Abstract
Background and objective: Albuminuria is a risk factor for cardiovascular disease, and excess urinary albumin is related to increased all-cause mortality. Diabetic dyslipidemia, such as low high-density lipoprotein (HDL) levels may be one of the factors responsible for albuminuria. Therefore, the purpose of this study was to find out the association and correlation between HDL level with albuminuria in Saudi patients with type 2 diabetes (T2DM).
Methods: For the present retrospective study, we analyzed 1574 participants whom are between the age 20 to 96 years. All patients were from the population of the diabetic centre and Primary health centre at King Fahad Armed Forces Hospital, Jeddah, Saudi Arabia.
Main results: A total of 1574 subjects (the mean age was 56.2 ± 12.1 years, minimum 20 years and maximum 96 years) were included in the analysis. There were 956 females (60.7%). Mean body mass index was 31.4 ± 6.1 kg/m2. Hypertension was present in 603 cases (57.4%). Mean HDL was 1.16 ± 0.3 mmol/l with frequency of low level of HDL (< 1.04 mmol/l) was 587 cases (37.3%). The frequency of female subjects with normoalbuminuria, microalbuminuria and macroalbuminuria were significantly higher than male (64.8% vs. 35.2%, 52.9% vs. 47.1% and 51.2% vs. 48.8% respectively, p < 0.0001). Subjects with macroalbuminuria were significantly older than subjects with microalbuminuria or normoalbuminuria (59.2 ± 11.7, 57.5 ± 12.4 and 55.5 ± 11.9 years respectively, p < 0.0001). Mean HDL in subjects with macroalbuminuria were significantly lower than subjects with normoalbuminuria or microalbuminuria subjects (1.06 ± 0.3, 1.18 ± 0.3 and 1.13 ± 0.3 mmol/l respectively, p < 0.0001). Moreover, the frequency of subjects with low level of HDL (< 1.04 mmol/l) was significantly higher in patients with macroalbuminuria than subjects with normoalbuminuria or microalbuminuria subjects (53.8%, 34.4% and 41.2% respectively, p < 0.0001). Moreover, the frequency of male subjects with low level of HDL in microalbuminuria and macroalbuminuria were significantly higher than female subjects and the mean HDL was significantly lower.
Conclusion: Our data clearly indicate low HDL levels as important risk factor for the development of albuminuria. The principal strategy is to slow the progression of renal disease with improving HDL levels.
key words
type 2 diabetes, high density lipoprotein and albuminuria
Introduction
Albuminuria is the major cause of end-stage renal disease worldwide [1]. Hyperglycemia and hypertension (HTN) are the main risk factors for chronic kidney disease (CKD) including albuminuria development and progression [2]. However, in spite of the achievement of recommended targets for blood glucose and blood pressure, the residual risk for diabetic nephropathy remains high among patients with type 2 diabetes (T2DM) [3,4].
T2DM as a cardio-vascular risk equivalent was confirmed by Framingham study and other landmark studies [5,6]. Characteristics of diabetic dyslipidemia include elevated triglyceride, elevated low-density lipoprotein cholesterol and low high density lipoprotein cholesterol (HDL) levels [7]. Diabetic dyslipidemia, high triglycerides and/or low HDL levels may be one of the factors responsible for this high residual risk [3,8].
HDL is an antiatherogenic, anti-inflammatory and antithrombotic particle [9]. Low levels of HDL are associated with chronic low-grade inflammation and oxidative stress in type 2 diabetic patients with dyslipidemia [10,11]. This systemic inflammation may change HDL properties into being dysfunctional and proinflammatory [12,13]. Epidemiological studies have demonstrated a link between diabetic dyslipidemia and albuminuria. Low HDL concentrations were associated with albuminuria in a post hoc analysis of large intervention studies of high-risk patients with diabetes [14-16]. The Action in Diabetes and Vascular Disease Study (ADVANCE) demonstrated that lower baseline HDL levels were a significant and independent predictor of albuminuria, whereas no association was found with the risk of diabetic retinopathy, suggesting that differences may exist in the pathophysiology of these microvascular complications [14]. A large, international, cross-sectional study of outpatients with diabetes recently demonstrated an independent association of low HDL with CKD in patients with T2DM after controlling for low density lipoprotein cholesterol levels and established risk factors for microvascular disease [17].
