Abstract
Background: Neurological disease may be a risk factor for COVID-19, in part because of the higher rate of comorbidities associated with these patients.
Aim of the study: To evaluate the association between chronic neurological diseases and clinical phenotypes of COVID-19 patients admitted to intensive care units (ICUs).
Methods: Data included 1048 ICU patients with COVID-19 in Cuban hospitals nationwide between January and August 2021. The optimal number of clusters (K) was estimated by modified v-fold cross-validation. Demographic variables such as age, symptoms, and comorbidities were used to define clinical phenotypes.
Results: One or more chronic neurological diseases (CND) were found in 178 patients (17%), of which cerebrovascular disease, dementia, epilepsy, and Parkinson’s disease were the most common. Although the proportion of CND was distributed among the six phenotypes, it was predominant in phenotypes class 3 (22.92%) [hypertension, diabetes, and cough], class 2 (20.37%) [asthma, cough, and fever], and class 4 (18%) [ hypertension without symptoms]. A significant association was found between CND occurrence and phenotypes (p = 0.05). The mortality rate in the total cohort was 178 (17 %). Of this number, 41 (23%) had preexisting CND. Survival analysis indicated that patients with CND have a similar probability of survival as patients without CND. Moreover, most deaths associated with CND were located in clusters 3 (34.15%), and 1 (29.26 %) [hypertension, coronary artery disease, cough, and fever].
Conclusions: This approach could help stratify COVID-19 patients with CND in ICUs to personalize therapy and rationally target healthcare services.
key words
neurological diseases, comorbidities, phenotypes, intensive care units, COVID-19
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is responsible for the 2019 coronavirus disease outbreak (COVID-19). Initially identified in Wuhan, China, in December 2019, it is now clear that the COVID-19 pandemic has become a global challenge [1,2].
Coronaviruses are a large class of viruses that typically cause mild to moderate upper respiratory tract infections; however, the clinical spectrum and severity of COVID-19 are broad and complex. Additionally, the clinical presentation and severity of COVID-19 patients can vary widely and extend outside the respiratory system. Some individuals are asymptomatic, whereas others may develop severe pneumonia with acute respiratory failure [3-6].
Intensive care units (ICUs) play an essential role in managing critically ill patients, as mortality is prominent in this group [7,8]. Older age and underlying chronic conditions or comorbidities, such as heart disease, hypertension, diabetes mellitus, chronic pulmonary disease, obesity, and cancer [9-12], have been associated with increased severity of COVID-19, hospitalization, ICU admission, and death [11,13-15].
Although most patients with COVID-19 develop primarily respiratory symptoms, the occurrence of neurological symptoms in 30-50% of patients has been associated with disease severity and mortality, suggesting a potential neurotropism of SARS-CoV-2 as a possible mechanism of neurological damage [16,17]. Moreover, some studies have indicated that neurological disease may be a risk factor for COVID-19, in part because of the higher rate of comorbidities associated with these patients. However, the frequency, type, and implications of preexisting chronic neurological diseases (CND) in patients with COVID-19 have been little explored and are still unknown [18].
A recent study confirmed the worsening of neurological symptoms associated with COVID-19 in patients with preexisting neurological diseases [19]. Furthermore, mortality due to COVID-19 is increased in patients with CND, particularly neurodegenerative diseases [20].
Although the heterogeneity of the clinical manifestations determines COVID-19 diagnosis and prognosis challenging, some studies have robustly explored and characterized the clinical phenotypes of the disease [21-23]. However, it is difficult for clinicians to consider the plethora of information on multiple symptoms and comorbidities, especially when patients have a less severe condition. There are few reports of the relationship between COVID-19 and CND. In fact, during the literature review, no publication was found on the use of phenotypic clusters to characterize COVID-19 patients with neurological disorders in ICUs.
Cluster analysis is considered one of the most popular unsupervised learning methods to identify subgroups that share similar characteristics. This method has been used to investigate some diseases’ heterogeneity and classify clinical phenotypes with similar traits [22,24,25].
This study aimed to characterize the association between the presence of CND and clinical phenotypes based on age, symptomatology, and comorbidities in patients with COVID-19 in Cuban ICUs.
Materials and methods
Patient selection and data collection
The Cuban Ministry of Health expert group conducted a retrospective observational cohort from January to August 2021. Data were collected from 1048 ICU patients with COVID-19 from 25 Cuban hospitals, and a structured database was created. Only ICU patients over 18 years of age were included in the study. The diagnosis of COVID-19 was confirmed by positive high-throughput sequencing or real-time reverse transcription-polymerase chain reaction (RT-PCR) assay with nasal and pharyngeal swab samples.
