An 87-year-old man presented with a 3.2-cm-sized tumor in the subcutis at the left axillary region with skin erosion and multiple lymph node metastases. Histologically, the excised tumor consisted of small solid nests of proliferation at high density. Partially, tumor cells with wide eosinophilic cytoplasm formed solid aggregations. The tumor resembled an invasive ductal carcinoma with apocrine differentiation derived from the axillary accessory breast based on the lack of decapitation secretion, abnormal accumulation of p53, and smooth muscle actin-positive stroma.
Apocrine carcinoma, Apocrine adenocarcinoma, Male breast cancer, Accessory mammary gland
Male breast cancer is a rare disease; however, male breast cancer derived from an accessory breast is an extremely rare entity. Moreover, apocrine sweat gland-derived adenocarcinoma is also extremely rare. Herein, we report a case of axillary carcinoma with apocrine differentiation in an elderly male patient, for which a differential diagnosis between axillary breast cancer and apocrine gland adenocarcinoma was needed.
An 87-year-old man was aware of an induration in his left axillary region for several years. Because the induration gradually increased to produce skin erosion, he visited Miyoshi Central Hospital.
A hard, subcutaneous tumor with an ill-demarcated border 3 cm in diameter was palpable in the left axillary skin. The tumor adhered to the skin, and an erosion was found in the center of the lesion. No tumor was felt bilaterally on the breast.
Computed tomography revealed multiple metastases to the lymph nodes in the left axillary and subclavian regions. Histopathological examination of a punch biopsy specimen showed invasive proliferation of tumor cells, which formed solid nests and strands. Tumor resection was performed under suspicion of accessory breast-derived carcinoma or cutaneous adnexal cancer. Considering his age and the presence of lymph node metastases, lymph node dissection was not performed.
Histopathological examination of the resected specimen revealed tumor cells that formed small solid nests and were proliferating at high density in the range of a diameter of 3.2 cm. No glandular structures were detected in the tumor. Partially, tumor cells with wide eosinophilic cytoplasm formed solid aggregations.
Tumor cells showed invasion into the epidermis, eccrine sweat glands, and subcutaneous fat tissue. Frequent lymphatic vessel infiltrations were recognized. The apocrine sweat gland was not observed in the whole specimen. Immunohistochemical examination revealed positive staining of the tumor cells for AE1/AE3, cytokeratin (CK) 7, androgen receptor (AR), in addition to gross cystic disease fluid protein 15 (GCDFP-15) in most tumor cells. In addition, p53 and Ki-67 were positive in 62% and 6% of the tumor cells, respectively. Meanwhile, the tumor tested negative for estrogen receptor (ER), progesterone receptor (PgR), and human epidermal growth factor receptor 2 (HER2). Smooth muscle actin (SMA)-positive tumor cells were not detected; however, SMA-positive fibers were observed in the tumor stroma. In the surrounding non-cancerous dermis, SMA-positive fibers were not found. In addition, tumor cells tested negative for CK 5/6, human melanoma black (HMB) 45, p63, vimentin, S100, cluster of differentiation (CD) 68, and CK 20.
From the histopathological findings, the patient was thought to harbor triple-negative invasive ductal carcinoma with apocrine differentiation derived from an accessory breast at the axilla. For residual cancer, chemoradiotherapy is considered.
Differential diagnosis between apocrine adenocarcinoma (AAC) and apocrine-type breast cancer is the most important aspect in this case. In nosology, AAC refers to primary cutaneous apocrine sweat gland carcinoma, whereas apocrine carcinoma (AC) refers to the subtype with apocrine differentiation of breast cancer (Molina, WHO).
Male breast cancer comprises <1% of all breast cancers; [1,2] accessory breast cancer (ABC) composes 0.3% of all breast cancer cases [3,4]. Most ABCs are derived from accessory breast tissue existing on the so-called milk line [5]; 70% of ABCs are located in the armpit [3,4]. ABCs in men are extremely rare. There are only several cases of male axillary breast cancer reported previously [6]. AAC is also rare, the incidence of which is 0.0173 cases/100,000 persons per year in the whole body [7], and only 50 cases have been reported previously [8].