In patients with T2DM, a reduction of HDL level is associated with an increased risk of renal injury; and lower HDL can be considered to be a risk factor for developing albuminuria in T2DM [18,19]. The impairment of HDL function has also been investigated in diabetic nephropathy [20]. Under this background, purpose of this study was to find out the association and correlation between HDL level with albuminuria in Saudi patients with T2DM.
Methods
For the present retrospective study, we analyzed 1574 participants whom are between the age 20 to 96 years. All patients were from the population of the diabetic centre and Primary health centre at King Fahad Armed Forces Hospital, Jeddah, Saudi Arabia. All data were collected on the basis of a review of electronic medical data. Weight (kg) and height (cm) were measured were recorded. Body mass index (BMI) was expressed as kg/m2 [21]. Participants were defined as having T2DM according to self-report, clinical reports, use of antidiabetic agents and HbA1c (≥ 6.5) [21]. HTN was defined when the systolic blood pressure was ≥ 130 mm Hg and/or diastolic blood pressure was ≥85 mm Hg in addition to receiving any medication for hypertension [22]. All patients in the present study fulfilled the revised National Kidney Foundation criteria for the diagnosis of albuminuria [23]. The urine albumin to creatinine ratio (ACR) was used as the index of urinary albumin excretion. A urine sample was collected during the first morning voiding. Conventionally, subjects with ACR < 30 mg/g were defined as having normalalbuminuria (NA). Microalbuminuria (MI) was defined as 30 ≤ ACR < 300 mg/g and macroalbuminuria (MA) as ACR ≥ 300 mg/g [23-25]. A polyclonal radioimmunoassay was used for albumin measurement.
Statistical analysis
Unpaired t-test analysis and Chi square (χ2) test (categorical data comparison) were used between variables to estimate the significance of different between groups for demographic and clinical laboratory. All statistical analyses were performed using SPSS Version 23.0. The difference between groups was considered significant when p < 0.05.
Results
A total of 1574 subjects (the mean age was 56.2 ± 12.1 years, minimum 20 years and maximum 96 years) were included in the analysis, table 1. There were 956 females (60.7%). Mean BMI was 31.4 ± 6.1 kg/m2. HTN was present in 603 cases (57.4%). Mean HDL was 1.16 ± 0.3 mmol/l with frequency of low level of HDL (< 1.04 mmol/l) was 587 cases (37.3%). The frequency of female subjects with NA, MI and MA were significantly higher than male (64.8% vs. 35.2%, 52.9% vs. 47.1% and 51.2% vs. 48.8% respectively, p < 0.0001). Subjects with MA were significantly older than subjects with MI or NA (59.2 ± 11.7, 57.5 ± 12.4 and 55.5 ± 11.9 years respectively, p < 0.0001). Mean HDL in subjects with MA were significantly lower than subjects with NA or MI Subjects (1.06 ± 0.3, 1.18 ± 0.3 and 1.13 ± 0.3 mmol/l respectively, p < 0.0001). Moreover, the frequency of subjects with low level of HDL (< 1.04 mmol/l) was significantly higher than subjects with NA or MI Subjects (53.8%, 34.4% and 41.2% respectively, p < 0.0001). Moreover, the frequency of male subjects with low level of HDL in MI and MA were significantly higher than female subjects and the mean HDL was significantly lower in figure 1.
