Abstract
Background: Inclusion of diverse populations in clinical trials for chronic hepatitis B (CHB) is an integral part of development of new treatments. However, the assessment of the willingness of diverse patients to participate in hepatitis B clinical trials is understudied.
Methods: The Hepatitis B Foundation conducted an online survey regarding the willingness to participate in clinical trials among people living with CHB in the U.S. and outside the U.S. Descriptive demographic and bivariate analyses were conducted to identify relationships between willingness to participate and demographic characteristics.
Findings: Among all respondents, the overall willingness to participate in clinical trials was high. 73% were willing to participate in clinical trials, 21% were undecided, and only 6% were unwilling to participate. Among all respondents, Black or African American respondents showed the highest rate of willingness to participate in clinical trials (83%), compared with White respondents (64%) or Asians (63%) (p<0.001). Among all respondents, higher willingness to participate in clinical trials was observed among those living outside the U.S. (77%), those who identified as Black (83%) or identified as male (77%) (p<0.001). Respondents from the U.S. were more likely to be undecided about their participation (34%), compared with international respondents (17%) (p<0·001).
Interpretations: This study showed there was a lower overall willingness to participate in clinical trials among those from the U.S. compared to those outside of the U.S. This highlights the need to increase the engagement with patients living with CHB within the U.S. and in particular with Black or African American and Asian U.S. populations.
Disclaimer: This publication reflects the views of the authors and should not be construed to represent FDA’s views or policies.
Keywords
chronic-hepatitis-B, clinical-trial-participation, diversity, under-represented-population, willingness-to-participate
Introduction
Hepatitis B is a vaccine-preventable liver infection, which in some individuals can progress to a chronic, life-long infection [1]. Chronic hepatitis B (CHB) can result in serious complications including cirrhosis, liver failure and liver cancer (hepatocellular carcinoma, HCC) [1]. Currently, there are approved therapies to control and manage symptoms, while slowing or preventing disease progression to cirrhosis and liver cancer. However, not every person living with CHB is eligible for treatment with these medications [2]. Additionally, current treatments typically need to be taken indefinitely once started, and while they can reduce liver cancer-risk, they cannot eliminate it [2]. Research and development of novel treatments to control or eliminate the virus and reduce age-adjusted liver cancer risk are ongoing [2,3].
Table 1: The demographic characteristics of those who responded to the question on clinical trial participation
Table (1) |
All Respondents
(771) |
U.S. Respondents
(178) |
International Respondents (593) |
|
|
|
|
Location |
|
|
|
U.S. |
23% (178) |
|
|
Outside U.S. |
77% (593) |
|
|
|
|
|
|
Age Group |
|
|
|
18-30 |
31% (243) |
12% (22) |
37% (221) |
31-45 |
48% (370) |
39% (69) |
51% (301) |
46-60 |
16% (121) |
33% (59) |
10.5% (62) |
>61 |
5% (37) |
16% (28) |
1.5% (9) |
|
|
|
|
Gender |
|
|
|
Female |
26% (199) |
50% (89) |
19% (110) |
Male |
72% (557) |
49% (88) |
79% (469) |
Preferred not to answer |
2% (15) |
1% (1) |
2% (14) |
|
|
|
|
Race |
|
|
|
Black or African American |
49% (379) |
16% (28) |
59% (351) |
AAPI* |
31% (239) |
39% (70) |
29% (169) |
White |
17% (134) |
42% (74) |
10% (60) |
Others |
3% (19) |
3% (6) |
2% (13) |
|
|
|
|
* AAPI: Asian, Asian American, or Pacific Islander |
An estimated 292 million people worldwide and 2·4 million people in the U.S. are living with CHB, with 80% of the global burden clustered in three regions: sub-Saharan Africa, Southeast Asia and the Western Pacific [4,5]. Only 10% of those living with CHB globally and 25% in the U.S. are diagnosed, and those who have been diagnosed face multiple challenges to accessing sustainable care and treatment [6,7]. In fact, only 5% of eligible patients globally and 10% in the U.S. are on treatment [4,5,8]. For many living with CHB, the burden of treatment and disease progression is significant, particularly in low-income countries [9].
CHB in the U.S. represents a significant health disparity for racial and ethnic minority populations including Asian Americans, Native Hawaiians, Pacific Islanders and Black or African Americans – and a particular burden for immigrants, with an overall CHB prevalence of 3.07% compared to U.S.-born individuals, with an overall prevalence of 0.15% [5]. It is important to consider the impact of the high prevalence of CHB among immigrants in the U.S., as they may face unique barriers to clinical trial participation [10]. This is particularly important to consider as Black or African American, as well as Asian populations continue to be among the largest growing population groups in the United States [11], including both immigrants and U.S. born individuals.
Although patient decision-making about trial participation has been frequently examined, differences in attitudes based on race, geography and gender have rarely been studied. Even rarer, are the studies that examine willingness to participate in clinical trials from a non-U.S. vs. U.S. perspective [12]. The world encompasses a heterogeneous mix of people and it is unlikely that a one-size-fits-all clinical trial strategy will succeed, as it assumes a monolithic population. It is likely that clinical trial barriers vary at the attitudinal (for both the patient and provider), structural, and clinical levels. Attitudes may vary across demographic variables as well, such as race and ethnicity, age, English proficiency, geography (U.S. vs. outside the U.S.), socioeconomic status, and gender [13]. It is important that people from racial and ethnic minorities and other diverse groups are represented in clinical research [14].
