Background: Diabetes mellitus (DM) is a worldwide health problem. The current study was designed to assess the protective effects of phenolic acids (gallic acid and p-coumaric acid) on oxidative stress and hematological alterations in an experimental rat model of type 2 diabetes mellitus.
Methods: Type 2 diabetes was induced by a single intraperitoneal (i.p.) injection of streptozotocin (65 mg/kg b.wt.), after 15 min of i.p. injection of nicotinamide (120 mg/kg b.wt.). Rats were randomly allocated into four groups as follows: group I (control), group II (diabetic), group III (diabetic rats administered gallic acid, 20 mg/kg body weight, daily for six weeks) and group IV (diabetic rats administered p-coumaric acid, 40 mg/kg body weight, daily for six weeks).
Results: In diabetic rats, it was found that the levels of malondialdehyde and nitric oxide significantly increased, while the activities of superoxide dismutase, catalase, glutathione peroxidase and glutathione-S-transferase, as well as reduced glutathione content, were markedly reduced as compared to those of the control ones. Diabetic rats also showed alterations in the red blood cells count, and its related indices indicating an anemic condition and in the total and differential leukocyte count. All these abnormalities were significantly alleviated following the administration of gallic acid and p-coumaric acid. In conclusion, treatment of diabetic rats with gallic acid and p-coumaric acid markedly diminished the oxidative stress and alleviated the hematological abnormalities that may be attributed to their strong antioxidant activities.
Conclusion: Therefore, both tested phenolic acids can be acted as powerful agents against the development of the oxidative stress status as well as the hematological alterations.
phenolic acids, oxidative stress, hematological alterations, diabetic complications, type 2 diabetes
Diabetes mellitus (DM) is a serious worldwide medical and social problem. DM is characterized by persistent hyperglycemia with impaired metabolism of glucose, lipids, and protein resulting from the malfunction in insulin secretion and/or insulin action [1, 2]. The 21st century has seen a rise in diabetes and its complications around the world. The incidence of diabetes has increased by 50% over the past 10 years and is accompanied by an increasing burden of morbidity and mortality that is attributable to diabetic complications [3].
Over the past decade, there has been increased interest in oxidative stress and its role in the development of complications of diabetes mellitus [4]. With diabetes hyperglycemia directly leads to reactive oxygen species (ROS) production, increased cellular stress, and glucotoxicity. In an upstream effect of hyperglycemia, production of increased amounts of ROS is also known to have detrimental effects on cellular function [5]. The formation of advanced glycation end products (AGEs), a group of modified proteins and/or lipids with damaging potential, is one contributing factor. On the one hand, it has been reported that AGEs increase reactive oxygen species formation and impair antioxidant systems. Free radicals and oxidative stress induced complications from diabetes include coronary artery disease, neuropathy, nephropathy, and retinopathy and stroke [6,7].
In the last years, there has been renewed interest in hematological parameters as predictors of endothelial dysfunction and inflammation, a relationship between HbA1c and hematological indices, and to evaluate the relationship between these parameters and microvascular complications of diabetes [8].
Gallic acid (GA, 3,4,5-trihydroxybenzoic acid) is a polyphenol from plants and a natural product of tannins hydrolysis found abundantly in grapes, different berries, and other fruits as well as in wine [9]. GA has many biological activities such as antihyperglycemic, antihyperlipidemic, antioxidant, anti-inflammatory and hepato-renal protective effects [10-13]. It received much attention because of its potent ability to scavenge ROS, such as superoxide anions, hydrogen peroxide, hydroxyl radicals, and hypochlorous acid [14]. This polyphenol is even more effective than ascorbic acid to prevent lipid peroxidation [15].
p-Coumaric acid (PCA, 4-hydroxyphenyl-2-propenoic acid) is a phenolic acid widely distributed in plants and form a part of the human diet. PCA is present in a plenty of foods, such as grapes, white and red wine, tomato, spinach, coffee, carrot and garlic [16]. PCA has attracted substantial attention due to its several pharmacological and biological actions, such as antidiabetic and anti-inflammatory activities [11,17,18]. However, few publications assess the effect of these phenolic compounds against diabetic complications. Thus, the present study was performed to evaluate the protective effects of both phenolic acids (gallic acid and p-coumaric acid) against oxidative stress, inflammation and hematological alterations in diabetic rats.
Experimental animals
Adult male albino rats (Rattus norvegicus) weighing about 130 ± 10 g were used in the present study. They were obtained from National Research Center (NRC), Doki, Giza, Egypt. They were kept under observation for two weeks before the onset of the experiment to exclude any inter-current infection. The chosen animals were housed in plastic good aerated cages at the normal atmospheric temperature (25 ± 5°C), humidity (55 ± 5%) and normal 12 hours light/dark cycle. During the entire period of study, the rats were provided with water and normal basal diet with known composition ad libitum. The animal procedures were conducted according to the principles and guidelines of the Canadian Council on Animal Care [19].