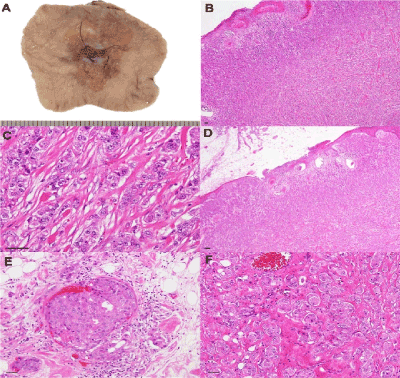
Figure 1. (A) Gross appearance of the excised axillary tumor. At the skin surface, a dip with erosion is observed. (B-F) Hematoxylin & eosin (HE)-stained photomicrogram. (B) The ill-demarcated tumor was located in the dermis and subcutaneous adipose tissue (4×). (C) The tumor comprised dense proliferation of solid nests (40×). (D) Tumor cells were invading the epidermis to form erosion (10×). (E) Venous and lymphatic-duct infiltration of the tumor was found (20×). (F) Tumor cells with wide, eosinophilic cytoplasm suggested apocrine differentiation (20×). Bar, 50 μm.
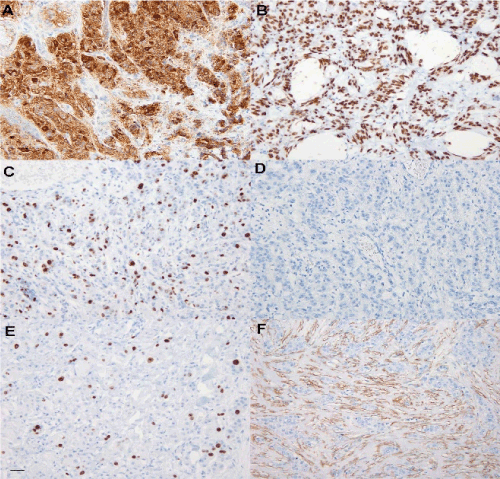
Figure 2. (A) Most of the tumor cells tested positive for gross cystic disease fluid protein-15 (GCDFP-15). (B) Most of the tumor cells tested positive for androgen receptor (AR). (C) Approximately 60% of the tumor cells tested positive for p53. (D) The tumor cells tested negative for estrogen receptor (ER). (E) Approximately 6% of the tumor cells tested positive for Ki-67. (F) Smooth muscle actin (SMA)-positive fibers were found in the tumor stroma, but not in non-cancer invaded dermis. Bar, 50 μm.
Male breast cancer is detected at advanced age in comparison with female breast cancer [2, 9]. Many ABCs are found in women aged ≥40 years [10]. Moreover, AAC occurs in those with an average age of 67 years, and the sex ratio is equal [7]. The present case was also of an 87-year-old, elderly patient.
Family history of breast cancer, obesity, excessive alcohol consumption, and liver cirrhosis are the most common risks for male breast cancer [9]. Some male breast cancers occur in those who carry gene abnormalities, such as BRCA2 mutations and Klinefelter's syndrome [11]. In our case, the patient did not exhibit these risk factors.
The diagnosis of male breast cancer often occurs late, when the stage is advanced [2]. Lymph node metastases are detected at the time of diagnosis in 80% of cases, and 14% of these are T4 [9]. The diagnosis of ABC also tends to be delayed by more than an average of 40 months [10]. In contrast, AAC shows a highly malignant phenotype; lymph node metastases are present in 69% of cases [7]. In the present case, the patient was diagnosed several years after he noticed a subcutaneous tumor, which caused dermal invasion and multiple lymph node metastases.
While most histological types of breast malignancies are reported in ABC [12], 70–80% of ABCs are invasive ductal carcinoma (IDC) [10, 13]. In male breast cancer, 80–90% of cases express hormone acceptors [2, 9] and 10% of cases express HER2 [9]. Our case was triple-negative, but positive for AR.
