The study explored the association of laboratory parameters with radiologic severity of RT-PCR SARS-CoV-2 infection in healthcare workers (HCWs).
We retrospectively studied the HCWs with a positive SARS-CoV-2 PCR. Clinical, laboratory and radiological data were retrieved from the health records. Lung opacities were assessed by two independent radiologists. Inferential statistics were used to determine association with CXR severity, laboratory parameters and demographic characteristics. Receiver operating characteristics (ROC) curves for sensitivity and specificity of laboratory parameters to distinguish CXR severity was analyzed.
Significant association of C-reactive protein (CRP) (p=0.0001), monocytes and eosinophil’s percentages with mild cases was observed. Lymphocyte to CRP Ratio (LCR) was found to be associated with hypertension (p=0.006) and diabetes (p=0.007). The ROC curve for LCR against mild CXR severity showed a sensitivity of 84.5% with a specificity of 60.0%. The neutrophil to lymphocyte ratio (NLR) and lymphocyte to monocyte ratio (LMR) were found to be non-significant.
CRP, monocytes, eosinophil’s and LCR correlated with mild disease severity in SARS-COV-2 infection confirmed HCWs cases in Qatar. LCR was found to have good sensitivity for discriminating mild cases and can serve as a marker to determine further referral in SARs-CoV-2 patients in a clinical setting.
SARS-CoV 2, healthcare workers, Chest X-ray scoring severity, biological markers, Radiological parameters, Lymphocyte to CRP ratio
In the latter half of 2019, the number of viral pneumonia cases seen in Wuhan, China rose exponentially with global spread throughout 2020. The virus was isolated from epithelial cells of the respiratory system of infected individuals and was termed as Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2). In January 2020, World Health Organization (WHO) declared it as a global pandemic and named it as coronavirus disease (SARS-COV-2 infection) [1].
Given the clinical ambiguity in presentation of SARS-CoV-2 infection with other common viral illnesses, early utilization of laboratory and radiological investigations play a useful clinic adjunct in appropriately managing suspected cases [2-4]. It is our aim that through piecing together these markers and further correlating with image presentation in early SARS-CoV-2 infection, Healthcare worker surveillance in future pandemics could be improved through health policy reformation and deeper understanding of early SARS-CoV-2 infection management.
In approximately 80% of those infected with SARS-CoV-2, a plethora of symptoms can develop. This can range from fever, cough, dyspnea, fatigue, myalgia, hemoptysis, headache, diarrhea, olfactory disorders, ischemic or hemorrhagic stroke, conjunctival hyperemia etc. [5,6]. However, 15% of those affected will go on to be hospitalized with critical disease & a further 5% will require admission into intensive care unit [7]. Abnormal Chest tomography (CT) used to monitor & facilitate diagnosis of Acute respiratory Distress Syndrome (ARDS) in the more sick infected population highlights the significant role imaging plays in SARS CoV-2 infection [2,4] suggesting that imaging plays a significant role in diagnosis and disease monitoring. Documented reports suggest that patients with severe SARS-COV-2 infection have ground-glass opacity (GGO), local or bilateral patchy shadowing, and interstitial abnormalities on CT indicating the clinical utility of chest CT as a predictive tool. In addition to chest CT, Chest X-Ray (CXR) is also considered as a useful adjunct in clinical care and in understanding severity of COVID-19 pneumonia [2]. Although X-ray being the less sensitive modality in the detection of COVID-19 lung disease compared to CT (69% compared to CT), its utilization in identifying radiological findings still serves a useful adjunct in clinical management (i.e. multifocal, bilateral ground glass opacities and consolidations with peripheral and basal predominance [8-11]. CXR is not limited by current infection control issues that limit CT utilization in hospital settings making it more accessible, it is available as a mobile unit and therefore more accessible in the early phase of SARS-COV-2 infection management [12].
Hematological and inflammatory markers too have been reported to provide useful clinical insight in the early phase stages of confirmed SARS-COV-2 infection [3]. Preliminary SARS-COV-2 infection research has already shown that in early stages of SARS-COV-2 infection, the leukocyte count either remains normal or is decreased while lymphocyte count remains low [13-15]. Furthermore, as the disease dynamics changes, an increase or decrease in leukocytes/lymphocytes is observed indicating that changes in blood parameters can serve as useful prognostic/predictive markers in SARS-COV-2 infection [5,14]. In lieu of this, changes in hematological circulating biomarkers associated with inflammation such as Neutrophil to Lymphocyte ratio (NLR), Lymphocyte to Monocyte ratio (LMR), Lymphocyte to C-Reactive Protein ratio (LCR) have been documented as useful prognostic makers to assess disease severity and predict inflammatory response in SARS-COV-2 infected patients [13,16-18].
The upsurge of SARS-COV-2 infectious cases for the 2nd time since the beginning of the pandemic in the various global hotspots including the Gulf, has presented increased pressures for the Healthcare systems. In Qatar, UK and South Africa variants have been reported and documented by the Ministry of Public Health (MoPH). These new strains are found to be exhibiting more mutations and are associated with increased risk of death and quicker spreading In March 2021, Qatar documented 82% and 58% increase in ICU and hospital admissions and 13 deaths since February 1st, 2021 [19]. Keeping these statistics in perspective, it becomes increasingly important for HCWs and policy makers facing these challenges to gain a deeper understanding of the clinical presentation, laboratory, and radiological findings in frontline staff. Correlation of clinical findings and tests, associations with patient demographic enrich clinical decision making for triaging processes in taking better care of Healthcare staff moving forward. Though, large number of studies on imaging and laboratory data have focused on COVID 19 infected patients, there is still paucity of respective data on SARS-COV-2 infected HCWs. HCWs are at highest risk for spread of infection and getting infected. They also struggle with long working hours, fatigue, increased psychological stress resulting in a worse disease prognosis. This can lead to increased lost workdays inducing additional pressure on the hospitals [20]. Therefore, it is imperative that robust biomarkers are identified and described in SARS-COV-2 infected HCWs to monitor and help manage the disease promptly.