Type 2 diabetes was induced in overnight fasted rats by single intraperitoneal (i.p.) injection of streptozotocin (STZ) (65 mg/kg b.wt.), freshly dissolved in cold citrate buffer (pH 4.5), after 15 min of i.p. injection of nicotinamide (NA) (120 mg/kg b.wt.) prepared in normal physiological saline [20]. After measuring plasma glucose concentration, seven days after injection, rats with a 2-hour plasma glucose level ranging from 200-300 mg/dl were considered mildly diabetic and included in the experiment.
Experimental design
Rats were randomly allocated into four groups as follows:
Group served as control and were orally administered an equivalent volume of vehicle.
Group served as diabeticand were orally administered an equivalent volume of vehicle.
Group acted as diabetic rats were orally treated with gallic acid (20 mg/kg b.wt.) for six weeks [10].
Group acted as diabetic rats were orally treated with p-coumaric acid (40 mg/kg b.wt.) for six weeks [17].
All treatments were dissolved in 0.5% carboxymethylcellulose (CMC) and given daily by gastric intubation. By the end of the sixth week, rats were sacrificed under diethyl ether anesthesia. Two blood samples were collected from each rat. The first was collected into a tube containing ethylene-diamine-tetra-acetic acid (EDTA) and immediately preserved in the refrigerator for subsequent analysis of blood glycosylated hemoglobin (HbA1c) and complete blood count (CBC). The second was allowed to coagulate at room temperature for further serum separation. The clear, sera were quickly removed, divided into three portions for each individual animal, and kept at -20°C for subsequent analysis. Part of liver homogenized at 4°C with ten times (w/v) in 0.9% saline. The homogenate was centrifuged at 3000 r.p.m. for five minutes to remove cellular debris, supernatant was kept at -20°C, and used for biochemical analysis.
Lab examinations
Hepatic levels of lipid peroxidation (LPO), nitric oxide (NO), and reduced glutathione (GSH) were estimated according to the methods of Preuss et al., Miranda et al. and Beutler et al. respectively [21-23]. Furthermore, the activities of hepatic superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPX) and glutathione-S-transferase (GST) were detected according to the methods of Marklund and Marklund, Cohen et al., Matkovics et al. and Mannervik and Gutenberg [24-27]. Serum tumor necrosis factor-α (TNF-α) was assayed by sandwich ELISA using reagent kit purchased from R&D Systems, Inc. (USA) according to the manufacturer’s protocol.
Red blood cells count (RBCs in 106/μl) and white blood cells (WBCs in 103/μl) were estimated according to the visual method of Dacie and Lewis [28]. Hematocrit (HCT %) was and the blood hemoglobin concentration (Hb in g/dl) in samples were assayed according to the method of Alexander and Grifiths [29]. Mean corpuscular volume (MCV in fl), mean corpuscular hemoglobin (MCH in pg) and mean corpuscular hemoglobin concentration (MCHC in %) were calculated as outlined in Dacie and Lewis [28]. Platelets count (103/μl) was detected as mentioned by Trowbridge et al. [30]. Differential leukocytes count [neutrophils (%), Lymphocytes (%), Monocytes (%), Eosinophils (%) and Basophils (%)] were estimated using the method of Osim et al. [31]. Blood HbA1c percentage was assessed according to the method of Bisse and Abraham using reagent kits purchased from Biosystems S.A. (Spain) [32].
The experimental results were expressed as mean ± standard error (SE) and subjected to One-Way Analysis of Variance (ANOVA), using a computer software package (SPSS version 20, IBM Corp., 2011) and followed by Duncan’s Multiple Range Test (DMRT) to determine the significant differences between groups, at P < 0.05.
HbA1c concentration exhibited a significant elevation in the diabetic rats and ameliorated after treatment with gallic acid or p-coumaric acid (Figure 1). Data regarding the effect of gallic acid and p-coumaric acid on oxidative stress markers in the liver of diabetic rats were shown in (Table 1). The level of malondialdehyde (MDA), a marker of lipid peroxidation, revealed a significant increase in diabetic rats as compared to the control rats. Treatment with either gallic acid or p-coumaric exhibited a significant amelioration in MDA concentration. NO is another marker of oxidative stress, expressed as nitrite, showed the same behavioral pattern as MDA.
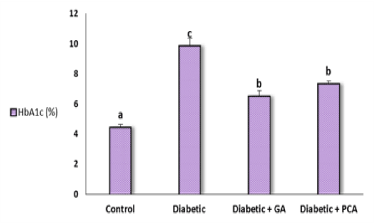
Figure 1. Glycosylated hemoglobin, HbA1c, (%) of control, diabetic and diabetic rat treated with gallic acid and p-coumaric acid.