Table 1. Baseline demographic and clinical characteristics of all subjects and patients with albuminuria subgroups [mean ± standard deviation (SD) or number (%)])
Parameters |
All subjects
(1775) |
Urine microalbumin (g/min) |
P value |
< 30
1213 (68.3) |
30-300
- (7.4)
|
> 300
76 (4.3) |
Gender |
Male |
618 (39.3) |
370 (35.2) |
209 (47.1) |
49 (48.8) |
< 0.0001 |
Female |
956 (60.7) |
680 (64.8) |
235 (52.9) |
41 (51.2) |
Age (years) |
56.2 ± 12.1 |
55.5 ± 11.9 |
57.5 ± 12.4 |
59.2 ± 11.7 |
0.001 |
Body mass index (kg/m²) |
31.4 ± 6.1 |
31.2 ± 6.0 |
31.8 ± 6.3 |
31.2 ± 6.9 |
0.2 |
Hypertension |
603 (57.4) |
546 (52.0) |
296 (66.7) |
61 (76.3) |
< 0.0001 |
High density lipoprotein (mmol/l) |
Mean ± SD |
1.16 ± 0.3 |
1.18 ± 0.3 |
1.13 ± 0.3 |
1.06 ± 0.3 |
< 0.0001 |
< 1.04 |
587 (37.3) |
361 (34.4) |
183 (41.2) |
43 (53.8) |
< 0.0001 |
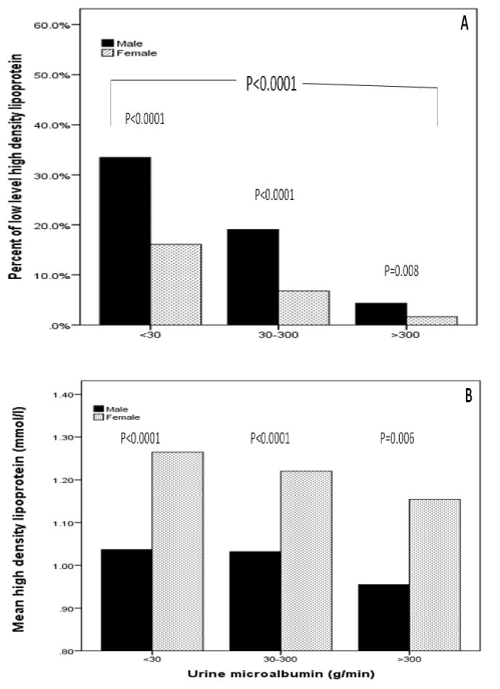
Figure 1. The percentage of albuminuria subgroups in subjects with type 2 diabetes and high-density lipoprotein level
Discussion
The major finding of the current study was that low HDL was frequently associated with albuminuria. Moreover, Saudi male patients with low HDL were more likely to present with MA and MI than patients with NA. MI is the first clinical sign of diabetic vascular damage and is a predictor for progressive kidney damage, myocardial infarction and cardiovascular disease (CVD) mortality [26]. Once present, MI progresses over 5-10 years to MA in 22-50% of patients [27,28]. The development of MA is usually followed by a further decline in glomerular filtration rates [27,28]. This is similar to what Afghahi et al. [19] reported a significant difference in HDL levels between normoalbuminuric and microalbuminuric patients in 3667 patients with T2DM which was lower in the microalbuminuric group [19]. On the contrary, Shen et al. [29] reported for HDL levels of microalbuminuric and normoalbuminuric groups in a 1069 hospital-based population study; Perassolo et al. [30] also reported this finding in 2003 [29,30]. The diversity of the results in these different reports may be due to the effect of gender on renal damage associated with HDL particles.
The importance of HDL particles in predicting renal damage in T2DM is still unknown, however, studies in endothelial cell cultures have demonstrated that HDL suppresses the expression of markers of inflammation and cell adhesion molecules in the early stages of diabetic nephropathy, which is compatible with the functional impairment of HDL molecules [31]. Moreover, Zhou et al. showed that the capacity of serum to induce cholesterol efflux is impaired in diabetic patients with incipient or overt nephropathy [20]. Low HDL is one of the clinical components of the metabolic syndrome and may be consequences of the underlying insulin resistance. Indeed, a growing body of evidence supports a pathogenic role of insulin resistance in kidney dysfunction through mechanisms involving glomerular hyperfiltration and increased vascular permeability caused by hyperinsulinemia, subclinical inflammation, or podocyte abnormalities [32,33]. These findings, mostly deriving from experimental studies, are supported by gene-association studies and interventional studies of the effect of insulin sensitizers on albuminuria progression [33]. On the other hand, dyslipidemia is not sufficient to initiate kidney damage since individuals without diabetes but with elevated cholesterol or TG levels rarely develop kidney disease; accordingly, it is plausible that the metabolic derangement typical of diabetes (hyperglycemia, insulin resistance) facilitates the lipotoxic effects on the microvascular bed and is necessary for DKD to develop.