Demographic variables included age, sex, and race. Comorbidities and symptoms presenting in all patients on admission were collected. The lead researcher (LMC) collected and verified each patient's clinical data, treatment, and outcome.
Clinical symptoms included fever, cough, nasal congestion, headache, fatigue, dyspnea, rhinorrhea, nausea and vomiting, diarrhea, arthralgia, myalgia, ageusia, anosmia, chills, chest pain, sore throat and loss of consciousness.
Regarding comorbidities, we analyzed the presence of hypertension, diabetes, coronary artery disease, chronic obstructive pulmonary disease (COPD), asthma, cancer, obesity, psychiatric illness, chronic renal disease, alcoholism, immunodeficiency disorders, smoking (current or in the last six months), as well as CND.
According to the World Health Organization, disabling CNDs can be defined as those neurological disorders that (a) cause persistent disability, (b) limit the individual’s functioning, and (c) interfere with the person’s ability to perform in activities [20]. In addition to these characteristics, conditions affecting mental and physical functions were also included. Thus, CND comprised dementia, epilepsy, movement disorders, previous stroke with long-term sequelae, neuromuscular disorders, spinal disorders, symptomatic central nervous system cancer, chronic encephalopathies, and neuroinflammatory disorders.
The presence of a history of neurological diseases was specifically evaluated, and only the conditions meeting criteria (a), (b), and (c) were included.
Clinical, radiological, and laboratory examinations were performed on all subjects during the first 24 h of ICU admission. Imaging test results included chest radiograph (CXR) abnormalities. Laboratory tests included white blood cells, lymphocyte and neutrophil count, platelet count, mean platelet volume, and neutrophil-to-lymphocyte ratio. Although not included in the data analysis, other variables such as ventilation and severity of illness on admission to the ICU were also included. Total days in the ICU and outcomes were also collected to compare survival rate and in-hospital mortality, representing the endpoint of this study. Patients were treated according to the Cuban protocol version 1.6 (available at https://covid19cubadata.github.io/protocolos/protocolo-version-6.pdf).
Ethical Statement
This study was revised and approved by the scientific committee and the ethics committee of the Innovative Commission of the Cuban Ministry of Health. Written informed consent was waived due to the use of identified retrospective data. All procedures performed followed the standards of the 1975 Declaration of Helsinki for human research.
Statistical Analysis
We performed unsupervised classification, commonly known as clustering, to identify clinical patterns related to symptoms, comorbidities, and age. Cluster analysis is widely used to identify subgroups with interesting biomedical data. It has proven to be an invaluable tool for the exploratory and unsupervised analysis of high-dimensional datasets [26,27].
The K-means algorithm based on unsupervised learning was used [28]. The optimal number of clusters (K) was estimated using modified V-fold cross-validation to use the K-means algorithm.
The steps of the algorithm are as follows:
- Determine the number of clusters (K)
- Establish the centroids by first shuffling the dataset and then randomly selecting data points for the centroids without replacement
- Calculate the distance between data points and all centroids
- Allocate each data point to the nearest cluster (centroid)
- Update the position of the centroid based on the assigned data
- Maintain the iteration until there are no changes in the centroids
At this point, values are assigned in K-clusters without any hierarchical structure by optimizing the minimum distance between points with each of the available clusters, applying Euclidean distance between data points and centroids as distance criterion.
Qualitative and ordinal variables are indicated in terms of frequency and percentage. Continuous variables are shown as medians and minimum-maximum values or mean and standard deviation (SD).
Statistical analysis included the χ2 test or Fisher’s exact test for contrasting categorical variables, adjusting the p-value by the Bonferroni correction of multiple comparisons. The Shapiro–Wilk test assessed the normality of the distribution. Due to the nature of the study, the sample size was not calculated. The significance threshold was set at 0.05 after appropriate adjustment.
The prognosis of COVID-19 patients was recorded based on ICU hospitalization days and outcomes. Outcomes of patients with CND and those with no clinical history of CND were followed up to hospital discharge or death. Total days in the ICU were compared, and survival analysis was performed using Kaplan-Meier curves. The Kaplan-Meier method and log-rank test were also used to examine similarities and differences in the prognosis of patients with COVID-19 between phenotypes.