Table 1. Liver LPO and NO levels of control, diabetic and diabetic rats treated with gallic acid and p-coumaric acid
 Parameter Parameter
Group |
LPO (nmol MDA/100mg tissue) |
NO (nmol nitrite/100mg tissue) |
Control |
22.14 ± 0.12a |
8.95 ± 0.20a |
Diabetic |
30.74 ± 0.36d |
15.44 ± 0.69c |
Diabetic + GA |
24.16 ± 0.11b |
10.02 ± 0.14a |
Diabetic + PCA |
26.82 ± 0.51c |
12.25 ± 0.51b |
Data are expressed as Mean ± SE of six rats from each group. Means not Sharing a common
superscript symbol(s) differ significantly at P < 0.05
Data describing the effect of treatments on the non-enzymatic antioxidant parameters, total thiols and reduced glutathione (GSH), in the liver of diabetic rats were presented in (Table 2). Both treatments showed a significant increase of total thiols and GSH concentrations which were decreased in the non-treated diabetic rats.
The activities of antioxidant enzymes, catalase, superoxide dismutase, glutathione peroxidase, and glutathione-S-transferase exhibited a significant decrease in the diabetic rats as compared to the control ones. The daily oral administration of gallic acid or p-coumaric acid produced a marked alleviation of these alterations. The effect of gallic acid on the above-mentioned antioxidant enzymes seemed to be more potent than p-coumaric (Table 2).
Table 2. Liver total thiols and GSH, SOD, CAT, GPx and GST activities of control, diabetic
and diabetic rats treated with gallic acid and p-coumaric acid
Parameter
Group |
Total thiols (nmol/100mg tissue) |
GSH (nmol/100mg tissue) |
SOD (U/g tissue) |
CAT (Kx102) |
GPx (mU/100mg tissue) |
GST (U/100mg tissue) |
Control |
493.86 ± 4.38d |
270.83 ± 12.66c |
16.79 ± 0.24d |
47.67 ± 5.15c |
98.90 ± 0.86d |
189.90 ± 1.02d |
Diabetic |
385.29 ± 3.17a |
92.08 ± 12.24a |
10.24 ± 0.21a |
19.56 ± 1.5a |
56.93 ± 1.66a |
145.95 ± 1.40a |
Diabetic + GA |
457.35 ± 4.26c |
212.02 ± 4.43b |
14.85 ± 0.32c |
38.40 ± 0.39b |
82.50 ± 1.32c |
167.52 ± 1.21c |
Diabetic + PCA |
425.73 ± 3.39b |
186.48 ± 5.14b |
13.86 ± 0.40b |
35.78 ± 0.96b |
73.33 ± 1.33b |
159.73 ± 0.74b |
Data are expressed as Mean ± SE of six rats from each group. Means not sharing a common superscript
symbol(s) differ significantly at P < 0.05 (DMRT)
The level of TNF-α in all experimental groups was shown in figure 1. Values of TNF-α obtained from diabetic rats revealed a significant increase as compared to control rats. Treatment of diabetic rats with gallic acid or p-coumaric acid showed a profound decrease in the TNF-α concentration (Figure 2).
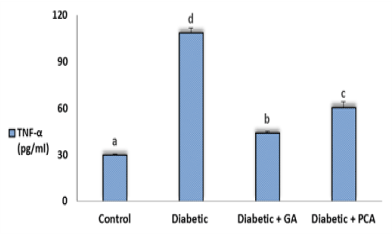
Figure 2. Tumor necrosis factor alpha (TNF-α) of control, diabetic and diabetic rats treated with gallic acid and p-coumaric acid.
Tables 3&4 described the effect of treatments on RBCs count and its indices. Results obtained from diabetic rats showed a significant decrease in RBCs count, Hb content, HCT, MCV, MCH, and MCHC when compared to corresponding control rats (Tables 3 and 4). Consequently, diabetic rats suffer from hypochromic anemia with anisocytosis. Orally treated diabetic rats returned the levels of these parameters nearly to the normal values. The platelets count of diabetic rats exhibited a significant decrease as compared to normal ones. Upon treatment, platelets count was improved and returned near to normal values.