Historically, racial and ethnic minorities have been consistently underrepresented in clinical trials. In assessing the demographics of participants in clinical trials that supported FDA approval of new molecular entities and original biologics from 2015-2019, participants were 76% White for all globally conducted clinical trials (including the U.S.) [15]. Globally, Asian individuals accounted for 11% of participants, while Black or African American individuals accounted for only 7%. Additionally, the majority of Asian participants were at non-U.S. sites, with only 2% of U.S. participants identifying as Asian (Asian Americans represent approximately 5% of the U.S. population) [15,16].
Clinical trials provide evidence for evaluating the safety and efficacy of new medical products, and efficacy and safety may differ among population subgroups depending on a mix of intrinsic and extrinsic factors [13]. Barriers to clinical trial participation are multifactorial, and may include structural, clinical, and attitudinal factors, which differ across demographic and socioeconomic factors [13,16-18]. This has led to only 31% of clinical trials completing patient recruitment on time, and with the planned number of participants [19-21]. There is little research to understand the difference in willingness to participate in clinical trials by demographic characteristics, with conflicting conclusions regarding the impact of these factors [15].
From 2019-2020, the Hepatitis B Foundation (HBF) conducted a large multi-aspect study to better understand the needs and experiences of people living with CHB, including an online survey that was distributed globally. The methodology and results of the PFDD and patient consult analysis have been previously reported and published [22,23].
In this study we seek to increase the understanding of perspectives among people living with CHB from both the U.S. and outside the U.S. on willingness to participate in clinical trials by demographic characteristics (e.g., race, age, geography, and gender) to advance diversity in hepatitis B clinical trials.
Methods
The key aims of the study were to answer: (1) What is the willingness to participate in clinical trials for people living with CHB, (2) How does this attitude differ between participants in the U.S. vs. participants living outside the U.S., and (3) Are there any differences in attitudes towards clinical trial participation among different demographic subgroups (race, gender, age, and U.S. vs. non-U.S. residence).
The online survey: Using an online survey, people 18 years or older living with CHB worldwide, were invited to anonymously answer questions about their experiences living with CHB. The survey was open for 6 months (12/23/2019 – 06/30/2020) and was shared with potential respondents through the Hepatitis B Foundation email listserv, Facebook and Twitter pages, and targeted Facebook advertisements. The survey was available in English only. IRB approval was obtained prior to conducting research activities (HIRB Project No. 191221-270).
The survey was designed as a decision tree, consisting of 29 to 39 questions. The number of questions a respondent encountered depended upon their responses to previous questions, such as country of residence and whether they had experience with hepatitis B medication or not. The survey included questions about demographic characteristics, diagnosis and CHB medication experiences, challenges living with CHB, and perspectives on future hepatitis B medications and clinical trials for hepatitis B. The questions on clinical trials focused on their willingness to participate in clinical trials for new hepatitis B treatments, including preferences regarding the route of administration, length of treatment, and acceptable potential side effects for future hepatitis B clinical trials. Questions included ranking options, multiple choice questions, and open-ended items. Some of the questions included several statements where respondents could express the emotional, psychosocial and physical impact of living with CHB on 5-point Likert scales. Each of these statements was later analyzed as a separate question. Questions were identical between the U.S. and global versions of the survey, except for a few modifications of response options regarding type of community lived in, level of education and health system/health insurance features. With multiple choice questions, participants were provided textboxes to submit answers they thought were not provided for them. These text boxes were later reviewed and coded accordingly. Data were downloaded from the survey tool as an Excel file, cleaned and coded, then imported into SPSS for analysis. The survey can be found in Annex 1 and the number of participants, and their responses can be found in Annex 2.
Analysis
The data were analyzed using SPSS Statistics 27 [24]. The survey was answered by 1,707 respondents from 100 countries, however only 771 respondents completed the section on participation in clinical trials for new CHB treatments (please see Annex 2). For this paper, our analysis consisted of only the 771 respondents who completed the clinical trials section. Frequency analysis was conducted to assess demographic information. Data were examined to understand the demographic make-up of participants in general, but also to compare these demographics between participants living in the U.S. (who will be referred to thereafter as “U.S. respondents”), and those living outside the U.S. (who will be referred to thereafter as “international respondents”). Age groups, gender, and race were examined and compared between U.S. respondents and international respondents. Additionally, percentage analysis for willingness to participate was conducted to understand the frequencies within this question prior to stratification.
Bi-variate analyses were performed using chi-square test to identify relationships between willingness to participate and demographic characteristics previously mentioned.
For descriptive and bivariate analysis purposes, Pacific Islanders were grouped with Asians as the number of respondents who self-identified as Pacific Islander was too small (1%) for analysis. Additionally, the number of Hispanic participants was very small (0.3%) reflecting the known prevalence of the disease, and were grouped with the “other races” category for analysis [2,6,25,26]. The option “Native Hawaiians” was provided in the survey, however, there were no survey respondents who chose this option.