Gender may be a factor that potentially influences the association of albuminuria risk with dyslipidemia [34]. Albuminuria occurs more frequently in male [35]. Females have significantly higher plasma levels of total cholesterol, triglycerides and HDL [36,37]. This is believed to be an important risk factor for cardiovascular events in females [38,39]. The effect of a worse lipid profile in females on the progression of diabetic nephropathy is less well studied. In keeping with our finding, male patients were significantly having lower HDL level. This is inconsistent with a previous study where we showed that different risk factors influence albuminuria in males and females; thus, HDL is considered as a risk factor for women, but not for men [40].
T2DM remains a tremendous challenge to public health worldwide. T2DM and HTN are common diseases that coexist at a greater frequency than chance alone would predict. HTN in the diabetic individual markedly increases the risk and accelerates the course of CVD, peripheral vascular disease, stroke, retinopathy, and nephropathy [41]. Patients with T2DM may be hypertensive for years prior to the onset of overt diabetes. At the time of diagnosis of T2DM, HTN is found in approximately 70-80% of patients. Still blood pressure rises further in those patients who subsequently develop diabetic nephropathy [42]. Reported prevalence of albuminuria in HTN have yielded variable results due to the chosen cutoff value, patient selection, and more importantly the duration of HTN and of prior treatment. In a cohort of 787 patients aged 18–72 years, a prevalence of MI of 8% was observed [43]. A prevalence of MI of 6% was found in a cohort of 1,041 younger patients (aged 18–45 years) with untreated mild HTN [44]. Both were lower than our report and this finding was also strongly associated with poor glycemic control. Ahmedani et al. [45] showed MI had a higher systolic and diastolic blood pressure compared to microalbuminuria negative group (p < 0.001) [45]. Similarly, Arkedani et al. [46] and Varghese et al. [47] reported a good statistical correlation between the prevalence of MI and the diastolic blood pressure [46,47]. Pasko et al. [48] found that microalbuminuric patients had higher systolic and diastolic blood pressure, suggesting that systolic blood pressure is a significant risk factor for diabetic nephropathy [48]. Svenson et al. [49] showed that high blood pressure increased the risk of developing signs of nephropathy [49,50].
Despite its complications, T2DM is largely a preventable and treatable disease. Annual screening for MI in all patients with T2DM is recommended, as early treatment that includes CVD risk reduction strategies is critical [21]. The recent availability of MI dipstick test strips provides a Simple method for primary screening and continued monitoring in patients without overt proteinuria. In those who test positive for MI by the dipstick test, quantitative analysis should be carried out to confirm the diagnosis and assist in planning the treatment regimen. The principal strategy is to slow the progression of renal disease with aggressive antihypertensive treatment which should include agents that block the renin-angiotensin system.
This study has some limitations. It was a retrospective and not longitudinal, preventing determination of whether any risk factors were the cause or result of albuminuria. Moreover, this study was based on hospital-based population; thus, the correct sampling weights were not used for insufficient data, thus limiting the generalization of our results to the general population of Saudi Arabia.
To conclude; our data clearly indicate low HDL levels as important risk factor for the development of albuminuria. The principal strategy is to slow the progression of renal disease with improving HDL levels.
Acknowledgment
We are grateful to the staff from the diabetic centre and Primary care department at King Fahad Armed Forces Hospital for their valuable contributions in data collection.
Conflict of interest<2021 Copyright OAT. All rights reserv
The authors have no conflict of interest to disclose.