Table 3. RBCs count, Hb content, HCT and platelets of control, diabetic and diabetic rats
Treated with gallic acid and p-coumaric acid
 Parameter Parameter
Group |
RBCs (106/μl) |
HCT (%) |
Hb (g/dl) |
Platelets (103/μl) |
Control |
6.80 ± 0.21b |
49.70 ± 0.75c |
15.55 ± 0.31c |
341.00 ± 15.24b |
Diabetic |
5.70 ± 0.21a |
35.60 ± 1.79a |
10.71 ± 0.53a |
214.16 ± 3.98a |
Diabetic + GA |
6.43 ± 0.16b |
46.60 ± 0.41b |
14.10 ± 0.11b |
311.50 ± 14.76ab |
Diabetic + PCA |
6.31 ± 0.16b |
45.50 ± 0.67b |
13.96 ± 0.47b |
281.00 ± 4.32ab |
Data are expressed as Mean ± SE of six rats from each group. Means not sharing a common superscript symbol(s) differs significantly at P < 0.05 (DMRT)
Table 4. MCV, MCH, and MCHC of control, diabetic and diabetic rats treated
with gallic acid and p-coumaric acid
 Parameter Parameter
Group |
MCV (fl) |
MCH (pg) |
MCHC (%) |
Control |
73.26 ± 2.63b |
22.95 ± 0.81b |
31.38 ± 0.45b |
Diabetic |
63.61 ± 2.62a |
18.71 ± 0.79a |
29.61 ± 0.39a |
Diabetic + GA |
70.96 ± 1.04b |
21.95 ± 0.71b |
30.23 ± 0.03ab |
Diabetic + PCA |
73.13 ± 1.84b |
22.06 ± 0.41b |
30.11 ± 0.81ab |
Data are expressed as Mean ± SE of six rats from each group. Means not sharing a common
superscript symbol(s) differ significantly at P < 0.05 (DMRT)
As showed in table 5, total WBCs count revealed a significant increase in diabetic rats as compared to control rats. Moreover, differential WBCs count of the diabetic group showed significantly increased in neutrophils, eosinophils, and monocytes as compared to control group. All these recorded changes were improved after treatments with the two tested agents. In contrast, there was no significant increase in both basophils and lymphocytes (Table 5).
Table 5. Total and differential WBCs count of control, diabetic and diabetic rats treated with gallic acid and p-coumaric acid
Parameter Group |
WBCs (103/mm3) |
Neutrophils (%) |
Lymphocytes (%) |
Monocytes (%) |
Eosinophils (%) |
Control |
7.33 ± 0.64a |
37.16 ± 2.30a |
52.33 ± 1.76a |
5.50 ± 0.42a |
4.00 ± 0.89a |
Diabetic |
10.71 ± 0.98b |
52.33 ± 1.83c |
47.50 ± 3.89a |
7.50 ± 0.67b |
2.00 ± 0.25b |
Diabetic + GA |
7.90 ± 0.44a |
41.16 ± 2.44ab |
50.33 ± 4.31a |
6.66 ± 0.49ab |
2.66 ± 0.33ab |
Diabetic + PCA |
8.41 ± 0.30a |
45.16 ± 1.95b |
46.50 ± 1.47a |
6.66 ± 0.61ab |
2.66 ± 0.33ab |
Data are expressed as Mean ± SE of six rats from each group. Means not sharing a common superscript symbol(s) differ significantly at P < 0.05 (DMRT)
Oxidative stress is defined as an imbalance between cellular antioxidant capacity and reactive oxygen species (ROS) production [33]. Oxidative stress plays a major role in the pathogenesis of diabetes and its complications [34]. Hyperglycemia causes tissue damage through multiple mechanisms including increased flux of glucose and other sugars through the polyol pathway, increased intracellular formation of advanced glycation end products (AGEs), increased expression of the receptor for AGEs and its activating ligands, activation of protein kinase C isoforms, and over-activity of the hexosamine pathway [35].
In the current study, MDA and NO levels in liver homogenate were significantly elevated in diabetic rats and declined by treatment with GA and PCA. Increased level of MDA in diabetics suggests that peroxidative injury may be involved in the development of diabetic complications. The increase in lipid peroxidation is also an indication of decline in defense mechanisms of enzymatic and nonenzymatic antioxidants [36]. In line with the previously published reports, our findings confirm the free radical scavenging activity of both GA and PCA [37,38]. In addition, GA showed more powerful radical scavenging activity than PCA [39]. Phenolic acids have been considered to have a high antioxidant ability and free radical scavenging capacity via several mechanisms including direct free radical scavenging activity, inhibiting the enzymes responsible for ROS production and upregulation of the antioxidant enzymes [40]. In addition, GA, PCA, gentisic acid and ferulic acid selectively induce hepatic mRNA transcripts for SOD, CAT, and GPx likely through upregulation of gene transcription as well as the Nrf2 transcription factor indicating their potential antioxidant role in liver [41]. Oxidative stress coexisted with a reduction in the antioxidant capacity, which could increase the deleterious effects of free radicals in diabetics. The accumulation of free radical observed in diabetic rats is attributed to chronic hyperglycemia that declines enzymatic and non-enzymatic antioxidant defense systems [42].