Findings
Respondent demographics: A total of 771 individuals living with CHB from 76 countries responded to the survey’s question about their willingness to participate in clinical trials for new hepatitis B medications. Among respondents, 77% were international respondents, 72% identified as male, 26% identified as female, and 2% chose not to answer. Most respondents (79.5%) were 45 years of age or younger. In terms of race, respondents were 49% Black or African American (Africans/of African descent), 31% Asian, South-East Asian, Asian-American, or Pacific Islander (AAPI), 17% White and 3% who identified as “other” (Table 1). For those who identified as Asian (including Asian/Asian-American/South-East Asian/Pacific Islander), the majority were from the U.S. (29.3%), India (23.4%), the Philippines (16·3%), and Pakistan (7·1%). The largest group of Black respondents was from Nigeria (46·7%), followed by Ghana (18·2%) the U.S. (7·4%) and Uganda (6·3%). Please see Figures 1 and 2.
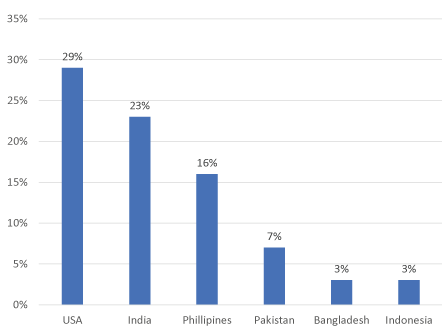
Figure 1. Country of residence of those who self-identified as Asian American, Asian and Pacific Islander (AAPI)
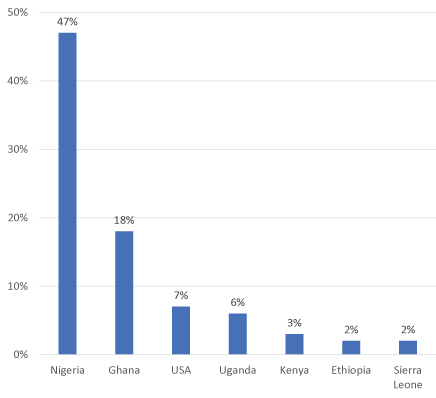
Figure 2. Country of residence of those who self-identified as Black or African American
Crosstabulation of the demographic characteristics by location showed international respondents were younger compared with U.S. respondents. While 88% of international respondents were 45 years-old or younger, only 51% of U.S. respondents were 45 years-old or younger (Figure 3). Among U.S. respondents, 49% identified as male and 50% identified as female. Among international respondents, 79% identified as male and 19% identified as female. Upon examining race, 42% of U.S. respondents identified as White, identified as White, 16% as Black or African American, and 39% as Asian-American or Pacific Islander. Among international respondents, 10% identified as White, 59% as Black, and 29% as Asian or Pacific Islander. Table 1 above shows these demographics stratified by location (U.S. vs. international).
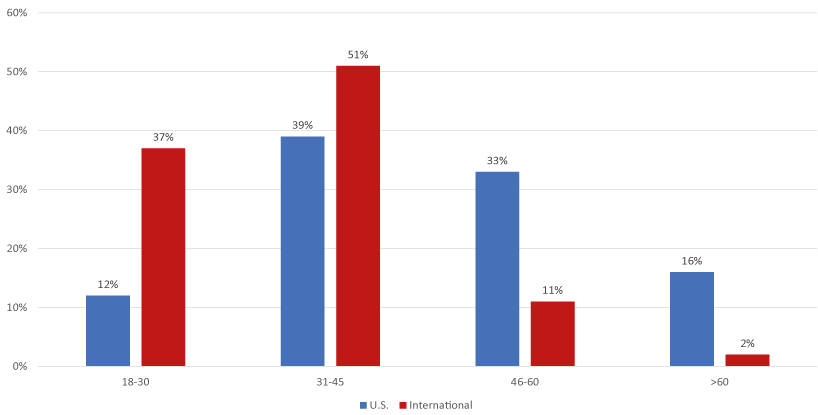
Figure 3. Distribution of age groups by location (U.S. vs. International) U.S. includes those who reported living in the United States, while International includes those who reported living in countries other than U.S.
Using location (U.S. vs. international), gender and race, three-way tabulation results show that male respondents participated more across all races of international respondents (Figure 4a). Among U.S. respondents, male Black or African American respondents showed a similar pattern to international respondents, while White and Asian respondents living in the U.S. exhibited almost equal gender representation (Figure 4b), similar to the overall distribution of U.S. respondents.