References
- Colhoun HM, Lee ET, Bennett PH, Lu M, Keen H, et al. (2001) Risk factors for renal failure: the WHO Mulitnational Study of Vascular Disease in Diabetes. Diabetologia 44: S46–S53. [Crossref]
- Retnakaran R, Cull CA, Thorne KI, Adler AI, Holman RR, et al. (2006) Risk factors for renal dysfunction in type 2 diabetes: U.K. Prospective Diabetes Study 74. Diabetes 55: 1832-1839. [Crossref]
- Fioretto P, Dodson PM, Ziegler D, Rosenson RS (2010) Residual microvascular risk in diabetes: unmet needs and future directions. Nat Rev Endocrinol 6: 19-25. [Crossref]
- Molitch ME, DeFronzo RA, Franz MJ, Keane WF, Mogensen CE, et al (2003) Diabetic nephropathy. Diabetes Care 26: S94-S98. [Crossref]
- Kannel WB, McGee DL (1979) Diabetes and cardiovascular disease: the Framingham study. JAMA 241: 2035-2038. [Crossref]
- Kannel WB, McGee DL (1979) Diabetes and glucose tolerance as risk factors for cardiovascular disease: the Framingham study. Diabetes Care 2: 120-126. [Crossref]
- Goldberg IJ (2001) Clinical review 124. Diabetic dyslipidemia: causes and consequences. J Clin Endocrinol Metab 86: 965-971. [Crossref]
- Aljabri KS, Bokhari SA, Alshareef MA, Khan PM, Alsaoud HA, et al. (2018) Effect of Hypertriglycridemia on the Frequency of Albuminuria in Saudi Populaion with Type 2 Diabetes Mellitus. Intern J Intern Emerg Med 4: 1-6.
- deGoma EM, deGoma RL, Rader DJ (2008) Beyond High-Density Lipoprotein Cholesterol Levels Evaluating High-Density Lipoprotein Function as Influenced by Novel Therapeutic Approaches. J Am Coll Cardiol 51: 2199-2211. [Crossref]
- Kontush A, Chapman MJ (2008) Why Is HDL Functionally Deficient in Type 2 Diabetes? Curr Diab Rep 8: 51-59. [Crossref]
- Leiter LA, Genest J, Harris SB, et al. Dyslipidemia in Adults with Diabetes. Can J Diab. 2006;30(3):230‒240.
- Navab M, Anantharamaiah GM, Reddy ST, Van Lenten BJ, Fogelman AM (2009) HDL as a Biomarker, Potential Therapeutic Target, and Therapy. Diabetes 58: 2711-2717. [Crossref]
- Ory DS, Schaffer JE (2010) ApoA-1 in Diabetes: Damaged Goods. Diabetes 59: 2358-2359. [Crossref]
- Morton J, Zoungas S, Li Q, Patel AA, Chalmers J, et al. (2012) Low HDL cholesterol and the risk of diabetic nephropathy and retinopathy: results of the ADVANCE study. Diabetes Care 35: 2201-2206. [Crossref]
- Davis TM, Ting R, Best JD, Donoghoe MW, Drury PL, et al. (2011) Fenofibrate Intervention and Event Lowering in Diabetes Study investigators. Effects of fenofibrate on renal function in patients with type 2 diabetes mellitus: the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) Study. Diabetologia 54: 280-290. [Crossref]
- Hermans MP (2011) Non-invited review: prevention of microvascular diabetic complications by fenofibrate: lessons from FIELD and ACCORD. Diab Vasc Dis Res 8: 180-189. [Crossref]