The present results also revealed that daily administration of GA and PCA to diabetic rats significantly increased the activities of antioxidant enzymes (SOD, CAT, GPx, and GST) and the levels of total thiols and GSH in the liver of diabetic rats. This could be due to decreased oxidative stress as evidenced by decreased LPO. In accordance with our results, Patel and Goyal, Ahad et al. and Huang et al. reported that GA reverted back the altered levels of these antioxidants in different tissues (liver, kidney, and heart) and different experimental animal models of diabetes [43-45]. GA significantly increased the activities of enzymatic antioxidants (SOD, CAT, GPx, GR, and GST) in the heart and non-enzymatic antioxidants (GSH, vitamin C, and E) in plasma and the heart in experimentally induced myocardial infarction model in Wistar rats [46]. Furthermore, Amalan and Vijayakumar reported that SOD, CAT, and GPx activities elevated and LPO level decreased indicating the efficacy of PCA in attenuating the oxidative stress in diabetic liver [47].
On the other hand, numerous evidences were correlated with oxidative stress and inflammation with the complications of diabetes as a result of NF-𝜅B activation [48]. The activated NF-κB regulates various pro-inflammatory mediators such as iNOS, and pro-inflammatory cytokines such as interleukins (ILs) and TNF-α [49].
The treatment of diabetic rats with GA and PCA significantly reduced the levels of serum TNF-α, proving the anti-inflammatory effects of both agents. GA also has an inhibitory activity on p38 MAPK activation and downstream TNF-α and IL-6 production [50]. Furthermore, PCA and UA enhanced the inhibitory effects against inflammation via inactivation of both NF-κB and MAPKs signaling pathways. Zhao et al. found that PCA may inhibit the production of inflammatory cytokines induced by lipopolysaccharide (LPS) in RAW264.7 macrophage cells through blocking NF-κB and MAPKs signaling pathways [51].
Hyperglycemia causes an increase in the oxidative stress that is responsible for the development of hematological complications in diabetic patients [52]. In the present study, the observed decreased levels of RBCs, Hb, HCT, MCV, MCH and MCHC in diabetic rats indicating an anemic condition. These findings are in accordance with the previous finding of Edet et al. [53]. The development of anemia in DM has been explained with increased non-enzymatic glycosylation of RBC membrane proteins [54]. Oxidation of these proteins and persistent hyperglycemia in DM causes an increase in the production of lipid peroxides leading to hemolysis of RBC [55]. The major pathological consequences of free radical induced membrane lipid peroxidation include increased membrane rigidity, decreased cellular deformability, reduced erythrocyte survival, and lipid fluidity [56]. Following GA and PCA administration, the alterations of RBCs and its related indices were considerably improved. This finding could be due to radical scavenging and antioxidant activity of both agents resulting in decreased hemolysis. It may also occur via stimulating formation or secretion of erythropoietin, which stimulates stem cells in the bone marrow of rats to produce new RBCs [57].
The present data also revealed obvious alterations in the total and differential leukocyte count of diabetic rats, especially an elevation of WBCs count, neutrophils, and monocytes. These results are in agreement with those of others [58,59]. The mechanism of leukocytosis in diabetes is exactly unknown. However, leukocytosis in diabetes may be activated through the release of cytokines such as TNF-α, NF-κB and transforming growth factor 1 [60]. Interleukins and TNF-α are released from activated leukocytes and cause endothelial dysfunction [61]. Moreover, Heidland et al. reported that leukocytes can be activated by glycation end products, oxidative stress, angiotensin II resulting from hyperglycemia, and can produce factors like tumor necrosis factor-α and interleukin β1 that involve chronic diabetes complication pathogenesis [62].
Platelets are the fragment of cells that participate in blood clotting, they initiate repair of blood vessels walls and are also considered as an acute phase reactant to infection or inflammation [63]. In line with the study of Zakrzeska et al., the obtained data indicated a reduction in the platelet count of diabetic rats relative to control ones [64]. Hyperglycemia may represent a causal factor for in vivo platelet activation and may be responsible for nonenzymatic glycation of platelet glycoproteins, causing changes in their structure and conformation, as well as alterations of membrane lipid dynamics. Furthermore, hyperglycemia‐induced oxidative stress is responsible for enhanced peroxidation of arachidonic acid to form biologically active isoprostanes, which represents an important biochemical link between impaired glycemic control and persistent platelet activation [65]. On the other hand, there was no significant change in platelet counts after treatment with GA or PCA although platelet counts moderately increased.
Gallic acid and p-coumaric acid are powerful antioxidants that revealed many beneficial effects in diabetic rats such as decreasing oxidative stress, improving antioxidant status, diminishing inflammation and relieving hematological abnormalities. Thus, both agents are good candidates for management of diabetes and diabetic complications.
The authors declare that they have no conflicts of interest.
- Zatalia SR, Sanusi H (2013) The role of antioxidants in the pathophysiology, complications, and management of diabetes mellitus. Acta Med Indones 45: 141-147. [Crossref]
- American Diabetes Association (ADA) (2014) Diagnosis and classification of diabetes mellitus. Diabetes Care 37: S81-S90.