The most important histopathological finding for the diagnosis of AAC is decapitation secretion in eosinophilic epithelial cells [14]. In the present case, glandular structures were not observed and decapitation secretion was not observed.
Based on immunohistochemical examination, AACs test positive for GCDFP-15 [15]. Regarding receptors, 36% of AACs are positive for AR, whereas <30% of these are positive for ER, PgR, and HER2 [16]. However, those with breast cancer also express GCDFP-15 at a high rate with 95% specificity and 74% sensitivity [17, 18].
Furthermore, AR is positive in 60% of all cases of breast cancer and 13% of triple-negative breast cancers (TNBCs) [19]. In apocrine metaplasia and apocrine carcinoma, AR overexpression is associated with decreases in ER and PgR expression [20]. In addition, AR expression in ER-negative breast cancer relates to apocrine differentiation [21], and AR expression in TNBC suggests an apocrine cancer [22].
From these, the expressions of GCDFP-15 and AR are not definitive diagnostic factors for distinguishing between AAC and AC. In contrast, 46% of the AC components in breast cancer are positive for p53 [23], the incidence of which is higher than the p53-positivity rate being 15% in AAC [16]. In addition, in breast cancer, SMA-positive stroma formation results from cancer-associated fibroblasts [24, 25]. In this case, the tumor tested positive for p53 and was accompanied by SMA-positive stroma.
As mentioned above, it is difficult to distinguish between AC and AAC based on patient backgrounds or clinical images. In addition, the characteristics of both greatly resemble each other upon examination of histopathology and immunohistochemistry. The present case was considered to be of an invasive ductal carcinoma with apocrine differentiation derived from the axillary accessory breast based on the lack of decapitation secretion, abnormal accumulation of p53, and SMA-positive stroma. However, tumor excision is performed in male ABC and AAC; the response to chemotherapy is not optimistic [7, 10]. The overall survivals of ABC and AAC are 40.5 and 51.5 months, respectively [7, 10].
- Fentiman IS, Fourquet A, Hortobagyi GN (2006) Male breast cancer. Lancet 367: 595-604.
- Gomez-Raposo C, Zambrana Tevar F, Sereno Moyano M, Lopez Gomez M, Casado E (2010) Male breast cancer. Cancer Treat Rev 36: 451-7. [Crossref]
2021 Copyright OAT. All rights reserv
- Evans DM, Guyton DP (1995) Carcinoma of the axillary breast. J Surg Oncol 59: 190-195. [Crossref]
- Wysokinska EM, Keeney G (2014) Breast cancer occurring in the chest wall: rare presentation of ectopic milk line breast cancer. J Clin Oncol 32: e35-36. [Crossref]
- Harris JR, Lippman MR, Morrow M, Osborne CK (2014) Diseases of the breast. 5th ed. Conshohocken: Wolters Kluwer Health Adis.
- Schneider S, Sariego J (2009) Male breast cancer presenting as an axillary mass: a case report and literature review. South Med J 102: 736-737. [Crossref]
- Hollowell KL, Agle SC, Zervos EE, Fitzgerald TL (2012) Cutaneous apocrine adenocarcinoma: defining epidemiology, outcomes, and optimal therapy for a rare neoplasm. J Surg Oncol 105: 415-419. [Crossref]
- Seong MK, Kim EK, Han K, Seol H, Kim HA, et al. (2015) Primary apocrine sweat gland carcinomas of the axilla: a report of two cases and a review of the literature. World J Surg Oncol 13: 59. [Crossref]
- Liukkonen S, Saarto T, Maenpaa H, Sjostrom-Mattson J (2010) Male breast cancer: a survey at the Helsinki University Central Hospital during 1981-2006. Acta Oncol 49: 322-327. [Crossref]
- Visconti G, Eltahir Y, Van Ginkel RJ, Bart J, Werker PM (2011) Approach and management of primary ectopic breast carcinoma in the axilla: where are we? A comprehensive historical literature review. J Plast Reconstr Aesthet Surg 64: e1-11. [Crossref]
- Agrawal A, Ayantunde AA, Rampaul R, Robertson JF (2007) Male breast cancer: a review of clinical management. Breast Cancer Res Treat 103: 11-21.