StatSoft, Inc. (2004). STATISTICA (data analysis software system), version 7. www.statsoft.com was used for statistical analyses. The Generalized Expectation Maximization (EM) and k-Means Cluster Analysis modules were applied to analyze clustering in data mining.
Results
Data on age, sex, symptoms, and comorbidities were collected from 1048 patients with COVID-19. Table 1 summarizes the clinical and demographic characteristics among COVID-19 patients with and without CND in the cohorts. One or more CND was present in 178 (17%) patients. According to the Cuban Society of Emergency Medicine Guidelines, 28.4% of these patients were considered critically ill on admission to the ICU.
Table 1. Demographic and clinical characteristics among COVID- 19 patients in cohorts with and without chronic neurological diseases
Variables |
No chronic neurological diseases
(n = 870) |
Chronic neurological diseases
(n = 178) |
p-values |
Age |
64.83 ± 16.16 |
73.84 ± 15.15 |
p = 0.00* |
Male sex, n (%) |
362 (41.64) |
81 (45.5) |
p = 0.34 |
White race, n (%) |
301 (74.79) |
76 (75.95) |
p= 0.51 |
Days in ICU |
4.33 ± 3.62 |
4.83 ± 4.49 |
p = 0.12 |
Hypertension, n (%) |
611 (70.2) |
127 (71.34) |
p = 0.36 |
Diabetes, n (%) |
243 (27.93) |
61 (34.2) |
p = 0.95 |
COPD, n (%) |
91 (10.45) |
18 (10.1) |
p = 0.52 |
Coronary disease, n (%) |
236 (27.1) |
46 (25.84) |
p = 0.89 |
Asthma, n (%) |
71 (8.16) |
12 (6.74) |
p = 0.18 |
Cancer, n (%) |
55 (6.32) |
14 (7.86) |
p = 0.39 |
Chronic kidney disease, n (%) |
86 (9.8) |
10 (5.52) |
p = 0.07 |
Alcoholism, n (%) |
- |
1 (0.5) |
p = 0.98 |
Smoking, n (%) |
11 (1.26) |
2 (1.12) |
p = 0.87 |
Psychiatric disease, n (%) |
9 (1.26) |
2 (1.12) |
p = 0.91 |
Pregnancy, n (%) |
14 (1.60) |
0 |
p = 0.11 |
Obesity, n (%) |
91 (10.4) |
8 (4.49) |
p = 0.003* |
Immunodeficiency, n (%) |
1 (0.11) |
- |
- |
Fever, n (%) |
290 (33.3) |
52 (29.2) |
p = 0.12 |
Cough, n (%) |
362 (41.6) |
76 (42.6) |
p = 0.90 |
Dyspnea, n (%) |
241 (27.7) |
37 (20.7) |
p = 0.05* |
Headache, n (%) |
62 (7.12) |
5 (2.8) |
p = 0.03* |
Rhinorrhea, n (%) |
49 (5.63) |
4 (2.24) |
p = 0.06 |
Diarrhea, n (%) |
30 (3.4) |
7 (3.93) |
p = 0.45 |
Fatigue, n (%) |
62 (7.12) |
20 (11.24) |
p = 0.06 |
Ageusia, n (%) |
28 (3.22) |
3 (1.69) |
p = 0.02* |
Anosmia, n (%) |
32 (3.6) |
2 (1.12) |
p = 0.07 |
Sore throat, n (%) |
17 (1.95) |
0 |
p = 0.03* |
Myalgia, n (%) |
17 (1.95) |
1 (0.56) |
p = 0.31 |
Chillness, n (%) |
15 (1.72) |
1 (0.56) |
p = 0.31 |
Vomiting, n (%) |
21 (2.41) |
3 (1.68) |
p = 0.50 |
Confusion, n (%) |
5 (0.57) |
3 (1.69) |
p = 0.12 |
Chest pain, n (%) |
9 (1.04) |
1 (0.58) |
p = 0.58 |
Severity of disease n (%) |
256 (31.40) |
46 (28.40) |
p = 0.44 |
Mortality rate, n (%) |
140/870 (16) |
41/178 (23) |
p = 0.50 |
COPD: chronic obstructive pulmonary disease; ICU: Intensive Care Unit.*significant difference vs. patients without neurological diseases Pearson Chi-square, and t value (p ≤ 0.05)
Although patients with CND were older, the rates of hypertension, coronary artery disease, and diabetes were similar to those of patients with no CND. However, obesity was more frequent in this last cohort. In terms of symptoms, there were no differences between the two groups of patients, except for dyspnea, headache, ageusia and sore throat, which showed a slightly higher trend in individuals with no CND (Table 1).