- Ahlqvist E, van Zuydam NR, Groop LC, McCarthy MI (2015) The genetics of diabetic complications. Nature Rev Nephrol 11: 277-287. [Crossref]
- Asmat U, Abad K, Ismail K (2016) Diabetes mellitus and oxidative stress- a concise review. Saudi Pharm J 24: 547-553. [Crossref]
- Finkel T, Holbrook NJ (2000) Oxidants, oxidative stress and the biology of aging. Nature 408: 239-247. [Crossref]
- Phillips M, Cataneo RN, Cheema T, Greenberg J (2004) Increased breath biomarkers of oxidative stress in diabetes mellitus. Clin Chim Acta 344: 189-194. [Crossref]
- Asfandiyarova N, Kolcheva N, Ryazantsev I, Ryazantsev V (2007) Risk factors for stroke in type 2 diabetes mellitus. Diab Vasc Dis Res 3: 57-60. [Crossref]
- Demirtas L, Degirmenci H, Akbas EM, Ozcicek A, Timuroglu A, Gurel A, Ozcicek E (2015) Association of hematological indicies with diabetes, impaired glucose regulation and microvascular complications of diabetes. Int J Clin Exp Med 8: 11420-11427. [Crossref]
- Senapathy JG, Jayanthi S, Viswanathan P, Umadevi P, Nalini N (2011) Effect of gallic acid on xenobiotic metabolizing enzymes in 1,2-dimethyl hydrazine induced colon carcinogenesis in Wistar rats-a chemopreventive approach. Food Chem Toxicol 49: 887-892. [Crossref]
- Latha RCR, Daisy P (2011) Insulin-secretagogue, antihyperlipidemic and other protective effects of gallic acid isolated from Terminalia bellerica Roxb. in streptozotocin-induced diabetic rats. Chem Biol Interact 189: 112-118. [Crossref]
- Abdel-Moneim AA, Yousef AI, Abd El-Twab SM, Abdel Reheim ES, Ashour MB (2017) Gallic acid and p-coumaric acid attenuate type 2 diabetes-induced neurodegeneration in rats. Metab Brain Dis 32: 1279-1286. [Crossref]
- Liao CL, Lai KC, Huang AC, Yang JS, Lin JJ, et al. (2012) Gallic acid inhibits migration and invasion in human osteosarcoma U-2 OS cells through suppressing the matrix metalloproteinase-2/-9, protein kinase B (PKB) and PKC signaling pathways. Food Chem Toxicol 50: 1734-1740. [Crossref]
- Abdel Moneim AA, Abd El-Twab SM, Ashour MB, Yousef AI (2016) Hepato-renal protective effects of gallic acid and p-coumaric acid in nicotinamide/streptozotocin-induced diabetic rats. Int J Bioassays 5: 4641-4649.
- Mansouri MT, Farbood Y, Sameri MJ, Sarkaki AR, Naghizadeh B et al. (2013) Neuroprotective effects of oral gallic acid against oxidative stress induced by 6-hydroxydopamine in rats. Food Chem 138: 1028-1033. [Crossref]
- Reckziegel P, Dias VT, Benvegnu D, Boufleur N, Silva Barcelos RC et al. (2011) Locomotor damage and brain oxidative stress induced by lead exposure are attenuated by gallic acid treatment. Toxicol Lett 203:74-81 [Crossref]
- Alamed J, Chaiyasit W, McClements DJ, Decker EA (2009) Relationships between free radical scavenging and antioxidant activity in foods. J Agric Food Chem 57: 2969-2976. [Crossref]
- Ambika S, Saravanan R, Thirumavalavan K (2013) Antidiabetic and antihyperlipidemic effect of p-hydroxycinnamic acid on streptozotocin-induced diabetic Wistar rats. Biomedicine and Aging Pathology 3: 253-257.
- Luceri C, Guglielmi F, Lodovici M, Giannini L, Messerini L et al. (2004) Plant phenolic 4-coumaric acid protects against intestinal inflammation in rats. Scand J Gastroenterol 39: 1128-1133. [Crossref]
- Canadian Council on Animal Care (CCAC) (1993) Guide to the care and use of experimental animals. Ontario: Canada 2: 1-298.