The aim of this observational study is to provide in-depth analysis of the demographics, clinical characteristics as well as correlation of change in lab parameters with CXR severity in HCWs who had confirmed SARS-CoV-2 infection via PCR testing. This will provide an insight on the relationship among CXR findings and inflammation to facilitate clinicians in early identification of HCWs/patients for further referrals and monitoring. In addition, our study will aid healthcare policy makers in healthcare staff deployment in future pandemics ultimately protecting those who may be most at risk.
Clinical significance
- In mild CXR severity cases, approximately six-fold increase in CRP levels was observed indicating the correlation of CRP levels with changes in the lung lesions
- Lymphocyte CRP Ratio (LCR) was found to be significantly associated with mild CXR severity. The ROC curves generated showed that this marker has fair ability to discriminate normal cases from mild CXR severity
- High CRP levels, low lymphocytes, and significant association of LCR with mild CXR severity indicate that LCR can be used as a biomarker of disease dynamics in SARS-CoV-2 infection in HCWs/patients.
Study population: The retrospective, observational study was conducted at the Central Staff Health Clinic, Hamad Medical Corporation (HMC), Doha, Qatar between March 1, 2020, and May 18, 2020. HMC is Joint Commission International Accredited (JCIA) public healthcare organization in the State of Qatar encompassing 14 hospitals with a total employed staff of >30,000. The Central Staff Health Clinic in Doha, Qatar, caters to healthcare workers (HCWs) from HMC associated hospitals.
Data collection: All HCWs at HMC facilities with RT PCR positive result for SARS-COV-2 infection on a nasopharyngeal swab were eligible to be included in this study. A total of 196 HCWs were included if they met the following criteria a) confirmed SARS-COV-2 infection RT-PCR positive result b) having baseline laboratory results including blood group, complete blood count, chemistry and C-Reactive Protein (CRP) c) CXR within 10 days of confirmed RT-PCR SARS CoV-2 infection. Data on demographics including age, gender, nationality, employment-related information, co-morbidities (Chronic Kidney disease, diabetes, chronic lung disease i.e. asthma, COPD, pulmonary fibrosis, Coronary heart disease (CHD), hypertension, asthma, allergies etc.) as well as laboratory, CXR related reports/images and symptoms (fever, dry cough, sore throat, headache, respiratory symptoms, gastrointestinal (i.e. diarrhea) disturbance, body ache and pain and rashes) were extracted from the electronic health record system (Cerner, Kansas City, USA). The HCWs who had missing laboratory parameters or CXR reports/imaging were excluded from the study (Figure 1).
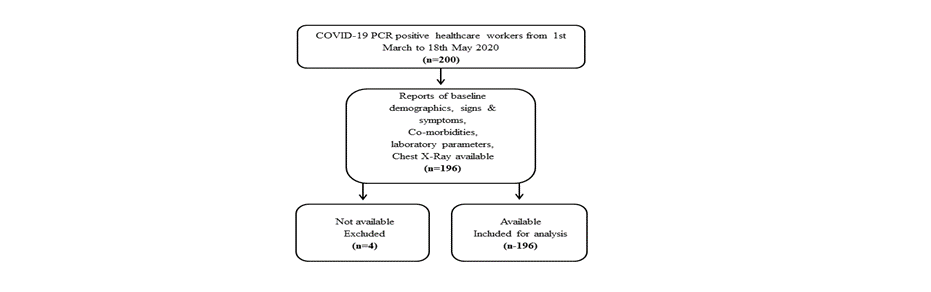
Figure 1. Flow diagram of patients
CXR assessment: For CXR assessment, a scoring system was devised based on the modified RALE scoring system (a scoring system previously utilized in published studies focusing on disease severity assessment of SARS-COV-2 infection on CXR) [21]. The initial frontal chest radiograph for each patient was scored independently by two radiologists to mitigate the risk of recall bias based on a devised scoring system of 0-24 and classified as normal/mild/moderate/severe depending on the extent of lung opacities. Both ground glass opacities (GGO) and consolidation were included under the term lung opacities and any opacification (GGO or consolidation) were used for scoring. Each lung was divided into 3 zones which were further divided into 4 sectors, resulting in a total of 24 sectors per patient. The upper, middle, and lower zones include the parts of the lungs above the level of the hilum, at the level of the hilum and below the level of the hilum, respectively (Figure 2a). A score of 0-24 was assigned depending on the number of sectors that contained airspace opacities. Opacities that extended over more than one sector were scored based on the number of sectors they filled. Finally, depending on the overall score, each chest radiograph was assigned a severity rating of either mild (0-8), moderate (9-16) or severe [17-24] (Figure 2b and Figure 2c).
