methanol for 20 minutes and stained with crystal violet. Colonies were observed under light microscopy.
Proliferation rate
The proliferation rates of MDPCs and iMDPCs cultured were determined by plating 10,000 cells (passage 3) per well on a six-well plate; each had three replicates and was passaged and counted every 72 hours. The proliferation rate was then calculated by dividing the total cell number after every 72 hours of culture by the initial plating number
Multilineage differentiation
Odonto/osteogenic differentiation: MDPCs and iMDPCs were seeded onto six-well plates, cultured to 70% confluence, and incubated in the induction medium containing 10 nmol/L dexamethasone, 10 mmol/L b-glycerophosphate, 50 mg/mL L-ascorbic acid phosphate, 10 nmol/L 1,25-dihydroxyvitamin D3, and 10% FBS for 1 to 4 weeks. Alkaline phosphatase (ALP) is considered an early osteogenic marker. After 1 and 2 weeks of induction, the cultures were fixed and stained for ALP (Sigma-Aldrich, Steinheim, Germany). After 2 weeks of induction, cultures were analyzed by immunofluorescence for the expression of dentin sialophosphoprotein (Santa Cruz Biotechnology). After induction for 4 weeks, cultures were fixed with 60% isopropanol for 20 minutes and stained for mineralization with 2% alizarin red stain.
Chondrogenic differentiation
MDPCs and iMDPCs that were cultured to 70% confluence on six-well plates were incubated in the neurogenic induction medium Basal insulin medium (Gibco-Invitrogen) with 20 ng/mL epidermal growth factor (BD Biosciences, Bedford, MA) and 40 ng/mL fibroblast growth factor (BD Biosciences) for 4 weeks. Cells were analyzed by alcian blue as previously described for the expression of Chondrogenic nodules. Images were analyzed under a light microscope.
Adipogenicdifferentiation
MDPCs and iMDPCs were seeded onto six-well plates, cultured to 70% confluence, and incubated in induction medium containing 1 mmol/L dexamethasone, 1 mg/mL insulin, 0.5 mmol/L 3-isobutyl-1-methylxanthine, and 10% FBS for 4 weeks. Cultures were fixed with 10% formalin for 60 minutes and washed with 70% ethanol, and lipid droplets were stained with 0.21% Oil Red O for 10 minutes and washed with deionized H2O four times.
Reverse transcription and real-time polymerase chain reaction
Total RNA from cell cultures of PDPC and I-PDPC was extracted with an RNeasy+ Mini kit (Qiagen, Crawley, UK); 1.5 mg of total RNA was used to synthesize complementary DNA by SuperScript VILO MasterMix(Invitrogen). The resulting complementary DNA concentrations were quantified using a NanoDrop ND-1000 (Thermo Fisher Scientific, Wilmington, DE) spectrophotometer. Quantitative real-time polymerase chain reaction (PCR) was carried out using the StepOnePlus Real-TimePCR system (Applied Biosystems) with SYBR green (Applied Biosystems). All of the samples were run in triplicate in 96-well plates, with each well containing 1.25 mL of complementary DNA diluted 1 to 10 in a total reaction volume of 20 mL. Reactions were carried out at 95°C for 10 minutes, followed by 40 cycles of 95°C for 15 seconds and 58°C for 1 minute. Primers for SV 40 T; Nestin; BMP1; DSPP; the osteogenic markers BSP, BMP, and ALP; and the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were designed using Primer Express software from Applied Biosciences. For data analysis, the StepOne software v2.0.2 (Applied Biosystems, Carlsbad, CA) calculated the levels of target (PDPC and I-PDPC) gene expression in samples relative to the level of expression in the calibrator (MDPC and iMDPCs) with the comparative cycle threshold (CT) method (DDCT). Expression values for target genes were normalized to the expression of GAPDH.
Tissue and cell recombinations
The lower molar tooth germs were carefully dissected from the mandible of the mouse embryo at E12. The tooth germs were incubated in Dispase II (Roche, USA) in PBA at 1.2 units/ml for 35 min at 37 ºC. After incubation, tooth germs were washed three times in a cold solution of DMEM (Bio Whittaker, USA, 12-640F) supplemented with 10% fetal bovine serum (FBS, Gibco, USA, 16000-044). Under a dissection microscope, the dental epithelium was separated from the dental mesenchyme with a fine tungsten needle.