- Masiello P, Broca C, Gross R, Roye M, Manteghetti M, et al. (1998) Experimental NIDDM: Development of a new model in adult rats administered streptozotocin and nicotinamide. Diabetes 47: 224-229. [Crossref]
- Preuss HG, Jarrel ST, Scheckenbach R, Lieberman S, Anderson RA (1998) Comparative effects of chromium, vanadium and Gymnema sylvestre on sugar-induced blood pressure elevations in SHR. J Am Coll Nutr 17: 116-123. [Crossref]
- Miranda KM, Espey MG, Wink DA (2001) A rapid, simple trophometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide 5: 62-71. [Crossref]
- Beutler E, Duron O, Kelly BM (1963) Improved method for determination of blood glutathione. J Lab Clin Med 61: 882-888. [Crossref]
- Marklund S, Marklund G (1974) Involvement of superoxide anion radical in the autooxidation of pyrogallol and convenient assay for superoxide dismutase. Eur J Biochem 47: 469-674. [Crossref]
- Cohen G, Dembiec D, Marcus J (1970) Measurement of catalase activity in tissue extracts. Anal Biochem 34: 30-38. [Crossref]
- Matkovics B, Sasvari M, Kotorman M, Varga IS, Hai DQ, Varga C (1998) Further prove on oxidative stress in alloxan diabetic rat tissues. Acta Physiol Hung 85:183-192. [Crossref]
- Mannervik B, Guthenberg C (1981) Glutathione Transferase (Human placenta). Methods Enzymol 77: 231-235. [Crossref]
- Dacie JV, Lewis SM (1991) Practical hematology. 7th ed., Edinburgh: ELBS with Churchill Livingstone, 37-85.
- Alexander RR, Grifiths JM (1993) Basic Biochemical Methods. 2nd ed., New York: Willey-Liss 186-187.
- Trowbridge EA, Martin JF, Slate DN, Kishk YT, Warren CW et al. (1984) The origin of platelet count and volume. Clin Phys Physiol Meas 5: 145-170. [Crossref]
- Osim EE, Akpogomeh BA, Ibu JO, Eno AE (2004) Experimental Physiology Manual. 3rd ed., Calabar: University of Calabar, Dept Physiol, 60-81.
- Bisse E, Abraham EC (1985) New less temperature-sensitive microchromatographic method for the separation and quantitation of glycosylated hemoglobins using a non-cyanide buffer system. J Chromatogr 344: 81-91. [Crossref]
- Kašparova S, Brezova V, Valko M, Horecky J, Mlynarik V, et al. (2005) Study of the oxidative stress in a rat model of chronic brain hypoperfusion. Neurochem Int 46: 601-611. [Crossref]
- Abdel-Moneim A, Bakery HH, Allam G (2018) The potential pathogenic role of IL-17/Th17 cells in both type 1 and type 2 diabetes mellitus. Biomed Pharmacother 101: 287-292. [Crossref]
- Brownlee M (2005) The pathobiology of diabetic complications: a unifying mechanism. Diabetes 54: 1615-1625. [Crossref]
- Saddala RR, Thopireddy L, Ganapathi N, Kesireddy SR (2013) Regulation of cardiac oxidative stress and lipid peroxidation in streptozotocin-induced diabetic rats treated with aqueous extract of Pimpinella tirupatiensis tuberous root. Exp Toxicol Pathol 65: 15-19. [Crossref]
- Shiromwar SS, Chidrawar VR (2011) Combined effects of p-coumaric acid and naringenin against doxorubicin-induced cardiotoxicity in rats. Pharmacognosy Res 3: 214-219. [Crossref]
- Kulkarni JM, Swamy AV (2015) Cardioprotective effect of gallic acid against doxorubicin-induced myocardial toxicity in albino rats. Indian J.Health Sci 8: 28-35.
- Karoui IJ, Marzouk B (2013) Characterization of bioactive compounds in Tunisian bitter orange (Citrus aurantium L.) peel and juice and determination of their antioxidant activities. Biomed Res Int 345415: 1-12. [Crossref]
- Saibabu V, Fatima Z, Khan LA, Hameed S (2015) Therapeutic potential of dietary phenolic acids. Adv Pharmacol Sci 823539: 1-10. [Crossref]
- Yeh CT, Yen GC (2006) Induction of hepatic antioxidant enzymes by phenolic acids in rats is accompanied by increased levels of multidrug resistance-associated protein 3 mRNA expression. J Nutr 136: 11-15. [Crossref]
- Rahimi R, Nikfar S, Larijani B, Abdollahi M (2005) A review on the role of antioxidants in the management of diabetes and its complications. Biomed Pharmacother 59: 365-373. [Crossref]
- Patel SS, Goyal RK (2011) Cardioprotective effects of gallic acid in diabetes-induced myocardial dysfunction in rats. Pharmacognosy Res 3: 239-245. [Crossref]
- Ahad A, Ahsan H, Mujeeb M, Siddiqui WA (2015) Gallic acid ameliorates renal functions by inhibiting the activation of p38 MAPK in experimentally induced type 2 diabetic rats and cultured rat proximal tubular epithelial cells. Chem Biol Interact 240: 292-303. [Crossref]
- Huang DW, Chang WC, Wu JSB, Shih RW, Shen SC (2016) Gallic acid ameliorates hyperglycemia and improves hepatic carbohydrate metabolism in rats fed a high-fructose diet. Nutr Res 36: 150-160. [Crossref]
- Priscilla DH, Prince PSM (2009) Cardioprotective effect of gallic acid on cardiac troponin-T, cardiac marker enzymes, lipid peroxidation products and antioxidants in experimentally induced myocardial infarction in Wistar rats. Chem Biol Interact 179: 118-124. [Crossref]
- Amalan V, Vijayakuma N (2015) Antihyperglycemic effect of p-coumaric acid on streptozotocin induced diabetic rats. Indian J Appl Res 5: 10-13. [Crossref]
- Patel S, Santani D (2009) Role of NF-κB in the pathogenesis of diabetes and its associated complications. Pharmacol Rep 61: 595-603. [Crossref]
- Tsai SJ, Lin CY, Mong MC, Ho MW, Yin MC (2010) S-Ethyl cysteine and S-propyl cysteine alleviateβ-amyloid induced cytotoxicity in nerve growth factor differentiated PC12 cells. J Agric Food Chem 58: 7104-7108.