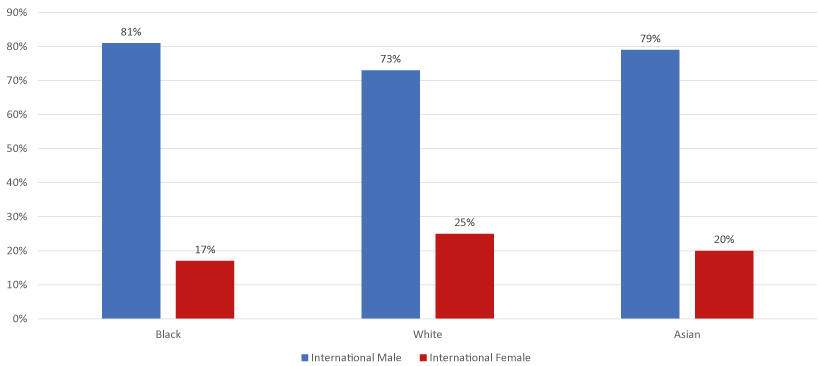
Figure 4a. Gender distribution by race and among international respondents only
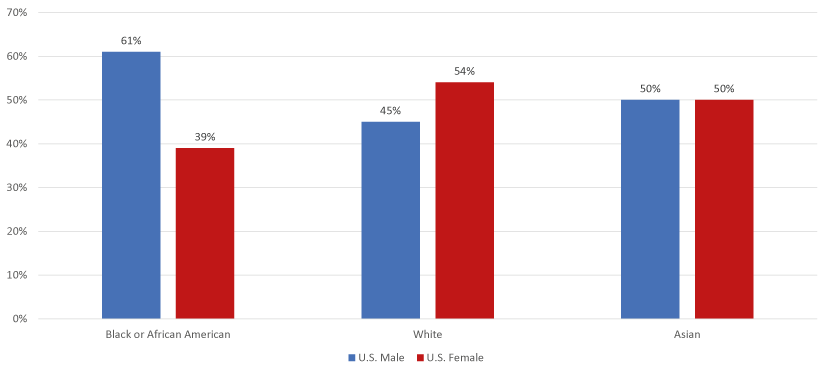
Figure 4b. Gender distribution by race among U.S. respondents only
Willingness to participate in clinical trials: Survey respondents were asked if they would be willing to participate in clinical trials for new hepatitis B medications. Among all respondents, 73% were willing to participate in clinical trials, 21% were undecided, and 6% were unwilling to participate (Figure 5).
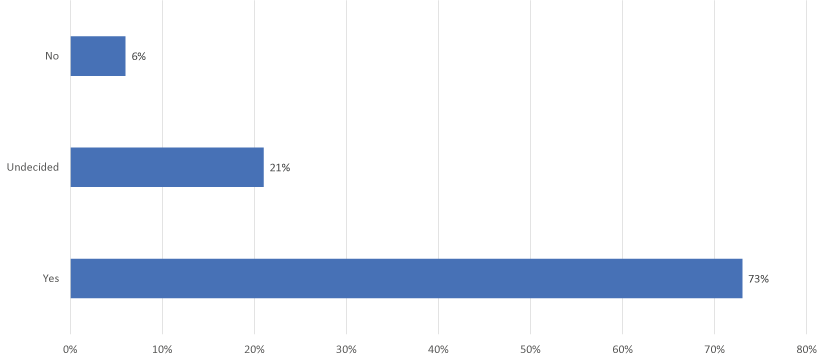
Figure 5. Willingness to participate in clinical trials for new Hepatitis B medication (all respondents). Participants who provided three possible responses (n) yes (563), undecided (162), no (46)
The decision to participate in clinical trials for new hepatitis B treatments varied among respondents by geographic location, gender, race, and age group (Table 2). Among all respondents, Chi-square (Chi2) test of independence showed that more male respondents (77%) expressed willingness to participate in clinical trials than female respondents (62%) (p<0·001). Additionally, respondents who were 18-30 years old expressed the highest rate of willingness to participate in clinical trials (77%) than other age groups (31-45 (74%), 46-60 (62%), and >60 (73%)) (p<0·05). Black or African American respondents also showed the highest rate of willingness to participate in clinical trials, compared with White respondents (64%) or Asians (63%) (p<0·001).
Table (2) |
Participation in Clinical Trials (771) |
|
Yes |
No |
Undecided |
Location*** |
|
|
|
U.S. |
59% (105) |
7% (12) |
34% (61) |
International |
77% (458) |
6% (34) |
17% (101) |
(strong association) |
|
|
|
Age Group* |
|
|
|
18-30 |
77% (188) |
6% (15) |
17% (40) |
31-45 |
74% (273) |
6% (23) |
20% (74) |
46-60 |
62% (75) |
5% (6) |
33% (40) |
>60 |
73% (27) |
5% (2) |
22% (8) |
(moderate association) |
|
|
|
Gender*** |
|
|
|
Female |
62% (124) |
6% (13) |
31% (62%) |
Male |
77% (426) |
7% (33) |
18% (98) |
(moderate association) |
|
|
|
Race/ Ethnicity*** |
|
|
|
Black or African American |
83% (314) |
5% (17) |
13% (48) |
AAPI* |
63% (151) |
10% (23) |
27% (65) |
White |
64% (86) |
3% (4) |
33% (44) |
(very strong association) |
|
|
Statistical significance levels: * p<0.05, ** p<0.01, *** p<0.001 |
* AAPI: Asian, Asian American, or Pacific Islander |
Table 2: The results of cross-tabulation of willingness to participate in clinical trials for new hepatitis B medication by demographics for all survey respondents
Attitudes towards clinical trial participation among people living with
chronic hepatitis B
Location
Comparing international respondents to U.S. respondents shows that international respondents were more willing to participate in clinical trials (77%) than U.S. respondents (59%). In addition, U.S. respondents were more likely to be undecided about their decision (34%), compared with international respondents (17%) (p<0·001) (Figure 6).