- Nihon-Yanagi Y, Ueda T, Kameda N, Okazumi S (2011) A case of ectopic breast cancer with a literature review. Surg Oncol 20: 35-42. [Crossref]
- Shin SJ, Sheikh FS, Allenby PA, Rosen PP (2001) Invasive secretory (juvenile) carcinoma arising in ectopic breast tissue of the axilla. Arch Pathol Lab Med 125: 1372-1374. [Crossref]
- Robson A, Lazar AJ, Ben Nagi J, Hanby A, Grayson W, et al. (2008) Primary cutaneous apocrine carcinoma: a clinico-pathologic analysis of 24 cases. Am J Surg Pathol 32: 682-690. [Crossref]
- Paties C, Taccagni GL, Papotti M, Valente G, Zangrandi A, et al. (1993) Apocrine carcinoma of the skin. A clinicopathologic, immunocytochemical, and ultrastructural study. Cancer 71: 375-381. [Crossref]
- LeLL LP, Dias-Santagata D, Pawlak AC, Cosper AK, Nguyen AT, et al. (2012) Apocrine-eccrine carcinomas: molecular and immunohistochemical analyses PLoS One 7: e47290.
- Fiel MI, Cernaianu G, Burstein DE, Batheja N (1996) Value of GCDFP-15 (BRST-2) as a specific immunocytochemical marker for breast carcinoma in cytologic specimens. Acta Cytol 40: 637-41. [Crossref]
- Wick MR, Lillemoe TJ, Copland GT, Swanson PE, Manivel JC, et al. (1989) Gross cystic disease fluid protein-15 as a marker for breast cancer: immunohistochemical analysis of 690 human neoplasms and comparison with alpha-lactalbumin. Hum Pathol 20: 281-287. [Crossref]
- Rakha EA, El-Sayed ME, Green AR, Lee AH, Robertson JF, et al. (2007) Prognostic markers in triple-negative breast cancer. Cancer 109: 25-32. [Crossref]
- Gatalica Z (1997) Immunohistochemical analysis of apocrine breast lesions. Consistent over-expression of androgen receptor accompanied by the loss of estrogen and progesterone receptors in apocrine metaplasia and apocrine carcinoma in situ. Pathol Res Pract 193: 753-758. [Crossref]
- Niemeier LA, Dabbs DJ, Beriwal S, Striebel JM, Bhargava R (2010) Androgen receptor in breast cancer: expression in estrogen receptor-positive tumors and in estrogen receptor-negative tumors with apocrine differentiation. Mod Pathol 23: 205-212. [Crossref]
- Tsutsumi Y (2012) Apocrine carcinoma as triple-negative breast cancer: novel definition of apocrine-type carcinoma as estrogen/progesterone receptor-negative and androgen receptor-positive invasive ductal carcinoma. Jpn J Clin Oncol 42: 375-386.
- Moriya T, Sakamoto K, Sasano H, Kawanaka M, Sonoo H, et al. (2000) Immunohistochemical analysis of Ki-67, p53, p21, and p27 in benign and malignant apocrine lesions of the breast: its correlation to histologic findings in 43 cases. Mod Pathol 13: 13-8. [Crossref]
- Cimpean AM, Raica M, Narita D (2005) Diagnostic significance of the immunoexpression of CD34 and smooth muscle cell actin in benign and malignant tumors of the breast. Rom J Morphol Embryol 46: 123-129. [Crossref]
- Kojima Y, Acar A, Eaton EN, Mellody KT, Scheel C, et al. (2010) Autocrine TGF-beta and stromal cell-derived factor-1 (SDF-1) signaling drives the evolution of tumor-promoting mammary stromal myofibroblasts. Proc Natl Acad Sci U S A 107: 20009-20014. [Crossref]