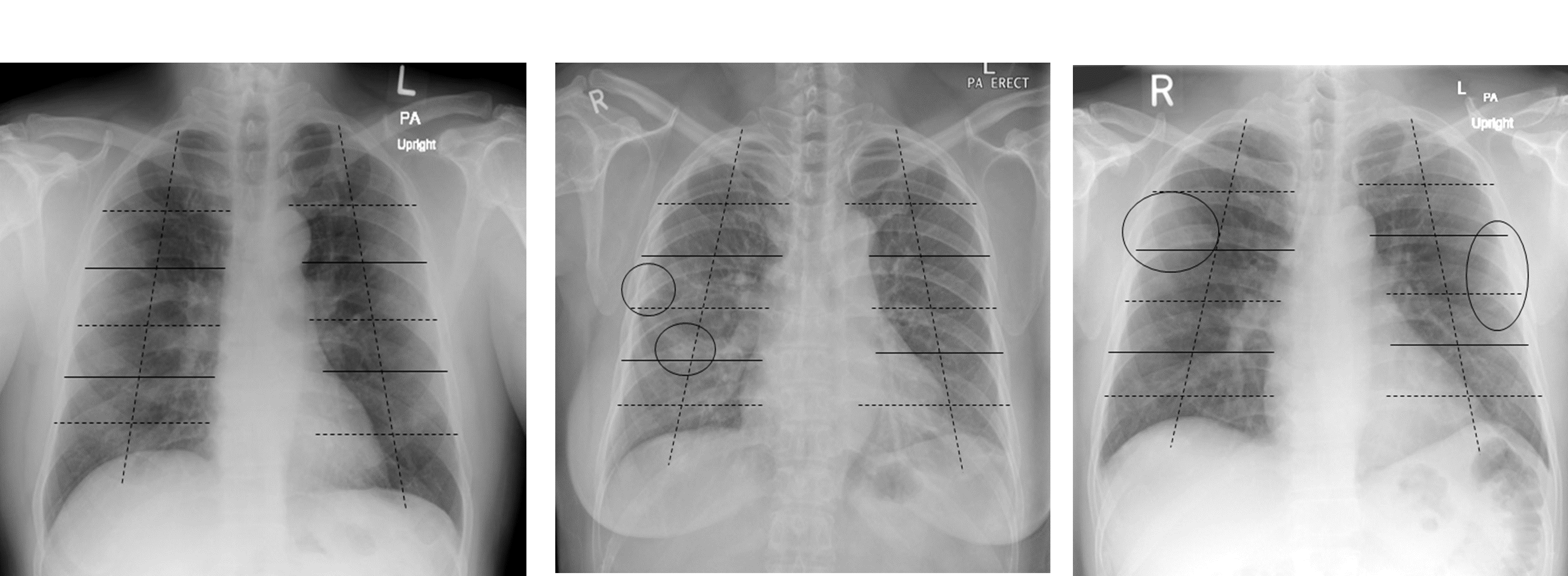
Figure 2a. Each lung is divided into three zones (indicated by lines) which are further divided into four sectors (indicated by dashed lines), resulting in a total of twenty-four sectors. The upper, middle, and lower zones include the parts of the lungs above the level of the hilum, at the level of the hilum and below the level of the hilum, respectively 2b) subtle patchy opacities in the right middle to lower lung zones (ellipse) which occupy a total area not exceeding one sector. This corresponds to a CXR severity score of 1/24 (Mild) 2c) subtle patchy opacities are seen peripherally in the right upper and left middle lung zones (ellipse) which occupy a total area of more than one sector but not exceeding the area of two sectors. This corresponds to a CXR severity score of 2/24 (Mild)
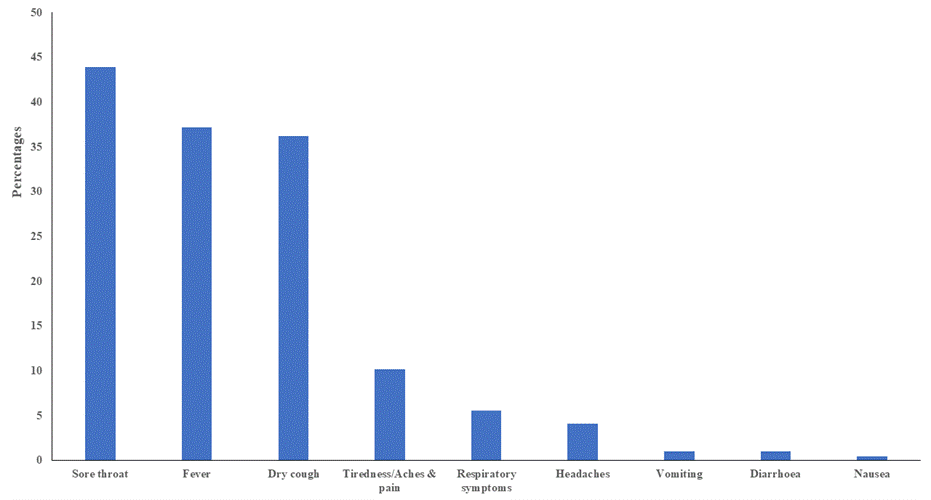
Figure 3. Types of symptoms reported in HCWs
Statistical analysis: Excel and SPSS v26 (IBM SPSS Statistics for Windows, Armonk, NY: IBM Corp.) programs were used for data management and analysis. Frequency (%), mean (SD) and median (IQR) were used to describe the data. Normality of data was confirmed with Kolmogorov-Smirnov test. Due to the non-normality of data, non-parametric tests were used for the inferential statistical analysis. Mann-Whitney, Kruskal-Wallis and Spearman rho tests were carried out at alpha level of 0.05. Interclass correlation was done to measure the level of agreement of the two radiologists for assessing the CXR images. The optimal cut-off values of the LCR, LNR and LMR for the differentiation of patients with different CXR levels were calculated by using the ROC analysis. Furthermore, the curve was established to measure the level of sensitivity and specificity of the laboratory parameters. P<0.05 was considered as statistically significant.
Ethics: The study was approved by the Institutional Review Board of Hamad Medical Corporation with a waiver of informed consent under a pandemic response framework adopted by the institution.
Patient characteristics: We identified 196 HCWs who met the study criteria. Table 1 shows the demographic profiles of HCWs. Most of the HCWs were males (n=136, 69.4%) with a median (IQR) age of 40 (33.0-49.0) years. These largest group was nurses (n=84, 42.9%) with direct contact (n=118, 60.5%) with patients. Most of the HCWs did not have any comorbidity (n=144, 73.5%). However, main co-morbidities observed included hypertension (n=29, 14.8%) and diabetes mellitus (n=25, 12.8%). Furthermore, majority of the HCWs were asymptomatic (n=171, 87.2%) while 12.8% (n=25) exhibited symptoms including sore throat (n=86, 43.9%), fever (n=73, 37.2%), dry cough (n=71, 36.2%), tiredness/aches/pain (n=20, 10.2%) and respiratory symptoms (n=11, 5.6%). (Table 1), (Fig 3).