Phenotypes defined by the k-means algorithm for ICU patients with COVID-19 and chronic neurological diseases
Six clusters (K) were estimated to be the optimal number using modified v-fold cross-validation for the k-means algorithm. Demographic variables, including age, symptoms (fifteen), and comorbidities (fourteen), were used to define clinical phenotypes as follows:
- Class 1 phenotype (n = 182): mean age 72.3 years—hypertension, coronary artery disease, cough, and fever (17.36% of the sample)
- Class 2 phenotype (n = 54): mean age 63 years—asthma, cough, and fever (5.1% of the sample)
- Class 3 phenotype (n = 192): mean age 74.5 years—hypertension, diabetes, and cough (18.3% of the sample)
- Class 4 phenotype (n = 349): mean age 67.8 years—hypertension and no symptoms (33.3% of the sample)
- Class 5 phenotype (n = 94): mean age 53 years—cough and no comorbidities (9% of the sample)
- Class 6 phenotype (n = 177): mean age 60 years—without symptoms or comorbidities (16.8% of the sample)
Clusters 3 and 4 were the largest groups. The most prominent symptom was coughing, which was identified in almost all individuals in clusters 1, 2, 3, and 5. In contrast, clusters 2 and 5 had the lowest COVID-19 cases.
From the total sample, CNDs were identified in 178 patients, of which cerebrovascular disease represented 38.2% (68 cases); dementia 36% (64 cases); epilepsy, 16.6% (30 cases); and Parkinson's disease, 4% (7 cases). Neuromuscular and spinal cord diseases, including polyneuropathy, Guillain-Barre syndrome, myasthenia gravis, muscular dystrophy, and radiculopathy, accounted for 6.8% (12 cases). We identified five cases of central nervous system tumors (2.8 %), eleven cases of mental retardation (6 %), one case of head trauma, one of vascular malformation, and one of migraine. More than one CND was identified in some patients; 5.6% (10/178) of these patients had cerebrovascular disease plus dementia.
As shown in Figure 1 although the proportion of CND was distributed among the six phenotypes, it was predominant in phenotypes class 3 (22.92%), class 2 (20.37%), and class 4 (18%). In contrast, the lowest proportions were found in class 1 (12.6%), class 5 (10.64%) and class 6 (15.25%) phenotypes (Figure 1). Moreover, a significant correlation was observed between CND occurrence and the phenotypes defined by the k-means algorithm (p = 0.051).
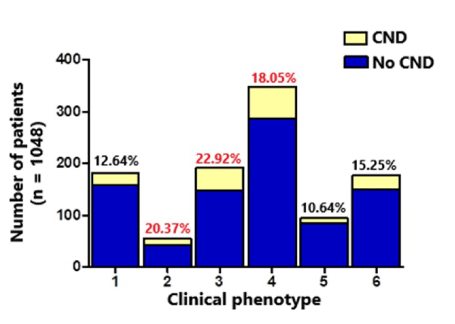
Figure 1. Distribution of patients with specific chronic neurological disorders in six phenotypes defined by the K-means algorithm. Class 1 phenotype (mean age 72.3 years): hypertension, coronary artery disease, and symptoms such as cough and fever (17.74% of the sample); class 2 phenotype (mean age 63 years): asthma, cough, and fever (5.3% of the sample); class 3 phenotype (mean age 74.5 years): hypertension and diabetes, cough (20.3% of the sample). Class 4 phenotype (mean age 67.8 years): hypertension and no symptoms (31.4% of the sample); class 5 phenotype (mean age 53 years): cough, no comorbidities (9.8% of the sample); class 6 phenotype (mean age 60 years): no symptoms or comorbidities (15.2% of the sample). The distribution of patients with chronic neurological disorders in each class phenotype was 12.64%, 20.37%, 22.92%, 18.05%, 10.64%, and 15.25%, respectively
Table 2 shows the distribution of specific CNDs in each phenotype. COVID-19 patients with cerebrovascular disease were found in phenotypes class 3 and 4. A notable association was observed between the presence of cerebrovascular disease and k-means algorithm-defined phenotypes (p = 0.0001). The number of patients affected by dementia was similar in phenotypes class 3 and 4. However, epilepsy was much higher in class 4 and 6 than in class 3. Parkinson’s disease was similarly dispersed in phenotypes 1, 2, 4, and 6. Moreover, 50% of the patients with neuromuscular diseases clustered in phenotype 4. Patients with tumors were placed in phenotypes 4, 5, and 6, while 50% of the subjects with mental retardation were grouped in phenotype 6. The remaining patients with the same condition were located in clusters 4 and 5.