- Sacks FM, Hermans MP, Fioretto P, Valensi P, Davis T, et al. (2014) Association between plasma triglycerides and high-density lipoprotein cholesterol and microvascular kidney disease and retinopathy in type 2 diabetes mellitus: a global case-control study in 13 countries. Circulation 129: 999-1008. [Crossref]
- Rutledge JC, Ng KF, Aung HH, Wilson DW (2010) Role of Triglyceride-Rich Lipoproteins in Diabetic Nephropathy. Nephrol 6: 361-370. [Crossref]
- Afghahi H, Cederholm J, Eliasson B, Zethelius B, Gudbjörnsdottir S, et al. (2010) Risk factors for the development of albuminuria and renal impairment in type 2 diabetes-- the Swedish National Diabetes Register (NDR). Nephrol Dial Transpl 26: 1236-1243. [Crossref]
- Zhou H, Tan KC, Shiu SW, Wong Y (2008) Cellular cholesterol efflux to serum is impaired in diabetic nephropathy. Diabetes Metab Res Rev 24: 617-623. [Crossref]
- Chen YM, Ho SC, Lam SS, Chan SS (2006) Validity of body mass index and waist circumference in the classification of obesity as compared to percent body fat in Chinese middle-aged women. Int J Obes (Lond) 30: 918-925. [Crossref]
- American Diabetes Association (2018) Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2018. Diabetes Care 41: S13-S27. [Crossref]
- Brook RD, Rajagopalan S (2017) Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults. A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 12: 238. [Crossref]
- Isakova T, Nickolas TL, Denburg M, Yarlagadda S, Weiner DE, et al. (2017) KDOQI US Commentary on the 2017 KDIGO Clinical Practice Guideline Update for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease–Mineral and Bone Disorder (CKD-MBD) American Journal of Kidney Diseases 70: 737-751. [Crossref]
- Mogensen CE, Chachati A, Christensen CK, Close CF, Deckert T, et al. (1985) Microalbuminuria: an early marker of renal involvement in diabetes. Uremia Invest 9: 85-95. [Crossref]
- Eknoyan G, Hostetter T, Bakris GL, Hebert L, Levey AS, et al. (2003) Proteinuria and other markers of chronic kidney disease: a position statement of the national kidney foundation (NKF) and the national institute of diabetes and digestive and kidney diseases (NIDDK). Am J Kidney Dis 42: 617-622. [Crossref]
- Stamler J, Vaccaro 0, Neaton JD, Wentworth D (1993) Diabetes, other risk factors, and 12-yr cardiovascular mortality for men screened in the Multiple Risk Factor Intervention Trial. Diabetes Care 16: 434-444. [Crossref]
- John L, Rao PS, Kanagasabapathy AS (1994) Rate of progression of albuminuria in type II diabetes. Five-year prospective study from south India. Diabetes Care 17: 888-890. [Crossref]
- Parving HH, Lehnert H, Brochner-Mortensen, Gomis R, Andersen S, Arner P, et al. (2001) The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med 345: 870-878. [Crossref]
- Shen FC, Chen CY, Su SC (2009) The prevalence and risk factors of diabetic nephropathy in taiwanese type 2 diabetes- a hospital- based study. Acta Nephrol 23: 90-95.