Table 1. Baseline characteristics of HCWs
Characteristics |
|
N |
% |
Median age years (IQR) |
40.00 (33.0-49.0) |
|
|
Males |
|
136 |
69.4 |
Nationality |
Asian
Arab
Others |
148
28
20 |
75.5
14.3
10.2 |
Occupation |
Nurse
Physician
Engineering
Paramedic
Pharmacist
Technician
Others |
84
15
12
9
9
10
53 |
42.9
7.7
6.6
4.6
4.6
5.1
27.6 |
Contact |
Direct contact
Indirect contact
No contact |
118
31
46 |
60.5
15.9
23.6 |
Comorbidities
|
Hypertension
Diabetes Mellitus
Chronic lung disease
Coronary heart disease
Chronic kidney disease
Other diseases |
29
25
7
4
2
3 |
14.8
12.8
3.6
2.0
1.0
1.5 |
Chest X-ray severity |
Normal
Mild
Score Ranges 0-9
Mean + SD: (0.22 + 0.74) |
175
21 |
89.3
10.7
|
Chest X-Ray: All HCWs underwent CXR within 10 days of confirmed diagnosis. Of the 196 HCWs, mild CXR severity was observed in 21 (10.7%) while the remaining 175 (89.3%) were found to have normal CXR. Scoring from both radiologists was considered for evaluation of severity. The scores ranged from 0 to 9 with an average score of 0.22 (0.74). The interclass correlation coefficient between the two radiologists was found to be 0.841 (p=0.000) which indicates good agreement.
Laboratory findings: Table 2 shows hematological and biochemical laboratory parameters of HCWs at the time of diagnosis. Of all 196 HCWs, mean CRP levels and monocyte percentage was found to be higher than normal ranges i.e. 8.26mg/ml and 9.82% respectively while mean eosinophil percentage was found to be lower than normal ranges i.e. 1.71%. Significant changes were observed in CRP (p=0.0001). The mean NLR, LMR and LCR in all 196 HCWs was found to be 2.00, 3.76 and 1.63 respectively while in 21 mild CXR severity cases, it was found to be 2.16, 3.86 and 0.64.
Table 2. Laboratory parameters of HCWs
Lab analytes
(reference ranges) |
All HCWs
(n=196) |
Mild CXR cases
(n=21) |
Mean (SD)
|
Mean (SD) |
Low
N (Mean) |
High
N (Mean) |
Urea
2.8-8.1 nmol/L |
3.77 (1.18) |
3.5 (0.76) |
4 (2.32)
|
- |
Creatinine
44-80 umol/L |
78.9 (17.03) |
80.71 (14.08) |
- |
11 (91.63) |
eGFR
>60 ml/min |
60.35 (7.20) |
60.00 (0.00) |
2 (48.5) |
- |
Sodium
136-145 mmol/L |
137.73 (9.94) |
137.91 (2.55) |
5 (134.4) |
- |
Potassium
3.5-5.1 mmol/L |
4.15 (0.40) |
4.11 (0.50) |
2 (3.2) |
1 (5.2) |
Bilirubin T
0-21 umol/L |
7.82 (4.12) |
8.76 (3.49) |
No Change |
Total Protein
66-87 gm/L |
78.22 (4.64) |
77.95 (5.42) |
- |
1 (91) |
Albumin
35-52 gm/L |
41.29 (3.36) |
39.43 (3.70) |
1 (30) |
- |
Alkaline Phosphatase
35-104 U/L |
68.58 (18.61) |
69.38 (16.08) |
|
1 (122) |
ALT
0-33 U/L |
30.14 (20.62) |
30.76 (15.82) |
- |
8 (46) |
AST
0-32 U/L |
27.41 (14.59) |
30.53 (20.65) |
- |
5 (48.4) |
CRP
0-5 mg/ml |
8.26 (12.60) |
19.96 (19.89) |
- |
14 (29.49) |
WBC
4-10x103/ul |
6.30 (1.97) |
6.17 (1.77) |
1 (3.7) |
1 (10.7) |
RBC
3.8-4.8x106/ul |
5.09 (0.55) |
5.03 (0.48) |
- |
13 (5.33) |
Hgb
12-15 gm/dL |
14.25 (1.46) |
14.37 (1.35) |
1 (11.4) |
7 (15.88) |
Hct
36-46 % |
43.10 (4.10) |
43.25 (3.70) |
- |
5 (48.74) |
MCV
83-101 fL |
85.00 (5.51) |
85.89 (4.28) |
6 (80.06) |
- |
MCH
27-32 pg |
28.12 (2.30) |
28.54 (1.63) |
4 (25.65) |
- |
MCHC
31.5-34.5 gm/dL |
33.05 (1.19) |
33.22 (0.92) |
1 (31) |
2 (34.7) |
RDW-CV
11.6-14.5 % |
12.94 (1.41) |
12.53 (1.03) |
2 (11.4) |
1 (15.5) |
Platelet
150-400 x 103/ul |
254.27 (68.50) |
244.71 (68.57) |
1 (113) |
- |
MPV
7.4-10.4 fL |
10.35 (0.90) |
10.19 (0.70) |
- |
4 (11.4) |
Absolute Neutrophil count Auto #
2-7 x 103/ul |
3.54 (1.48) |
3.71 (1.47) |
1 (1.3) |
1 (8.3) |
Lymphocyte #
1-3 x 103/ul |
2.00 (0.74) |
1.90 (0.59) |
2 (0.85) |
- |
Monocyte #
0.2-1.0 x 103/ul |
0.59 (0.22) |
0.53 (0.18) |
- |
1 (1.1) |
Eosinophil #
0.0-0.5 x 103/ul |
0.11 (0.15) |
0.05 (0.07) |
No Change
|
Basophil #
0.02-0.10 x 103/ul |
0.03 (0.46) |
0.02 (0.01) |
8 (0.008) |
- |
Neutrophil %
50% - 70 % |
55.18 (10.75) |
58.85 (10.13) |
2 (41.7) |
4 (74.75) |
Lymphocyte %
25 % - 40 % |
32.75 (9.55) |
31.25 (9.00) |
5 (19.38) |
1 (51.6) |
Monocyte %
2% - 8% |
9.82 (3.72) |
8.58 (2.31) |
- |
10 (10.43) |
Eosinophil %
2%-4% |
1.71 (2.07) |
1.02 (0.95) |
16 (0.64) |
- |
Basophil %
0-1% |
0.57 (1.11) |
0.31 (0.18) |
No change
|
Upon stratification of laboratory parameters with CXR severity (n=21), changes in other laboratory parameters (below or above the normal ranges) were also observed in several mild cases. Higher values were observed in creatinine, total protein, alkaline phosphatase, ALT, AST, CRP, RBC, lymphocyte% and monocyte % while lower values of e GFR, albumin, MCV, MCH, platelets, absolute neutrophil number, lymphocyte number and neutrophil%, lymphocyte%, eosinophil% were observed (Table 2).