The number of the aggregated cells including iMDPCs and dental mesenchymal primary cells that were obtained from E14 tooth germs was adjusted to 2.5 × 105, which corresponds to 10 times the average number of dental mesenchymal cells in one molar of E14 mice as previously described [20]. The aggregated cells were placed in a hole that was made by a blunt yellow tip in 2% agar semi-solid medium, and the epithelium was overlaid on the center of the aggregated cells. These recombinants were incubated at 37ºC in DMEM with 20% FBS for 2 days.
Transplantation of recombinants into the renal subcapsular layer of nude mice
For calcification, the recombinants were cultured for 2 days in vitro and transplanted into the renal capsular layer of adult nude mice. After 5 weeks, the host mice were sacrificed, and the kidneys were dissected to obtain the calcified teeth.
Results
Generation of reversibly immortalized MDPCs
To create immortalized MDPCs, primary cells were infected with a SV40Tag-containing lentivirus. The GCV-sensitive colonies were selected and expanded. The iMDPC cells exhibited the typical fibroblast-like morphological characteristics of normal MDPCs (Figure 1). These cells were homogenously polygonal and had characteristic ovoid nuclei with one or two nucleoli (Figure 1). The confirmation of the successful transduction of SV40Tag, genome PCR and western blotting were performed. The SV40Tag was integrated and expressed in iMDPCs and not in the primary cultured PDPCs (Figure 2). Moreover, SV40Tag was detected in only iMDPCs cells by genomic PCR and western blotting. To characterize their proliferation ability, immortalized MDPCs were compared to primary cultured PDPCs. PDPCs (passage 5) showed a rapid increase in cell proliferation 2 d after passage and produced a sinusoidal growth curve. However, the primary cells did not enter log phase until 3 d after passage (Figure 1). Primary MDPCs stopped dividing and entered crisis by 14 days, but the I-PDPCs bypassed senescence and grew for more than 30 days without significant growth retardation. To determine the lineage of immortalized cells, real time PCR was used to detect odontoblastic markers, such as BMP1, DSPP, and NESTIN. I-PDPCs and PDPCs were positive for these three markers (Figure 1). The differentiation state of these cells was further determined by the expression level of odontoblastic markers. However, a significantly lower expression of BMP1, DSPP, and NESTIN was observed in iMDPCs (Figure 1)
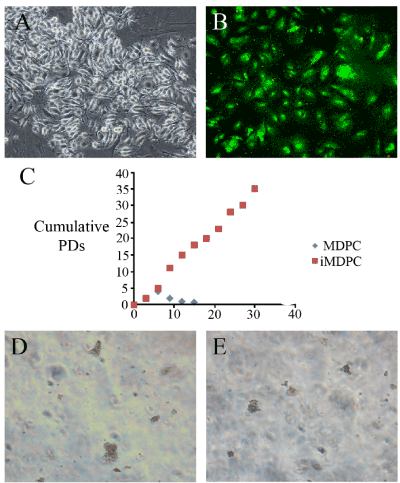
Figure 1.The immortalized mouse dental papilla mesenchymal cells (B). The cells were transfected together with SV40 T Ag conjugated with GFP. Thus, the transfected cells presented green fluorescence (B). The cell proliferation assay was conducted by calculating the cumulative value of the number of cells presented in figure 1C. Colony formation was observed in the gel assay, and both the immortalized and primary cell cultures showed colony formation (D&E).
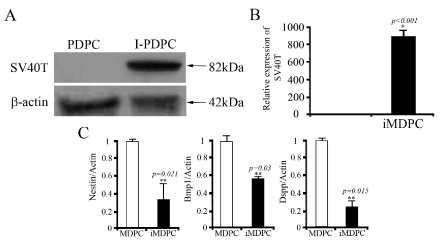
Figure 2.The immortalized cells were validated for the presence of SV40 T using western blotting and RT-PCR (A & B).
Multilineage differentiation
Staining for alkaline phosphatase, an early marker of odonto/osteogenesis, showed a higher ALP activity in induced iMDPCs and MDPCs (Figure 3). Alizarin red staining showed mineralization in both 3- and 4-week-induced cultures (Figure 3). As shown by PCR analysis, both cell types showed a significantly enhanced expression of ALP in 2-3 weeks. The non-induced MDPCs did not show much higher expression. Chondrogenic induction caused morphological changes in cells, and alcian blue staining showed stained chondrogenic nodules in 4-week-old cultures of both iMDPCs and MDPCs. Both cells types presented equal staining and duration throughout the induction (Figure 3). According to the results, the adipogenic potential of both cells types was weaker. However, the production of lipid goblets within 3-4 weeks of induction was apparent but lower compared to that of other reported studies. Tiny globules appeared in the induced cells cultures with significantly altered cell morphology. The cytoplasm was observed to be filled with lipid droplets (Figure 3).