Table 2. Distribution of patients with the most prominent chronic neurological disorders, severity of disease and mortality in each clinical phenotype
|
Clinical Phenotype |
p-values |
Class 1 |
Class 2 |
Class 3 |
Class 4 |
Class 5 |
Class 6 |
Chronic neurological disorders (n = 178) |
23/178 (12.9%) |
11/178 (6.1%) |
44/178 (24.7%) |
63/178 (35.3%) |
10/178 (10.64%) |
27/178 (15.1%) |
p = 0.05* |
Cerebrovascular disease (n = 68) |
9/68 (13.23%) |
2/68 (3%) |
28/68 (41.1%) |
26/68 (38.23%) |
3/68 (4.4%) |
0 (0%) |
p = 0.000* |
Dementia (n = 64) |
13/64 (20%) |
5/64 (7.8%) |
16/64(25%) |
16/64 (25%) |
2/64 (3%) |
12/64 (18.7%) |
p = 0.21 |
Epilepsy (n = 30) |
2/30 (6.6%) |
0 (0%) |
5/30 (16.60%) |
14/30 (46.6%) |
2/30 (6.6%) |
7/30 (23.3%) |
p = 0.28 |
Parkinson’s disease
(n = 7) |
2/7 (28.5%) |
2/7 (28.5%) |
0 (0%) |
2/7 (28.5%) |
0 (0%) |
1/7 (14.28%) |
p = 0.07 |
Neuromuscular
(n = 12) |
1/12 (8.3%) |
2/12 (16.6%) |
1/12 (8.3%) |
5/12 (41.6%) |
2/12 (16.6%) |
1/12 (8.3%) |
p = 0.29 |
Illness disease
(n = 46) |
8/46 (17.3%) |
5/46 (10.8%) |
Severity of disease
(n=46) |
1/46 (2%) |
5/46 (10.48%) |
p = 0.152 |
|
8/46
(17.3%) |
5/46 (10.8%) |
15/46 (32.6%) |
12/46
26.06 % |
1/46
(2%) |
5/46 (10.48%) |
p = 0.15 |
In-patient mortality
(n = 41) |
12/41 (%) |
0 (0%) |
14/41 (34.15%) |
10/41 (24.3%) |
2/41 (4.8%) |
3/41 (7.32%) |
p = 0.001* |
Results are reported as frequencies and percentages. *significant difference among groups Pearson ,Chi-square, (p ≤ 0.05)
Clinical prognosis of the different CNDs and phenotypes of patients with COVID-19 in the ICU
The mortality rate of the total sample was 17% (178 cases). Of this number, 41 patients (23%) had preexisting neurological diseases.
As for neurological diseases, about 56% of the deaths corresponded to cerebrovascular disease (23/41) and dementia 39% (16/41,), followed by epilepsy 7.32% (3/41,), neuromuscular diseases 7.32%), (3/41, and Parkinson’s disease (2/41, 4.88%). Only one patient with an aneurysm was confirmed to have died. Additionally, no deaths associated with mental retardation were recorded in this cohort. Mortality of each neurological disease was as follows: 33.8% in cerebrovascular disease (23/68), 28.6% in Parkinson’s disease (2/7), 25% in neuromuscular disease (3/12), 25% in dementia (16/64), and 10% in epilepsy (3/30).
Similar to the mortality rate associated with the six phenotypes defined for COVID-19, previously described by our group (data in press), most of the patients with CND who died were grouped in clusters class 3 (34.15%), class 1 (29.27%) and class 4 (24.3%), and to a lesser extent in class 5 (4.8%), and 6 (7.32%). Surprisingly, no deaths were reported in patients with CND in cluster 2. A significant relationship was found between patients with CND of each phenotype and inpatient mortality (p = 0.001) (Table 2).
To evaluate patient outcomes across clusters, we first compared the length of hospital stay among the six clusters. The mean ICU stay across all phenotypes was 4.08 ± 3.68 days (range: 2 hours to 39 days). In terms of ICU stay, no differences were detected between patients with CND and those without CND (p = 0.125).