Relationship between NLR, LMR, LCR with HCW characteristics and mild CXR severity: The association of NLR, LMR, LCR with HCW characteristics and CXR severity showed that only LCR was significantly associated with hypertension (p=0.006), diabetes (p=0.007) and mild CXR severity (p=0.001). (Table 3).
Table 3. Relationship between HCW characteristics and laboratory parameters (n=196)
Parameters |
Hypertension |
Diabetes |
Mild CXR severity |
Neutrophil/Lymphocyte ratio |
p=0.276 |
p=0.874 |
p=0.361 |
Lymphocyte/Monocyte ratio |
p=0.124 |
p=0.429 |
p=0.719 |
Lymphocyte/CRP ratio |
p=0.006** |
p=0.007** |
p=0.001**** |
Mann-Whitney U test. p<0.05 (statistically significant), * significant, **moderately significant, ****highly significant
Sensitivity and Specificity of mild CXR severity with NLR, LMR, LCR (ROC Curves): To determine the sensitivity and specificity of NLR, LMR, and LCR in distinguishing between normal and mild CXR severity cases, ROC curves were developed. It was observed that LCR showed a fair ability to discriminate mild CXR severity (AUC: 0.718, 95% CI: 0.589 - 0.848, p-0.001) while the NLR and LMR were not able to distinguish (AUC: 0.438, p=0.362 and AUC: 0.476, p=0.722, respectively). The optimal cut–off value for the LCR for the differentiation of normal/abnormal CXR of SARS-COV-2 infected patients was 0.1407 (sensitivity: 85% and 1-specificity: 40%). (Figure 4).
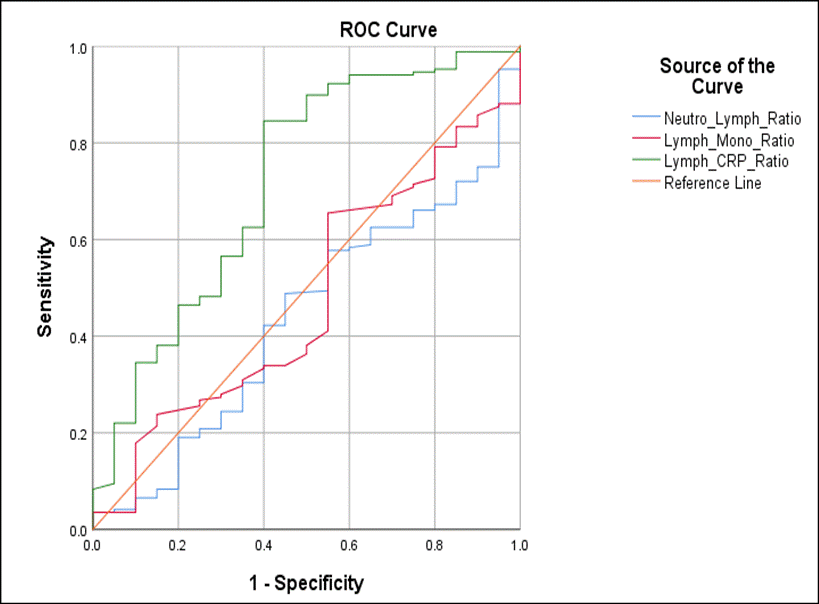
Figure 4. Receiver operating characteristics (ROC) curves for the abilities of LCR, NLR and LMR to distinguish CXR image level among COVID-19 infected patients
In this observational study, we have explored and described the clinical characteristics, laboratory parameters and radiological features of SARS-COV-2 infection in HCWs in governmental hospitals in Qatar. There are two published papers from Qatar focused primarily on prevalence of SARS-COV-2 infection and risk factors in healthcare workers (HCWs) [22,23]. This study provides an in-depth analysis of the demographics, clinical characteristics as well as correlation of changes in lab parameters with CXR severity in HCWs. The results also provide an insight into laboratory-based biomarkers that could help clinicians in early identification of SARS-COV-2 infection. This coupled with CXR correlations observed in the study could aid in piecing together the SARS-COV-2 infection presentations in HCWs more confidently.