- Perassolo MS, Almeida JC, Prá RL, Mello VD, Maia AL, et al. (2003) Fatty Acid Composition of Serum Lipid Fractions in Type 2 Diabetic Patients with Microalbuminuria. Diabetes Care 26: 613-618. [Crossref]
- Rutledge JC, Ng KF, Aung HH, Wilson DW (2010) Role of Triglyceride-Rich Lipoproteins in Diabetic Nephropathy. Nephrol 6: 361-370. [Crossref]
- Jauregui A, Mintz DH, Mundel P, Fornoni A (2009) Role of altered insulin signaling pathways in the pathogenesis of podocyte malfunction and microalbuminuria. Curr Opin Nephrol Hypertens 18: 539-545. [Crossref]
- De Cosmo S, Menzaghi C, Prudente S, Trischitta V. Role of insulin resistance in kidney dysfunction: insights into the mechanism and epidemiological evidence. Nephrol Dial Transplant 28: 29-36. [Crossref]
- Penno G, Solini A, Zoppini G, Fondelli C, Trevisan R, et al. (2015) Renal Insufficiency and Cardiovascular Events (RIACE) Study Group. Hypertriglyceridemia is independently associated with renal, but not retinal complications in subjects with type 2 diabetes: a cross-sectional analysis of the renal Insufficiency and Cardiovascular Events (RIACE) Italian multicenter study. PLoS One 10: e0125512. [Crossref]
- Hanai K, Babazono T, Yoshida N, Nyumura I, Toya K, et al. (2012) Gender differences in the association between HDL cholesterol and the progression of diabetic kidney disease in type 2 diabetic patients. Nephrol Dial Transplant 27: 1070-1075. [Crossref]
- Carnevale Schianca GP, Fra GP, Colli E, Bigliocca M, Mella R, et al. (2012) Sex differences in lipid profiles in relation to the progression of glucose abnormalities. J Diabetes 4: 95-101. [Crossref]
- Eriksson M, Zethelius B, Eeg-Olofsson K, Nilsson PM, Gudbjörnsdottir S, et al. (2010) Blood lipids in 75 048 type 2 diabetic patients: a population-based survey from the Swedish National diabetes register. Eur J Cardiovasc Prev Rehabil 8: 97-105. [Crossref]
- Mora S, Buring JE, Ridker PM, Cui Y (2011) Association of HDL Cholesterol with Incident Cardiovascular Events in Women, by LDL Cholesterol and Apo B100 Levels: A Cohort Study. Ann Intern Med 155: 742-750. [Crossref]
- Lee WL, Cheung AM, Cape D, Zinman B (2000) Impact of Diabetes on Coronary Artery Disease in Women and Men: A meta-analysis of prospective studies. Diabetes Care 23: 962-968. [Crossref]
- Nakhjavani M, Morteza A, Jenab Y, Ghaneei A, Esteghamati A, et al. (2012) Gender Difference in Albuminuria and Ischemic Heart Disease in Type 2 Diabetes. Clin Med Res 10: 51-56. [Crossref]
- Epstein M, Sowers JR (1992) Diabetes mellitus and hypertension. Hypertension 19: 403-418. [Crossref]
- Christensen CK, Mogensen CE (1985) The course of incipient diabetic nephropathy-Studies of albumin excretion and blood pressure. Diabet Med 2: 97-102. [Crossref]
- Pontremoli R, Sofia A, Ravera M, Nicolella C, Viazzi F, et al. (1997) Prevalence and clinical correlates of microalbuminuria in essential hypertension: the MAGIC Study: Microalbuminuria: A Genoa Investigation on Complications. Hypertension 30: 1135-1143. [Crossref]
- Palatini P, Mormino P, Mos L, Mazzer A, Dorigatti F, et al. (2005) Microalbuminuria, renal function and development of sustained hypertension: a longitudinal study in the early stage of hypertension. J Hypertens 23: 175-182. [Crossref]
- Ahmadani MY, Fawaad A, Basit A, Hydrie ZI (2008) Microalbuminuria prevalence study in hypertensive patients with type 2 diabetes in Pakistan. J Ayub Med Coll Abbottabad 20: 17-20. [Crossref]
- Afkhami-Ardehani M, Modarresi M, Amirchaghmaghi E (2008) Prevalence of microalbuminuria and its risk factors in type 2 diabetic patients. Int J Neph 18: 112-117. [Crossref]
- Varghese A, Deepa R, Rema M, Mohan V (2001) Prevalence of microalbuminuria in type 2 DM at a diabetes center in southern India. Post Grad Med J 77: 399-402. [Crossref]
- Pasko N, Toti F, Zekollari E, Strakosha A, Kacori V, et al. (2013) Prevalence of microalbuminuria in type 2 diabetic patients in Tirana, a preliminary multicenter study. J Diab Mell 3: 145-149.
- Svensson M, Sundkvist G, Arnqvist HJ, Björk E, Blohmé G, et al. (2003) Signs of nephropathy may occur early in young adults with diabetes despite modern diabetes management. Results from the nationwide population-based Diabetes incidence study in Sweden. Diabetes Care 26: 2903-2909. [Crossref]