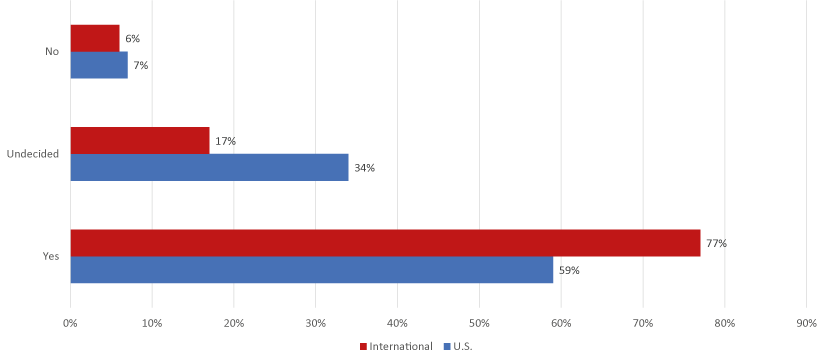
Figure 6. Willingness to participate in clinical trials for new Hepatitis B treatment by location (U.S. vs. International)
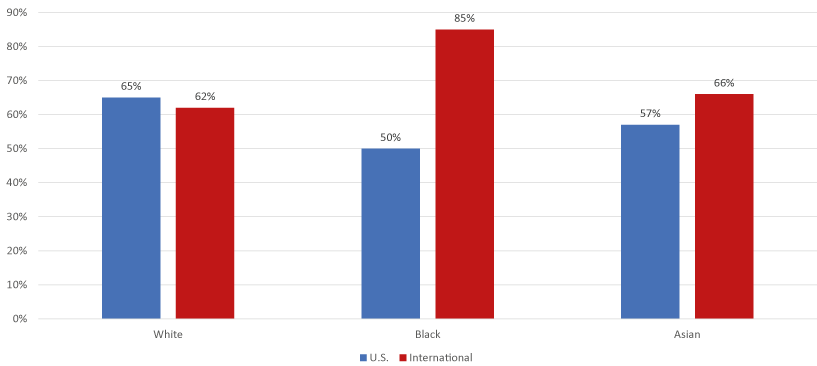
Figure 7. Those willing to participate in clinical trials by race and location (U.S. vs. International)
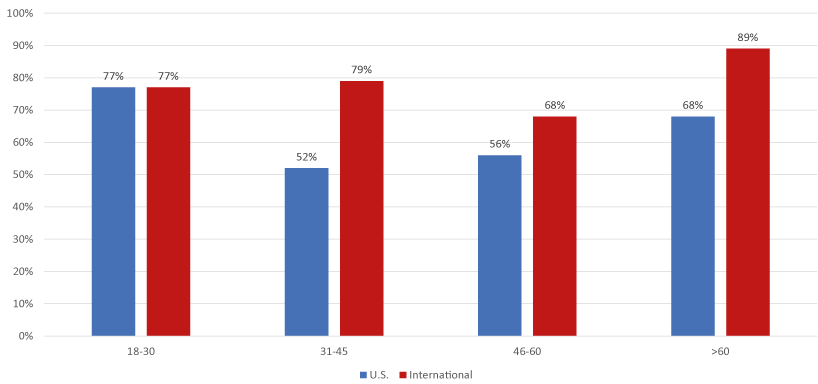
Figure 8. Those willing to participate in clinical trials by age-group and location (U.S. vs. International)
Further examination showed wide variability in willingness to participate in clinical trials by race among international respondents (Figure 7). The highest rates were found to be among Black respondents, with 85% willing to participate in clinical trials, 4% not willing to participate in clinical trials, and 11% undecided. Among international White respondents, 62% were willing to participate in clinical trials, 2% were not willing to participate, and 36% were undecided. For Asians, 66% were willing to participate in clinical trials, 10% were not willing to participate, and 24% were undecided.

Figure 7. Those willing to participate in clinical trials by race and location (U.S. vs. International)

Figure 8. Those willing to participate in clinical trials by age-group and location (U.S. vs. International)
There was wide variability in willingness to participate in clinical trials by race among U.S. respondents, as well (figure 7). Among Black or African American respondents, 50% were willing to participate in clinical trials, 11% were not willing to participate in clinical trials, and 39% were undecided. For Asian Americans, 57% were willing to participate in clinical trials, 8% were not willing to participate, and 35% were undecided. The highest rates were found to be among Whites, with 65% willing to participate in clinical trials, 4% not willing to participate, and 31% undecided.
Looking at willingness to participate in clinical trials for new hepatitis B medications by age and geographic location (Figure 8), we see that U.S. respondents’ ages 31-45 and 46-59 were mainly driving the lack of willingness/ undecidedness to participate in clinical trials. This breakdown by age and location was not statistically significant; however, it highlights the remarkable divide in responses between the different age groups, especially among those living in the U.S.
Interpretation
Clinical trials are critical for testing novel hepatitis B therapies, advancing the science of hepatitis B, and determining the strategies to improve outcomes for patients living with hepatitis B. Barriers to trial participation have been the subject of frequent study, but the rate of trial participation has not changed substantially over time [11].