Furthermore, Kaplan-Meier distributions indicated a similar probability of survival in both groups of patients (80.5 vs. 75.75, respectively; p = 0.646) (Figure 2A). Subsequently, Kaplan-Meier survival analysis of CNDs included in the defined clusters was performed. Since there were no deaths in patients with CND in cluster 2 and non-censored cases in cluster 5, these clusters were not included in the analysis. In addition, survival rates of COVID-19 patients with CND in the ICU were assessed by log-rank test. No significant differences were observed between clusters 1, 3, 4 and 6 [33 non-censored (22.60%), 113 censored (77.40%); p = 0.097] (Figure 2B).
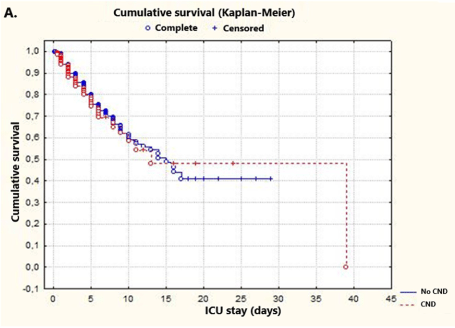
Figure 2A. Kaplan-Meier plots illustrating the period of ICU admission. Log-rank test p-values were p = 0.64676 in comparisons of patients with and with no CND comorbidities. Survival times in this plot are indicated with circles (○), and censored observations are specified with crosses (+). Note that COVID-19 patients in the ICU (with or without CND) show a similar probability of survival at day 8 (CND in red)
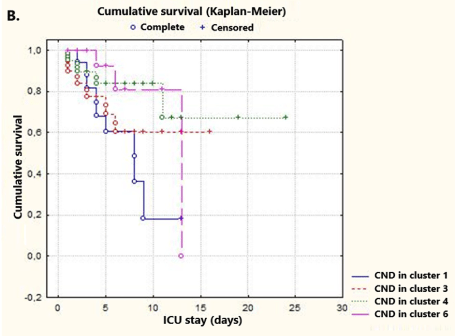
Figure 2B. Kaplan-Meier plots illustrating cumulative survival curves of four clusters, including patients with CND. The p-values of the log-rank tests were p = 0.09 when comparing the four phenotypes. Survival times in this plot are indicated with circles (○), and censored observations are shown with crosses (+). Note that patients with CND in cluster 1 exhibit the lowest probability of survival at day 8. Period: days from ICU admission to death or discharge
Discussion
This is the first study to demonstrate an association between chronic neurological disease and clinical phenotypes and in-hospital mortality in patients with COVID-19. CNDs were distributed among the six phenotypes (according to age, symptoms, and comorbidities), predominating phenotypes 3, 2 and 4. A significant relationship was shown between the occurrence of CND and defined phenotypes. Survival analysis revealed that patients with CND had a similar probability of survival as patients without CND. However, most CND-related deaths were located in clusters 1 (29.27%) and 3 (34.15%). Additionally, ICU patients with CND in cluster 1 showed the lowest survival probability at day 8.
Our results corroborate the notion that COVID-19 is associated with various comorbidities often related to endothelial dysfunction, such as cardiovascular disease, hypertension, diabetes, liver, kidney, and lung diseases, obesity, and neurological diseases [29-31], indicating that the endothelium may be targeted by SARS-CoV-2.
In the present study, CNDs represented 17% of ICU patients with COVID-19. A recent review found a frequency of CND between 14 and 40%, with co-occurrence of a pre-existing neurological disease of 8.0% [18]. Possible reasons for the mixed results were underreported frequency, variable definitions, and the absence of specific studies.
In this study, patients with CND were distributed across the six phenotypes, although predominately in classes 3, 2 and 4. This finding is consistent with Guan et al., who found hazard ratios for worse infection outcomes as high as the number of concomitant diseases [32].
Additionally, patients with CND were older, and approximately 30% had diabetes or coronary artery disease. Overall, symptoms in this group were similar to those of subjects with no CND. These results are consistent with a cohort study in which patients with CND were older, more disabled, and had more vascular risk factors and comorbidities, and the same need for ventilatory support [33].
In terms of symptoms, no differences were found between the two groups, except for dyspnea, rhinorrhea, and headache, which showed a slightly higher trend in individuals without CND. A recent PRISMA guideline-based systematic review showed that headache, musculoskeletal injuries, psychiatric disorders, altered consciousness, and gustatory/olfactory dysfunction were the most common neurological symptoms in COVID-19 patients. Altered consciousness and acute cerebrovascular events were significantly higher among patients with severe infection [34].