We observed that demographically, the frequency of infection was higher in Asian males (Indians and Filipinos) working in nursing profession with direct contact to patients. Given that the Filipino and Indian ethnic group make up the largest cohort in HCWs in Qatar this was an expected finding. Hypertension and diabetes mellitus were the main co-morbidities observed while the manifested symptoms were generally non-specific with fever, sore throat and cough being most common followed by tiredness/aches, respiratory symptoms, and headaches. Accordingly, our results are consistent with published data from Qatar on HCWs [22,23].
CXR was assed rather than CT in the study as it was considered a validated imaging modality in HMC for initial assessment of patients who present mild symptoms of suspected COIVD-19. CT imaging was reserved for use in the most severe cases of SARS-CoV-2 infection. Several studies have demonstrated the value of CXR in assessment of patients with SARS-COV-2 infection and the association of extent of lung abnormalities on CXR with clinical parameters and/or disease severity [24,25]. Usually, typical CXR findings in SARS-COV-2 infection include patchy or diffuse asymmetric airspace opacities (consolidation and ground-glass opacities) [11,26]. 10% (21/196) of the HCWs showed lung opacities (both GGO and consolidation) that ranged from 0-9 on the devised scoring system and were designated as mild cases. The remaining 175 were considered normal on CXR. To mitigate recall bias, two radiologists had independently scored the CXRs and the interclass correlation coefficient was calculated which was found to 0.841 (p=0.000) indicating good reliability for the CXR severity scoring. No HCW exhibited moderate or severe CXR severity in the study. Several factors that may have contributed to this observation include; HMC COVID-19 Policy for testing suspected cases result in increased early presentation in RT-PCR SARS CoV-2infection cases, our CXR scoring system did not incorporate additional scores based on the density of airspace opacification which may have influenced the assessment as less subjective and scoring of only the initial chest radiograph might have contributed to the relatively low number of abnormal CXR severity/lung involvement. This inference is based on reports that note that the sensitivity of CXR in the first 2 days after symptom onset is approximately 55% which subsequently rises to 79% after 11 days [27,28]. Therefore, it is important to keep these factors in perspective when planning future large scale prospective/retrospective studies to have a better understanding of the disease dynamics.
Amongst the most notable laboratory parameters, CRP was found to be the main indicator of inflammation with consistently higher than normal ranges observed in all 196 HCWs (8.26mg/ml) as well as in all 21 mild CXR severity cases (19.96 mg/ml, p value=0.0001***). Further stratification showed that 14/ 21 mild CXR cases exhibited approximately six-fold increase in CRP levels (29.49mg/ml), indicating that infection, inflammation, and changes in the lungs can lead to high CRP levels. This finding is clinically important as it provides evidence that CXR changes can be captured early on by variation in CRP levels indicating the utility of this marker in acute-phase inflammatory response. Other studies have also documented similar observations for CRP in SARS-COV-2 Infection. For e.g. a study by Wang on the correlation of CRP levels with lung lesions and severe presentation showed that increased CRP levels were positively correlated with largest diameter of lung lesions and diseaseseverity (28). Studies by Hiu TC, et al. and Orsi MA, et al. also showed significant positive correlation between CXR severity and high CRP levels [29,30]. On the other hand, a number of studies have documented on the importance of CRP as an inflammatory marker that can be used to monitor and predict disease severity/progression [28,31,32].
Monocyte percentages were also found to be higher (9.82%) in all HCWs as well as in 10/21 mild CXR cases (10.43%). These findings are consistent with reported studies that document significantly higher levels of monocyte % at the time of diagnosis in SARS-COV-2 infected patients [33]. Biamonte, et al. utilized baseline lab parameters to stratify patients’ response and survival in Sars-CoV-2 infection [34]. The study presented compelling data which showed that low risk patients (defined as 1 death over 17 patients with good overall survival) had higher monocyte count as compared to high/intermediate risk patients. The study results indicated the discriminatory ability of monocytes and its utility as a biomarker of disease severity.
We noted low eosinophil percentages (1.71%) in all HCWs as well as in 16/21 mild CXR cases (0.64%). A similar trend was observed in a study reported by Liu, et al. in which eosinophil’s exhibited lower threshold at baseline diagnosis that increased significantly with therapeutic intervention [35]. A further study in Qatar depicted similar findings in which patients with eosinophilia exhibited milder clinical course and fewer radiological abnormalities as compared to those without eosinophilia, indicating that eosinophilia is associated with a favorable outcome in SARS-CoV-2 infected patients [36]. Therefore, findings from published literature indicate that eosinophilia may be an indicator of COVID-19 recovery and thus eosinophil’s can be potential candidates as biomarkers of response.
In mild CXR severity cases other laboratory parameters also showed difference in values from the normal ranges. For the sake of clarity, only those parameters that showed significant difference in values from normal ranges are being discussed here. Higher values of creatinine, total protein, alkaline phosphatase, ALT, AST, CRP, RBC, lymphocyte% and monocyte % while lower values of e GFR, albumin, MCV, MCH, platelets, absolute neutrophil number, lymphocyte number and neutrophil%, lymphocyte%, eosinophil% were observed in a number of mild cases (Table 2). These findings are consistent with various studies that have correlated high CRP, neutrophils, ALT, AST, bilirubin and creatinine and low lymphocytes, eosinophil’s, platelets, albumin with immune-mediated inflammation and disease severity in SARS-COV-2 infection [14,18,37-43]. This study, in addition to the existing medical research looking specifically at the lab hematology & biochemistry in SARs-CoV-2 infection, supports the use of CRP, monocyte, eosinophil percentages in serving as early indicators of disease severity. Since these tests are done routinely at early onset as well as during therapeutic intervention, their utility is unprecedented. We advise caution in data interpretation given the limitation of sample sizing in our study and advocate larger scale work to gain deeper insight into the parameters described.