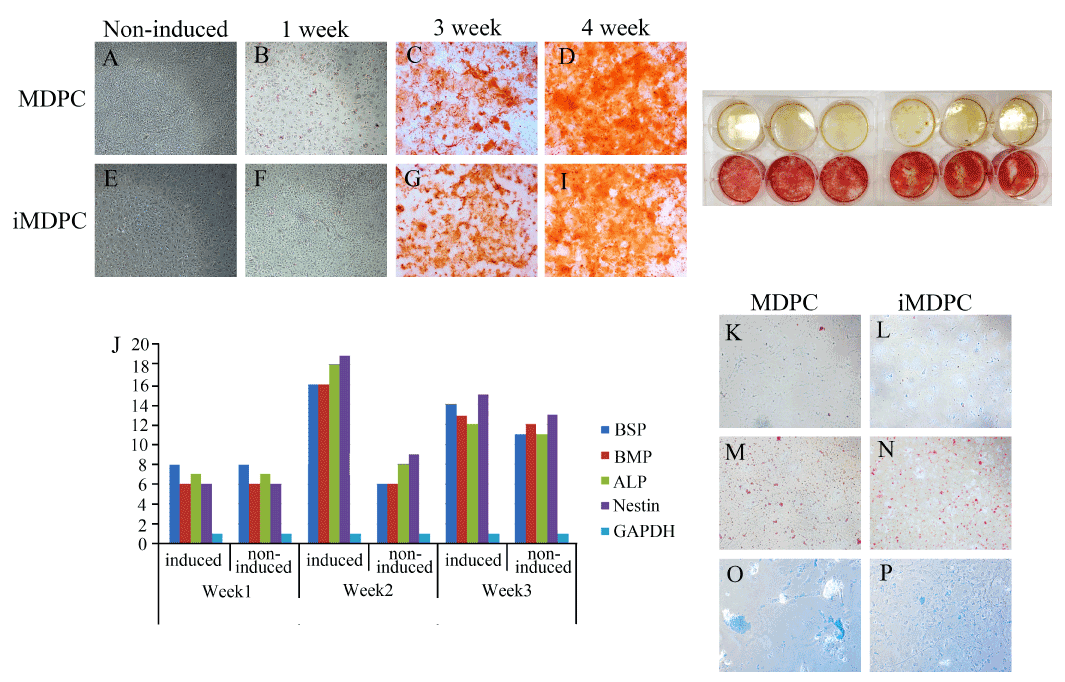
Figure 3.The alizarin red assay of the I-PDPC cells and PDPC cells is shown in 3.1. The cells that were induced chemically for osteogenesis showed evidence of mineralization in the alizarin red assay. However, the cells that were not induced did not produce any mineralization. The osteogenic markers increased from the second week of induction (3.2). Adipogenesis was observed as the formation of lipid globules observed by the oil red assay (3.3). Some chondrogenesis was also visible upon chemical induction (3.4).
Bone-like structures generated from iMDPCs and E12 tooth epithelium
The reaggregated iMDPCs were recombined with the dental epithelium at E12 and transplanted into the mouse kidney capsule. After 5 weeks, we observed bone-like structures with fibrous cysts and the surrounding bone (Figure 4). Furthermore, the bone-like structure was confirmed by Masson’s trichrome staining in cross sections of recombinants without decalcification (Figure 4). As a control, we isolated dental mesenchymal cells from E14 tooth germ, recombined them with the mouse dental epithelium at E12, and transplanted them into mouse kidney capsule. After 5 weeks, we observed tooth-like structures with the surrounding bone in the kidney under a microscope and by microCT imaging (Figure 4A).
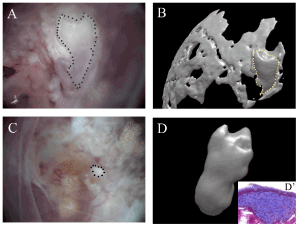
Figure 4.A bone-like structure with a fibrous cyst and the surrounding bone after 5 weeks of transplantation are presented in figure 4. The micro-CT image of the samples presented a tooth-like structure (4A) Furthermore, the bone-like structure was confirmed by Masson’s trichrome staining in cross sections of recombinants without decalcification (4D).