The most frequent CNDs in this study were dementia, cerebrovascular disease, epilepsy, and Parkinson’s disease. Similar to the mortality rate associated with the phenotypes defined for COVID-19 patients in the ICU described previously by our group (data in press) most deaths related to CND were observed in clusters 1 and 3. The mortality rate for these phenotypes, which included symptomatic elderly with two different comorbidity patterns, was approximately 70%, indicating the highest percentage. This finding underlines the relevance of the association between age and hypertension and other comorbidities, such as cardiovascular disease and diabetes in in-hospital mortality. Jakhmola et al. showed that deaths associated with cardiovascular disease and diabetes are significantly higher than those of hospitalized patients in countries such as Italy, France, and Spain, in contrast to the Netherlands. In addition, they reported that losses due to renal diseases and neurological conditions are significantly higher than the total of hospitalized patients affected by specific comorbidity [30]. A recent systematic review and meta-analysis also concluded that advanced age and pre-existing comorbidities were related to mortality [35].
Based on the SARS and MERS epidemics, evidence shows that these comorbidities predispose to the risk of severe infections, leading to critical care and mortality. The same trend has been observed with SARS-CoV-2 and COVID-19 disease [32,36-38]. The results of this study indicated that the mortality rate in the ICU has been considerably high for patients with at least two comorbidities, such as coronary artery disease, diabetes, and CND. As in SARS and MERS, angiotensin-converting enzyme-2 (ACE2) receptors play a crucial role in determining disease severity and up-regulation of ACE2 [39,40].
A fundamental area of concern for neurologists is how COVID-19 affects patients with underlying neurological diseases. Interestingly, we found a higher mortality rate in patients with cerebrovascular disease, followed by dementia and epilepsy. Moreover, one report demonstrated that the presence of CND is an independent predictor of mortality in patients hospitalized for COVID-19 [41].
Concerning cerebrovascular disease, previous reports have noted that patients with COVID-19 requiring ICU admission present with a history of cerebrovascular disease more frequently [42]. Another study found that these patients are more commonly hospitalized, require ICU admission in more than 20% of cases, and present with a severe infection in two-thirds of cases [20]. The findings observed in this study mirror those reported in two meta-analyses by Aggarwal et al. [43] and Wang et al. [44], which showed that underlying cerebrovascular disease is related to higher disease severity in patients with COVID-19. These findings are consistent with a recent study by Romagnolo et al., who stated that cerebrovascular disease and cognitive impairment are determinants of COVID-19 severity [20]. Another article by the same group demonstrated that preexisting neurological diseases, especially neurodegenerative diseases, increase lethality in COVID-19 cases [45]. In addition, Du et al. reported a 2.4-fold increased mortality risk in patients with cerebrovascular or cardiovascular disease, although they did not specify the distinction between these two conditions [36].
In the present study, dementia was considered the second leading cause of mortality among CND. Similarly, two systematic reviews and meta-analyses showed that dementia is a high-risk factor for the prevalence and mortality of COVID-19 [46,47]. In one of these studies, the association between dementia and mortality in patients with COVID-19 was affected by age and comorbidities [47]. Patients with Alzheimer’s disease and other types of dementia are at increased risk, given their advanced age, the coexistence of other comorbidities, and poor cognition [48].
Moreover, patients with Parkinson’s disease represented 4% of CND comorbidities, equally distributed in phenotypes 1, 2, and 4 (primarily associated with other comorbidities, advanced age, and respiratory symptoms). Parkinson’s disease-related mortality was 4.8%. There is limited evidence on whether patients with Parkinson’s disease are at increased risk of COVID-19 or worse outcomes. In the first-mentioned systematic review and meta-analysis, the pooled rates were 4.7% for ICU admission and 25.1% for mortality. They concluded that the prevalence and prognosis of patients with COVID-19 appear to be comparable in patients with Parkinson’s disease and those without this condition. Therefore, the increased hospitalization and mortality may be attributed to advanced age and comorbidities [49].
The percentage of patients with active epilepsy who died was 7.3 %. Several studies have suggested that epilepsy is a potential risk factor for morbidity and mortality in patients with COVID‐19; however, the association is still unclear [50-52]. Cabezudo-García et al. observed that epilepsy could be an independent risk factor for mortality. They also stated that this result could be explained by the higher rate of risk factors for a severe form of COVID-19 in patients with epilepsy, such as hypertension [50]. These findings were further confirmed by other authors [53,54]. In our study, the frequency of epilepsy in clusters 4 and 6 was higher than in cluster 3, suggesting that hypertension was a recurrent underlying chronic condition in most of these patients.