Emerging evidence suggests that peripheral blood neutrophil-to-lymphocyte ratio (NLR), Lymphocyte-to monocyte ratio and Lymphocyte-to CRP ratios can be used as a markers of systemic inflammation, and may serve as a reliable predictor of disease severity by differentiating between mild, moderate, and severe SARS-COV-2 infection [44-48]. LCR was found to be significantly associated with diabetes (p=0.006), hypertension (p=0.007) and mild CXR severity (p=0.001). The ROC curves generated showed that AUC for LCR was 0.718 and the optimal cut–off value for the LCR for the differentiation of normal/abnormal CXR of SARS-CoV-2 infection was 0.1407 (sensitivity: 85% and 1-specificity: 40%) indicating fair ability of this parameter to discriminate normal cases from mild CXR severity. For NLR and LMR, the AUC were low (0.438 and 0.476) and were therefore not reliable parameters for distinguishing mild CXR cases.
LCR is a well-known surrogate marker of immunological interactions and systemic inflammatory process with favorable prognostic value in various diseases [49,50]. A study by Waqas Ullah, et al. reported that LCR can serve as a predictive marker for disease severity in SARS-CoV-2 infection due to several factors [47]. It is well known that CRP levels rise early in SARS-CoV-2 infection while lymphocyte levels reduce significantly. Therefore, it was postulated that due to this rise in CRP levels and reduced lymphocyte levels early on into the disease, LCR may be a more sensitive in capturing the early inflammatory cascade. Since CRP is not deregulated by steroids (given during infection) or immune suppression etc., it can truly represent induced inflammatory changes thus making LCR a robust predictive marker for disease severity. Similarly, a meta-analysis on six studies also concluded that decline in LCR might correlate with enhanced inflammatory process, poor prognosis and disease severity of SARS-CoV-2 infection [51]. Since we observed similar findings in our study i.e. high CRP levels, low lymphocytes, and significant association of LCR with mild CXR severity, we concur with the reported data. We observed acceptable sensitivity and specificity for LCR; therefore, we can carefully deduce that LCR can be used in clinical settings to facilitate healthcare teams by assisting early triage and guiding prognosis in SARS-CoV-2 infection in HCWs/patients.
The limitation of this study is the small sample size and retrospective design. Or recommendation is that large prospective studies exploring timeline associated changes in both CXR and laboratory parameters need to be performed. Such a study is more significant now as the second wave of COVID-19 is in full force in Qatar and early markers may allow better understanding and management of disease within the population of Qatar, especially amongst HCWs.
The study concludes that laboratory parameters can serve as predictive and prognostic marker in SARS-CoV-2 infection disease severity. In a pandemic situation, it is essential that tools such as CRP, LCR and changes in laboratory parameters be tested and validated to understand their utility as indicators of disease dynamics. These aspects should be explored further in large scale studies to understand and manage disease dynamics.
The views presented in this manuscript are those of the authors and do not necessarily represent the views or official policy of Hamad Medical Corporation or the Ministry of Public Health, Qatar.
Study concept and design: MUR, KP, AR, MI, SU. Data acquisition: MI, ME, RMU, KP, AR. Data analysis: MI, ME, MUR, KP, AR. Radiological analysis: NM, HS. Manuscript writing: MUR, KP, AR, MI, NM, HS. Critical review and scientific input: MM, FA, SD, SU.
No potential conflict of interest was reported by the author(s).
We would like to acknowledge and appreciate Dr. Adeel Ajwad Butt, Professor of Medicine, and Professor of Healthcare Policy and Research, Weill Cornell Medicine-Qatar, Vice Chair, Department of Medicine, Director, Clinical Epidemiology Research Unit, Hamad Medical Corporation, Doha, Qatar for his critical review and input on the manuscript. The publication of this article was funded by the Qatar National Library.
The study was funded by the Hamad Medical Corporation, Medical Research Center grant, MRC 01-20-598 titled “Biological & Radiological markers Exploration & Association in SARS-CoV-2 Infected Healthcare workers in Governmental Hospitals, Qatar. (BREACH-A Pilot Study)”.
The study was approved by the Institutional Review Board of Hamad Medical Corporation with a waiver of informed consent under a pandemic response framework adopted by the institution.
- WHO Coronavirus (COVID-19) Dashboard Accessed on 08 July 2021. [Available from: https://covid19.who.int/].
- Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, et al. (2020) Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med 382: 1708-1720. [Crossref]
- Wang C, Deng R, Gou L, Fu Z, Zhang X, et al. (2020) Preliminary study to identify severe from moderate cases of COVID-19 using combined hematology parameters. Ann Transl Med 8: 593. [Crossref]
- Wang D, Hu B, Hu C, Zhu F, Liu X, et al. (2020) Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA 323: 1061-1069.
- Huang C, Wang Y, Li X, Ren L, Zhao J, et al. (2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395: 497-506.
- Vetter P, Vu DL, L'Huillier AG, Schibler M, Kaiser L, et al. (2020) Clinical features of covid-19. BMJ 369: m1470. [Crossref]
- Wu Z, McGoogan JM (2020) Characteristics of and Important Lessons from the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72314 Cases from the Chinese Center for Disease Control and Prevention. JAMA 323: 1239-1242. [Crossref]
- Bernheim A, Mei X, Huang M, Yang Y, Fayad ZA, et al. (2020) Chest CT Findings in Coronavirus Disease-19 (COVID-19): Relationship to Duration of Infection. Radiology 295: 200463. [Crossref]
- Meng H, Xiong R, He R, Lin W, Hao B, et al. (2020) CT imaging and clinical course of asymptomatic cases with COVID-19 pneumonia at admission in Wuhan, China. J Infect 81: e33-e39.
- Rousan LA, Elobeid E, Karrar M, Khader Y (2020) Chest x-ray findings and temporal lung changes in patients with COVID-19 pneumonia. BMC Pulm Med 20: 245.