Discussion
In this report, we describe the establishment and characterization of an immortalized MDPC cell line; the iMDPCs cells stained SV40Tag positive, retained mineralization ability, and expressed identification markers of odontoblastic-related genes. These data suggest that iMDPCs cells are functionally active and show most of the phenotypic characteristics of primary dental papilla cells. The immortalized cells with SV40Tag were able to undergo replicative senescence.
The immortalized MDPC cell line was characterized by detecting odontoblast-specific markers and determining proliferation kinetics. The real time PCR results showed the expression of DMP1, DSPP, and NESTIN in iMDPCs cells. DSPP is the marker of odontoblasts [21,22]. Moreover, the expression of NESTIN, which is specific for odontoblasts but not for osteoblasts, further excluded the potential osteoblastic lineage [23,24]. Because all cells were positive for these markers, phenotypic uniformity was confirmed. I-PDPC and PDPC cells were able to undergo odontoblastic differentiation and form mineralized nodules when cultured in odontoblastic induction medium. This finding was similar to the primary cells, although the immortalized cells showed a more rapid proliferation rate.
The induction of iMDPCs cells resulted in replicative senescence and a higher differentiation state. This result indicates the function of SV40Tag in cell cycle progression. SV40Tag interacts with the two major cellular tumor suppressor proteins, P53 and PRB, and causes the activation of E2F/DP transcription factors, thus promoting cell proliferation and avoiding senescence [25,26].Once the SV40Tag is excised, immortalized cells will lose the ability to actively proliferate, with the bonus of escaping from senescence. We also observed upregulated mineral deposition and some odontoblastic-related gene expression in immortalized cells. As a type of odontoblast progenitor cell, dental papilla mesenchymal cells possess the potential to differentiate into odontoblast cells. This type of cell could spontaneously differentiate into odontoblast-like cells under normal in vitro culture conditions [27]. As with the transduction of SV40Tag, the cells showed a dedifferentiation state [28]. The low proliferation rate and high differentiation state of the immortalized cells could greatly simulate the terminal differentiation of odontoblasts.
Although tumor-forming ability and chromosomal abnormalities were not detected in iMDPCs cells, the oncogene SV40Tag was still a potential threat to cell biosafety. Hopefully, iMDPCs will facilitate a broad range of research, including in vivo studies. In conclusion, we established and characterized an immortalized MDPC cell line. The immortalized cells retained similar phenotypes to those of primary cultured MDPCs. When reverted, these cells showed phenotypes similar to those of terminally differentiated odontoblasts. Because the reverse procedure is easier to perform than traditional methods, the ready-to-use immortalized cell line not only can be used in basic research to study the mechanism of odontoblastic differentiation but can also be potentially applied in drug toxicity tests and material biocompatibility examinations in the future.
Various cell lines have been established with SV40 transfection for investigating dental morphogenesis, such as cell lines from dental epithelium [29,30]and dental mesenchyme [31,32].Dental papilla cells have the tendency to differentiate into odontoblasts. Recent studies have demonstrated that immortalized dental papilla cells can maintain their genotypic and phenotypic characteristics similar to primary cells [33,34].
The microenvironment in some tissues reprograms the already-made program in foreign cells and allows them to take a natural program [35,36].When bone marrow cells were mixed with dental epithelial cells prepared from embryos and proceeded to tooth germ formation, the bone marrow cells subsequently differentiated into ameloblasts in the tooth [37].Similarly, we reconstituted the tooth germ with epithelial cells from E12 tooth germs of the mice and the immortalized dental papilla cells from E14.5. The tooth germs were transplanted in the kidney capsule for five weeks and developed into tooth-like structures (Figure 4). However, the histological sections showed bone formation instead of tooth tissues. Thus, it is not clear how the microenvironment of the E12 primary epithelial cells interacts with the trans-differentiation of iMDPCs.
The microenvironment for the tooth germs was properly created as a pellet of epithelial cells sitting on mesenchymal tissue [38]. The ratio of cell proportions was according to a previously published study conducted in the same laboratory [39].However, the conditions may not be feasible for maintaining the microenvironment for the primary epithelial cells to reprogram the iMDPCs for tooth formation.
A previous study reported that mouse dental mesenchyme transplanted without recombination formed bone-like structures, confirmed by BSP staining [20]. Our iMDPC recombinant approach, which did not display tooth formation but rather bone formation, indicates that the iMDPCs have dental mesenchymal cell characteristics and high potential for tooth formation. Moreover, these cells could be applied to tooth regeneration. Of course, further study should be conducted for the odontogenic potential of our iMDPCs with a detailed analysis of epithelial-mesenchymal interactions. Thus, it is important to reassess the factors that are important in tooth formation. The reversible immortalization of dental papilla cells to enhance more primary characteristics may be a solution to this problem [40].