- Kim SH, Jun CD, Suk K, Choi BJ, Lim H, et al. (2006) Gallic acid inhibits histamine release and pro-inflammatory cytokine production in mast cells. Toxicol Sci 91: 123-131. [Crossref]
- Zhao Y, Liu J, Liu C, Zeng X, Li X (2016) Anti-inflammatory effects of p-coumaric acid in LPS-stimulated RAW264.7 cells: Involvement of NF-κB and MAPKs pathways. Med Chem 6: 327-330.
- Asmah RH, Yeboah G, Asare-Anane H, Antwi-Baffour S, Archampong TN et al. (2015) Relationship between oxidative stress and haematological indices in patients with diabetes in the Ghanaian population. Clin Diabetes Endocrinol 1: 1-5. [Crossref]
- Edet AE, Patrick EE, Olorunfemi A (2013) Hematological parameters of alloxan-induced diabetic rats treated with ethanol extracts and fractions of Nauclea lafiloia leaf. Eur Sci J 9: 203-210.
- Oyedemi SO, Adewusi EA, Aiyegoro OA, Akinpelu DA (2011) Antidiabetic and hematological effect of aqueous extract of stem bark of Afzelia africana (Smith) on streptozotocin-induced diabetic Wistar rats. Asian Pac. J Trop Biomed 1: 353-358. [Crossref]
- Arun GS, Ramesh KG (2002) Improvement of insulin sensitivity by perindopril in spontaneously hypertensive and streptozotocin diabetic rats. Indian J Pharmacol 34: 156-64.
- Kolanjiappan K, Manoharan S, Kayalvizhi M (2002) Measurement of erythrocyte lipids, lipid peroxidation, antioxidants and osmotic fragility in cervical cancer patients. Clin Chim Acta 326: 143-149. [Crossref]
- Ohlsson A, Aher SM (2006) Early erythropoietin for preventing red blood cell transfusion in preterm and/or low birth weight infants. Cochrane Database Syst Rev 3: CD004865. [Crossref]
- Mahmoud AM (2013) Hematological alterations in diabetic rats-role of adipocytokines and effect citrus flavonoids. EXCLI J 12: 647-657. [Crossref]
- Rajeswari P, Krishnakumari S (2013) Potent antihyperglycaemic activity of Calocybe indica in streptozotocin induced diabetic rats antihyperglycemic activity of Calocybe indica. Int J Pharm Pharm Sci 5: 512-515.
- Kanter M, Altan MF, Donmez S, Ocakc A, Kartal ME (2007) The effects of quercetin on bone minerals, biomechanical behavior, and structure in streptozotocin-induced diabetic rats. Cell Biochem Funct 25: 747-752. [Crossref]
- Henry CBS, Duling BR (2000) TNF-a increases entry of macromolecules into luminal endothelial cell glycocalyx. Am J Physiol Heart Circ Physiol 279: H2815-2823. [Crossref]
- Heidland A, Sebekova K, Schinzel R (2001) Advanced glycation end products and the progressive course of renal disease. Am J Kidney Dis 38: S100-106. [Crossref]
- Ganong WF (1999) A Review of Medical Physiology. 19th ed., Appleton and Lange, Stanford, USA, 187-241.
- Zakrzeska A, Gromotowicz-Popławska A, Szemraj J, Szoka P, Kisiel W et al. (2015) Eplerenone reduces arterial thrombosis in diabetic rats. J Renin Angiotensin Aldosterone Syst 16: 1085-1094. [Crossref]
- Ferroni P, Basili S, Falco A, Davı G (2004) Platelet activation in type 2 diabetes mellitus. J Thromb Haemost 2: 1282-1291. [Crossref]