The results of this survey support the idea that willingness to participate in CHB clinical trials vary by race, gender, age, and geographical location. We found that the largest discrepancy between U.S. and international respondents was in those undecided (p<0·001). While 17% of international respondents reported being undecided, 34% of U.S. respondents were undecided. This significant percentage of U.S. undecided respondents represents a key group to target with recruitment efforts.
Comparing international respondents to U.S. respondents shows that international respondents were more willing to participate in clinical trials (77%) than U.S. respondents (59%). The highest rates of willingness were found to be among international Black respondents, with 85% willing to participate in clinical trials. This could be due to a multitude of variables, including disease prevalence, and clinical trials being perceived as a means of access to health care, especially in geographic locations where access to hepatitis B care and treatment is limited [12].
In the U.S., there was less willingness to participate in hepatitis B clinical trials by both Black or African Americans (50%) and Asians (57%), compared to Whites (65%). Prior research has shown that minorities often did not participate in clinical trials because they were simply not asked, were not aware of the trials, did not have sufficient education about trials, or did not have access to trial sites [27, 28]. The lack of recruitment for racial and ethnic groups may supersede cultural barriers or lack of willingness to participate [27, 28].
Several studies have assessed the specific factors contributing to low levels of Asian-Americans participating in clinical trials [16,29]. Largely, these studies have concluded that Asian Americans are generally trusting of the clinical trial process, and cultural or religious beliefs do not contribute to decreased participation [29]. Studies have found that financial, physical, communication and language barriers, and cultural attitudes account for additional barriers that emigrant Asian Americans face [30].
While Asians are underrepresented in clinical trials, several small studies have assessed specifically targeted strategies for engagement and recruitment among Asian-Americans and have shown that culturally competent approaches have the potential to improve willingness and involvement [29,31]. Some of these methods include a focus on logistical support and communication services. Additionally, a study in the United Kingdom found that South Asian individuals, when informed of the lack of representation of South Asians in clinical trials, were altruistically more likely to be willing to participate [31]. Further studies involving much larger cohorts and different disease states are warranted to evaluate more culturally competent approaches to outreach.
Compared to Asians, the literature suggests that Black or African Americans in the U.S. have a historic distrust and lack of faith in scientific research and clinical trials leading to a decreased willingness to participate [32,33]. Our study noted that there was a significant difference in willingness to participate between U.S. and international Black respondents, where 85% of international Black respondents were willing to participate, while that number dropped to 50% among U.S. Black or African American respondents. In the U.S., 38.5% of Black or African American respondents were undecided about participating, the largest discrepancy among any racial group in the study. Although several studies associate similar findings among U.S. Black or African American participants with the Tuskegee Syphilis Study and other examples of racial discrimination [32-34]. other studies have shown that the hesitancy to participate in clinical trials among Black or African Americans in the U.S. has more to do with awareness and access [27,28]. Additionally, the high willingness to participate in clinical trials among international black respondents, especially those from West Africa, is likely driven by the significant financial barriers to accessing care and treatment, and the discrimination they face due to their hepatitis B status, leading to an inability to access education or employment opportunities. This leads to a perception that participating in clinical trials would facilitate access to care [12,35].
When assessing age in subgroup analysis, there were differences in willingness amongst groups. For both U.S. and international respondents, those between age 46-59 were the least likely to be willing to participate in clinical trials. This age group reported 40% unwillingness to participate in the overall sample, with 44% unwilling to participate among U.S. respondents and 32% unwillingness among international respondents. The lack of willingness to participate among those age 46-59 could be for a multitude of reasons, including potential competing demands of raising a family and working, and pursuing career goals [12,22]. Further research is warranted to investigate age-based and gender-based differences in willingness to participate in clinical trials.
Although the study found that there was variability in willingness to participate based on geography, race, age, and gender, the overall willingness to participate in clinical trials was quite high. Among all respondents, 73% were willing to participate in clinical trials, 21% were undecided, and only 6% were unwilling to participate. Among all respondents, Black or African American respondents showed the highest rate of willingness to participate in clinical trials, compared with White respondents (64%) or Asians (63%) (p<0.001). These findings illustrate the need to re-examine the way we think about patient participation in clinical trials, and support the possibility that patient willingness may not be the greatest barrier to clinical trial participation, but rather structural barriers (i.e., availability of a clinical trial), clinical barriers (i.e., not meeting eligibility), and healthcare providers’ attitudes may be greater contributors to the barriers to enrollment in clinical trials.
A hepatitis B patient’s decision about what hepatitis B treatment to undergo is complex and deeply personal. The prospect of incorporating clinical treatment into a patient’s care adds another level of complexity. Barriers may be structural (i.e., availability of a clinical trial), clinical (i.e., not meeting eligibility), or attitudinal (both patient and physician). In cases such as hepatitis B, additional barriers due to disease-related stigma, immigration and sociocultural status also need to be considered [36,37].