In summary, this study demonstrates that immortalized dental papilla cells induce multilineage differentiation and have the potential for tooth formation. Although it could not form a complete tooth within this study, using a mixture of primary and immortalized cells in different proportions could induce tooth formation. We are continuing our research through this direction to determine the ideal ratios of mixing primary and immortalized cells. However, our findings suggest that immortalized dental mesenchymal cells could be a candidate source for tooth regeneration and improve dental regenerative therapies with high efficacy.
Disclosure
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI14C1817). This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI14C1817). The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Ikeda E, Tsuji T (2008) Growing bioengineered teeth from single cells: potential for dental regenerative medicine. Expert OpinBiolTher8:735-44. [Crossref]
- Tucker A. Sharpe P (2004) The cutting-edge of mammalian development; how the embryo makes teeth. Nat Rev Genet 5:499-508. [Crossref]
- Avery J (2002) Oral development and histology. New York: Thieme Press.
- Proffit W, Fields H, Sarver D (2004) Contemporary orthodontics. St. Louis: Mosby Press.
- Pokorny PH, Wiens JP, Litvak H (2008) Occlusion for fixed prosthodontics: a historical perspective of the gnathological influence. J Prosthet Dent99:299-313. [Crossref]
- Burns DR, Beck DA, Nelson SK (2003) A review of selected dental literature on contemporary provisional fixed prosthodontic treatment: report of the Committee on Research in fixed prosthodontics of the academy of fixed prosthodontics. J Prosthet Dent90: 474-497.[Crossref]
- Huang GT, Sonoyama W, Liu Y, Liu H, Wang S, et al. (2008) The hidden treasure in apical papilla: the potential role in pulp/dentin regeneration and bioroot engineering. J Endod34: 645-651.[Crossref]
- Atala A (2005) Tissue engineering, stem cells and cloning: current concepts and changing trends. Expert OpinBiolTher5:879-892.[Crossref]
- Brockes JP, Kumar A (2005) Appendage regeneration in adult vertebrates and implications for regenerative medicine. Science 310: 1919-1923.[Crossref]
- Kørbling M, Estrov Z (2003) Adult stem cells for tissue repair - a new therapeutic concept? N Engl J Med 349: 570-582.[Crossref]
- Thesleff I (2003) Epithelial-mesenchymal signalling regulating tooth morphogenesis. J Cell Sci 116: 1647-1648.[Crossref]
- Purnell B (2008) New release: the complete guide to organ repair. Introduction. Science 322: 1489.[Crossref]
- Sharpe PT, Young CS (2005) Test-tube teeth. Sci Am 293: 34-41.[Crossref]
- Volponi AA, Pang Y, Sharpe PT (2010) Stem cell-based biological tooth repair and regeneration. Trends Cell Biol20:715-722. [Crossref]
- Gridelli B, Remuzzi G (2000) Strategies for making more organs available for transplantation. N Engl J Med 343: 404-410.[Crossref]
- Kaukua N, Shahidi MK, Konstantinidou C, Dyachuk V, Kaucka M, et al. (2014) Glial origin of mesenchymal stem cells in a tooth model system. Nature 513: 551-554.[Crossref]
- Zaret KS, Grompe M (2008) Generation and regeneration of cells of the liver and pancreas. Science 322: 1490-1494.[Crossref]
- Hu B, Nadiri A, Kuchler-Bopp S, Perrin-Schmitt F, Peters H, Lesot H (2006) Tissue engineering of tooth crown, root, and periodontium. Tissue Eng12:2069-2075. [Crossref]
- Oshima M, Ogawa M, Yasukawa M, Tsuji T (2012) Generation of a bioengineered tooth by using a three-dimensional cell manipulation method (organ germ method). Methods MolBiol887: 149-165.[Crossref]
- Cai J, Zhang Y, Liu P, Chen S, Wu X, et al. (2013) Generation of tooth-like structures from integration-free human urine induced pluripotent stem cells. Cell Regen (Lond) 2: 6.[Crossref]