Evidence has shown an increased incidence, rapid progression, and poor prognosis of COVID-19 in patients with underlying comorbidities such as diabetes and epilepsy. One case report showed a COVID-19 patient with a history of multiple underlying diseases, including diabetes, epilepsy, and gout. The patient developed multiple organ failure and died one week after admission to the ICU [55]. Diabetes is a significant risk factor for hospitalization and adverse outcome in these patients [56,57] and is also associated with an increased risk of thromboembolism among patients with COVID-19. Additionally, D‐dimer levels have been confirmed to be directly proportional to the severity of the disease [58].
In this study, 12 patients had neuromuscular and spinal diseases (5.5%), including polyneuropathy, Guillain-Barré syndrome, muscular dystrophy, myasthenia gravis, and radiculopathy, which were mainly located in cluster 4 (hypertension as underlying comorbidity). The category of neuromuscular disorders (NMD) encompasses a wide range of diagnoses with widely varying levels of disability, even in individuals with the same diagnosis. Infections are known to unmask or exacerbate several autoimmune neuromuscular diseases, such as myasthenia gravis, chronic inflammatory demyelinating polyneuropathy, multifocal acquired demyelinating sensory, and motor neuropathy, and some degenerative disorders, such as amyotrophic lateral sclerosis and spinal muscular atrophy [17,59]. Some studies have shown that the risk of a severe course of COVID-19 is increased in patients with NMD due to chest and diaphragm muscle weakness, use of ventilator supports, tracheostomy, weak airway clearance, cardiac involvement, rhabdomyolysis, comorbidities, and steroid and immunosuppressant treatments [60,61]. A multi-data-based study conducted in the United States showed a higher incidence of contracting infections in patients with multiple sclerosis (MS) than in patients without MS [62]. However, only 7% of patients with neuromuscular and spinal diseases died in the present study.
To summarize, in this study, the prevalence of CND in patients with COVID-19 in the ICU was approximately 17%. Furthermore, the mortality rate in this group was around 25%, which is reasonably low and most likely multifactorial. The potential neurotropism of SARS-CoV-2, with a detrimental effect on pre-existing neurological diseases, advanced age, and the higher burden of comorbidity observed in these patients, should be considered [20,63]. Notably, the Cuban healthcare system emphasizes preventing and controlling chronic diseases in the elderly population from primary healthcare, which is crucial to mitigate the impact of severe COVID-19 and lethality in this age group [64]. The results of this study emphasize that elderly patients with neurological diseases and at least two other underlying comorbidities (hypertension with diabetes or hypertension with coronary artery disease) are a high-risk population for severe COVID-19 and thus need close health follow-up.
Conclusion
To date, this is the first study documenting the use of phenotypic clusters to characterize COVID-19 patients with CND admitted to the ICU. We consider that the association of CND with clinical phenotypes could help clinicians to quickly identify individuals with higher severity and worse prognosis based on their age, symptoms and comorbidities. This approach cannot only be used for newly admitted subjects but to evaluate COVID-19 patients under medical treatment and who require a re-evaluation of their conditions.
Limitations
Our study has some unavoidable shortcomings. First, neither pulmonary imaging findings nor laboratory test results were included in the evaluated algorithm in order to obtain the cluster definition based on clinical issues. Second, the study results are limited due to the retrospective cohort design. Despite the limitations, the present study allows determining the severity of disease in ICU patients with COVID-19 from a novel perspective suggesting prognoses with the help of the model so that the treatment schemes can be adjusted appropriately.
Data availability statement
The datasets produced for this study are available on request to the corresponding author.
Author contributions
LMC designed the study, analyzed the results, and wrote the manuscript. LGG participated in the statistical analysis of the results. OMC revised the English and contributed to the organization of the manuscript. NPF and TCH played a part in the layout of the manuscript. LRA contributed to the manuscript’s organization, EAM created the database and CMR, collaborated to collect data.
Conflicts of interest
The authors declare that the research was conducted without any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank the medical assistance experts across the nation for their close and active collaboration. We wish to extend our appreciation to Abel Sánchez Coroneaux, Daimi Orta Izquierdo and the COVID-19 expert group of the Cuban Health Ministry and to all the ICU medical staff who have treated COVID-19 patients in Cuba for their constant group effort and support.