There is currently limited data on the perception and attitudes of ethnic and racial patient perceptions towards clinical trials and even more limited data on patient perceptions/attitudes towards clinical trials by geography (U.S., versus outside of the U.S.) [12]. This study adds to the current limited knowledge by examining patient attitudes towards willingness to participate in clinical trials based on race, geography, age, and gender.
Limitations
There were several limitations to this study. The first limitation relates to survey access. Our survey was online, and only those who have access to the internet were able to participate; however, the online survey also made it feasible for many individuals living all over the globe to participate. Additionally, the survey was promoted on HBF social media platforms that may not be available in all countries. For example, it was promoted via Facebook and Twitter, which are not available in China. Moreover, the survey was offered in English only, which would be a significant obstacle for those with limited or no English proficiency. With advances in survey platforms, the investigators anticipate future studies utilizing surveys with multiple language capabilities.
The second limitation was the small sample size among U.S. respondents and the lack of information on the U.S. respondent’s country of origin. This information would have improved our understanding of underrepresented populations regarding clinical trials and should be incorporated in future studies. However, the race-based results obtained from our study do conform with findings from previous studies about participation in clinical trials.
Conclusion
Our study showed certain populations were less willing to participate in clinical trials, it did not address why that was the case. Additional research on structural, or clinical barriers are needed [13,16-18]. It may be a lack of access to clinical trials, lack of awareness on participation in clinical trials, misperceptions, or mistrust about participation in clinical trials, or trials that didn’t address their needs driving respondents’ unwillingness to participate or provider and health system barriers. Additional research on industry barriers, specifically recruitment methods, is also needed to understand their impact on individual’s indecision and unwillingness to participate in clinical trials for hepatitis B. All these factors warrant further investigation to advance diverse participation in clinical trials.
The results of this survey highlight that the willingness to participate in CHB clinical trials varies by race, gender, age, and geographical location. Additionally, this paper suggests that patients’ willingness to participate in clinical trials may not be the main reason for decreased clinical trial participation among minority groups, given the overall willingness to participate in clinical trials was high.
Author contributionship
Yasmin Ibrahim: Writing – concept development, original draft, formal analysis
Chari Cohen: Writing – concept development, original draft (has access to the data)
Richardae Araojo: concept development, supervision
Christine Merenda: review
Sarah Ballou: Writing – review and editing
Christine Lee: Writing – concept development, original draft (attended data analysis sessions)
Non-Endorsement statement
The views expressed and the contents are those of the author(s) and do not necessarily represent the official views of, nor an endorsement, by FDA/HHS, or the U.S. Government.
Acknowledgement
The authors thank Dr. Milena Lolic for reviewing and proofreading the manuscript.
Funding
This study was supported by general operating funds from the Hepatitis B Foundation.
Competing interests
The author(s) declare that they have no competing interests.
References
- Hepatitis B - FAQs, statistics, data, & guidelines [Internet]. Centers for Disease Control and Prevention. Centers for Disease Control and Prevention; 2020 [cited 2021 Apr]. Available from: https://www.cdc.gov/hepatitis/hbv/index.htm
- Terrault NA, Lok ASF, McMahon BJ, Chang KM, Hwang Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Clin Liver Dis 12: 33-34.JP, et al. (2018) Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Clin Liver Dis 12: 33-34. [Crossref]
- Papatheodoridis GV, Chan HL, Hansen BE, Janssen HL, Lampertico P, et al. (2015) Risk of hepatocellular carcinoma in chronic hepatitis B: assessment and modification with current antiviral therapy. J Hepatol 62: 956-967. [Crossref]
- Lim JK, Nguygen MH, Kim WR, Gish R, Perumalswami P, et al. (2020) Prevalence of chronic hepatitis B virus infection in the United States. Am J Gastroenterol 115: 1429-1438.
- Wong RJ, Brosgart CL, Welch S, Block T, Chen M, et al. (2021) An updated assessment of chronic hepatitis B prevalence among foreign‐born persons living in the United States. AASLD Hepatology 74: 607-626. [Crossref]
- World Health Organization. Global hepatitis report 2017. Geneva: World Health Organization; 2017. License: CC BY-NC-SA 3.0 IGO.
- National Academies of Sciences, Engineering, and Medicine. A national strategy for the elimination of hepatitis B and C: Phase two report. Washington, DC: The National Academies Press, 2017.
- Cohen C, Holmberg SD, McMahon BJ, Block JM, Brosgart CL, et al. (2011) Is chronic hepatitis B being undertreated in the United States? J Viral Hepat 18: 377-383. [Crossref]
- Zampino R, Boemio A, Sagnelli C, Alessio L, Adinolfi LE, Sagnelli E. et al. Hepatitis B virus burden in developing countries. World J Gastroenterol 21: 11941-11953.
- Steinberg EM, Valenzuela-Araujo D, Zickafoose JS, Kieffer E, DeCamp LR, et al. (2016) The "battle" of managing language barriers in health care. Clin Pediatr (Phila) 55: 1318-1327.
- Bureau, US Census. “Local Population Changes and Nation’s Racial and Ethnic Diversity.” The United States Census Bureau, www.census.gov/newsroom/press-releases/2021/population-changes-nations-diversity.html
- Walsh E, Sheridan A (2016) Factors affecting patient participation in clinical trials in Ireland: a narrative review. Contemp Clin Trials Commun 3: 23-31.