- Fisher LW, Fedarko NS (2003) Six genes expressed in bones and teeth encode the current members of the SIBLING family of proteins. Connect Tissue Res44:33-40.[Crossref]
- Narayanan K, Srinivas R, Ramachandran A, Hao J, Quinn B, George A (2001) Differentiation of embryonic mesenchymal cells to odontoblast-like cells by overexpression of dentin matrix protein 1. Proc Natl Acad Sci U S A 98:4516-4521. [Crossref]
- Fujita S, Hideshima K, Ikeda T (2006) Nestin expression in odontoblasts and odontogenic ectomesenchymal tissue of odontogenic tumours. J ClinPathol59: 240-245.[Crossref]
- Struys T, Krage T, Vandenabeele F, Raab WH, Lambrichts I (2005) Immunohistochemical evidence for proteolipid protein and nestin expression in the late bell stage of developing rodent teeth. Arch Oral Biol50:171-176. [Crossref]
- Ali SH, DeCaprio JA (2001) Cellular transformation by SV40 large T antigen: interaction with host proteins. Semin Cancer Biol11:15-23. [Crossref]
- Sullivan CS, Pipas JM (2002) T antigens of simian virus 40: molecular chaperones for viral replication and tumorigenesis. MicrobiolMolBiol Rev 66: 179-202.[Crossref]
- Yu JH, Shi JN, Deng ZH, Zhuang H, Nie X, Wang RN (2006) Cell pellets from dental papillae can reexhibit dental morphogenesis and dentinogenesis. BiochemBiophys Res Commun346:116-124. [Crossref]
- Thenet S, Benya PD, Demignot S, Feunteun J, Adolphe M (1992) SV40-immortalization of rabbit articular chondrocytes: alteration of differentiated functions. J Cell Physiol150: 158-167.[Crossref]
- Kawano S, Saito M, Handa K, Morotomi T, Toyono T, Seta Y (2004) Characterization of dental epithelial progenitor cells derived from cervical-loop epithelium in a rat lower incisor. J Dent Res83:129-133. [Crossref]
- Nakata A, Kameda T, Nagai H, Ikegami K, Duan Y, et al. (2003) Establishment and characterization of a spontaneously immortalized mouse ameloblast-lineage cell line. BiochemBiophys Res Commun308: 834-839.[Crossref]
- Arany S, Kawagoe M, Sugiyama T (2009) Application of spontaneously immortalized odontoblast cells in tooth regeneration. BiochemBiophys Res Commun381: 84-89.[Crossref]
- Hanks CT, Sun ZL, Fang DN, Edwards CA, Wataha JC, et al. (1998) Cloned 3T6 cell line from CD-1 mouse fetal molar dental papillae. Connect Tissue Res 37: 233-249.[Crossref]
- Liu C, Wang X, Zhang H, Xie X, et al. (2015) Immortalized Mouse Floxed Fam20c Dental Papillar Mesenchymal and Osteoblast Cell Lines Retain Their Primary Characteristics. J Cell Physiol230: 2581-2587.[Crossref]
- Wu L, Wang F, Donly KJ et al. (2015) Establishment of immortalized mouse Bmp2 knock-out dental papilla mesenchymal cells necessary for study of odontoblastic differentiation and odontogenesis. J Cell Physiol230: 2588-2595.[Crossref]
- Booth BW, Mack DL, Androutsellis-Theotokis A, McKay RD, Boulanger CA, et al. (2008) The mammary microenvironment alters the differentiation repertoire of neural stem cells. Proc Natl Acad Sci U S A 105: 14891-14896.[Crossref]
- Boulanger CA, Mack DL, Booth BW, Smith GH (2007) Interaction with the mammary microenvironment redirects spermatogenic cell fate in vivo. Proc Natl Acad Sci U S A 104: 3871-3876.[Crossref]
- Hu B, Unda F, Bopp-Kuchler S, Jimenez L, Wang XJ, Haikel Y (2006) Bone marrow cells can give rise to ameloblast-like cells. J Dent Res5:416-421.[Crossref]
- Komine A, Tomooka Y (2012) Successful reconstruction of tooth germ with cell lines requires coordinated gene expressions from the initiation stage. Cells1:905-925. [Crossref]
- Cai J, Cho SW, Kim JY, Lee MJ, Cha YG, Jung HS (2007) Patterning the size and number of tooth and its cusps. Dev Biol304:499-507. [Crossref]
- Lin H, Liu H, Sun Q, Yuan G, Zhang L, Chen Z (2013) Establishment and characterization of a tamoxifen-mediated reversible immortalized mouse dental papilla cell line. In Vitro Cell Dev BiolAnim49:114-116.[Crossref]