- Unger JM, Cook E, Tai E, Bleyer A (2016) The role of clinical trial participation in cancer research: barriers, evidence, and strategies. Am Soc Clin Oncol Educ Book 35: 185-198.
- Clark LT, Watkins L, Piña IL, Elmer M, Akinboboye O, et al. (2019) Increasing diversity in clinical trials: overcoming critical barriers. Curr Probl Cardiol 44: 148-172. [Crossref]
- U.S. Food and Drug Administration. 2015-2016 Drug Trials Snapshots Summary Report – Five-Year Summary and Analysis of Clinical Trial Participation and Demographics. November 2020. Available from: https://www.fda.gov/media/143592/download
- Liu Y, Elliott A, Strelnick H, Aguilar-Gaxiola S, Cottler LB, et al. (2019) Asian Americans are less willing than other racial groups to participate in health research. J Clin Transl Sci 28: 90-96.
- Brandenberger J, Tylleskär T, Sontag K, Peterhans B, Ritz N, et al. (2019) A systematic literature review of REPORTED challenges in health care delivery to migrants and refugees in high-income countries - the 3C model. BMC Public Health 19: 755.
- Portes A, Light D, Fernández-Kelly P (2009) The U.S. health system and Immigration: an institutional interpretation. Sociological Forum 24: 487-514. [Crossref]
- Noirmain C, Gil-Wey B, Pichon I, Brindel P, Haller G, et al. (2020) Factors associated with patient willingness to participate in anaesthesia clinical trials: a vignette-based cross-sectional study. BMC Med Res Methodol 20: 67. [Crossref]
- Easterbrook PJ, Matthews DR (1992) Fate of research studies. J R Soc Med 85: 71-76.
- Kadam RA, Borde SU, Madas SA, Salvi SS, Limaye SS, et al. (2016) Challenges in recruitment and retention of clinical trial subjects. Perspect Clin Res 7: 137-143.
- The Hepatitis B Foundation (HBF). The voice of the patient living with chronic hepatitis B. In: Externally-Led Patient-Focused Drug Development Meeting [Internet]; 2020 Jun 9. HBF; 2020 Oct [cited 2021 Apr]. Available from: https://www.hepb.org/assets/Uploads/ExPFDD-Report-HBF-10-1-2020.pdf
- Kumar P, Freeland C, Bodor S, Farrell S, Cohen C, et al. (2020) Needs of individuals living with hepatitis delta virus and their caregivers, 2016–2019. Prev Chronic Dis 17: E159.
- IBM Corp (2020) Released 2020. IBM SPSS Statistics for Windows, Version 27.0. Armonk, NY
- Cornberg M, AS Lok, NA Terrault, Zoulim F (2019) EASL-AASLD HBV Treatments endpoints conference faculty. (2020). Guidance for design and endpoints of clinical trials in hepatitis B from the 2019 EASL-AASLD HBV treatment endpoints conference. Hepatology 71: 1070-1092.
- Haber P, Schillie S (2021) The pink book: hepatitis B [Internet]. Cdc.gov. 2021[cited 2021 Apr]. Available from: https://www.cdc.gov/vaccines/pubs/pinkbook/hepb.html#Virus
- Sheikh A (2006) Why are ethnic minorities under-represented in US research studies? PLoS Med 3: e49.
- Wendler D, Kington R, Madans J, Van Wye G, Christ-Schmidt H, et al. (2006) Are racial and ethnic minorities less willing to participate in health research? PLoS Med 3: e19.
- Ma GX, Seals B, Tan Y, Wang SY, Lee R, et al. (2014) Increasing Asian American participation in clinical trials by addressing community concerns. Clin Trials 11: 328-335.
- Lee S, Martinez G, Ma GX, Hsu CE, Robinson ES, et al. Barriers to health care access in 13 Asian American communities. Am J Health Behav 34: 21-30.
- Hussain-Gambles M (2004) South Asian patients' views and experiences of clinical trial participation. Fam Pract 21: 636-642. [Crossref]
- Shavers VL, Lynch CF, Burmeister LF (2002) Racial differences in factors that influence the willingness to participate in medical research studies. Ann Epidemiol 12: 248-256.
- Corbie-Smith G, Thomas SB, Williams MV, Moody-Ayers S (1999) Attitudes and beliefs of African Americans toward participation in medical research. J Gen Intern Med 14: 537-546. [Crossref]
- Corbie-Smith G, Williams IC, Blumenthal C, Dorrance J, Estroff SE, et al. (2007) Relationships and communication in minority participation in research: multidimensional and multidirectional. J Natl Med Assoc 99: 489-498. [Crossref]
- Hepatitis B Foundation. Data. Unpublished.
- Lee GE, Ow M, Lie D, Dent R (2016) Barriers and facilitators for clinical trial participation among diverse Asian patients with breast cancer: a qualitative study. BMC Women's Health 16: 43. [Crossref]
- Soh IPH, Tay CKJ, Ho GY, Pang A, Voon PJ, et al. (2012) Perceptions and barriers towards clinical trials participation in Asian cancer patients. J Clin Oncol 30: e19523-e19523. [Crossref]