- Wong HYF, Lam HYS, Fong AH, Leung ST, Chin TW, et al. (2020) Frequency and Distribution of Chest Radiographic Findings in Patients Positive for COVID-19. Radiology 296: E72-E8. [Crossref]
- Jacobi A, Chung M, Bernheim A, Eber C (2020) Portable chest X-ray in coronavirus disease-19 (COVID-19): A pictorial review. Clin Imaging 64: 35-42.
- Liu G, Zhang S, Hu H, Liu T, Huang J (2020) The role of neutrophil-lymphocyte ratio and lymphocyte-monocyte ratio in the prognosis of type 2 diabetics with COVID-19. Scott Med J 65: 154-160.
- Velavan TP, Meyer CG (2020) Mild versus severe COVID-19: Laboratory markers. Int J Infect Dis 95: 304-307.
- Qin C, Zhou L, Hu Z, Zhang S, Yang S, et al. (2020) Dysregulation of Immune Response in Patients with Coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis 71: 762-768. [Crossref]
- Xu JB, Xu C, Zhang RB, Wu M, Pan CK, et al. (2020) Associations of procalcitonin, C-reaction protein and neutrophil-to-lymphocyte ratio with mortality in hospitalized COVID-19 patients in China. Sci Rep 10: 15058. [Crossref]
- Yang AP, Liu JP, Tao WQ, Li HM (2020) The diagnostic and predictive role of NLR, d-NLR and PLR in COVID-19 patients. Int Immunopharmacol 84: 106504.
- Liu Y, Yang Y, Zhang C, Huang F, Wang F, et al. (2020) Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci 63: 364-374. [Crossref]
- Tribune Q (2020) Available from: https://www.qatar-tribune.com/latestnews-article/articleid/5924].
- Barranco R, Ventura F (2020) Covid-19 and infection in health-care workers: An emerging problem. Med Leg J 88: 65-66.
- Li MD, Arun NT, Gidwani M, Chang K, Deng F, et al. (2020) Automated assessment and tracking of COVID-19 pulmonary disease severity on chest radiographs using convolutional siamese neural networks. Radiology: Artificial Intelligence 2: e200079.
- Al Kuwari M, AbdelMalik M, Al Nuaimi A, AbdelMajeed J, Romaihi H (2021) Epidemiology of COVID-19 infection amongst workers in Primary Healthcare in Qatar. medRxiv.
- Alajmi J, Jeremijenko AM, Abraham JC, Alishaq M, Concepcion EG, et al. (2020) COVID-19 infection among healthcare workers in a national healthcare system: The Qatar experience. Int J Infect Dis 100: 386-389. [Crossref]
- Monaco CG, Zaottini F, Schiaffino S, Villa A, Della Pepa G, et al. (2020) Chest x-ray severity score in COVID-19 patients on emergency department admission: a two-centre study. Eur Radiol Exp 4: 68.
- Toussie D, Voutsinas N, Finkelstein M, Cedillo MA, Manna S, et al. (2020) Clinical and Chest Radiography Features Determine Patient Outcomes in Young and Middle-aged Adults with COVID-19. Radiology 297: E197-E206.
- Rodrigues JCL, Hare SS, Edey A, Devaraj A, Jacob J, et al. (2020) An update on COVID-19 for the radiologist - A British society of Thoracic Imaging statement. Clin Radiol 75: 323-325. [Crossref]
- Stephanie S, Shum T, Cleveland H, Challa SR, Herring A, et al. (2020) Determinants of chest x-ray sensitivity for covid-19: A multi-institutional study in the united states. Radiology Cardiothoracic Imaging 2: e200337.
- Wang L (2020) C-reactive protein levels in the early stage of COVID-19. Med Mal Infect 50: 332-324.
- Hui TC, Khoo HW, Young BE, Mohideen SMH, Lee YS, et al. (2020) Clinical utility of chest radiography for severe COVID-19. Quantitative imaging in medicine and surgery 10: 1540.
- Orsi MA, Oliva G, Toluian T, Pittino CV, Panzeri M, et al. (2020) Feasibility, reproducibility, and clinical validity of a quantitative chest X-ray assessment for COVID-19. The American Journal of Tropical Medicine and Hygiene 103: 822-827.
- Liu F, Li L, Xu M, Wu J, Luo D, et al. (2020) Prognostic value of interleukin-6, C-reactive protein, and procalcitonin in patients with COVID-19. J Clin Virol 127: 104370. [Crossref]
- Shi Y, Wang Y, Shao C, Huang J, Gan J, et al. (2020) COVID-19 infection: the perspectives on immune responses. Cell Death Differ 27: 1451-1454.
- Yun H, Sun Z, Wu J, Tang A, Hu M, et al. (2020) Laboratory data analysis of novel coronavirus (COVID-19) screening in 2510 patients. Clin Chim Acta 507: 94-97. [Crossref]
- Biamonte F, Botta C, Mazzitelli M, Rotundo S, Trecarichi EM, et al. (2021) Combined lymphocyte/monocyte count, D-dimer and iron status predict COVID-19 course and outcome in a long-term care facility. J Transl Med 19: 79. [Crossref]
- Liu F, Xu A, Zhang Y, Xuan W, Yan T, et al. (2020) Patients of COVID-19 may benefit from sustained Lopinavir-combined regimen and the increase of Eosinophil may predict the outcome of COVID-19 progression. Int J Infect Dis 95: 183-191.
- Nair AP, Soliman A, Al Masalamani MA, De Sanctis V, Nashwan AJ, et al. (2020) Clinical Outcome of Eosinophilia in Patients with COVID-19: A Controlled Study. Acta Biomed 91: e2020165.
- Yang X, Yu Y, Xu J, Shu H, Liu H, et al. (2020) Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. The Lancet Respiratory Medicine 8: 475-481.
- Chen L, Liu H, Liu W, Liu J, Liu K, et al. (2020) Analysis of clinical features of 29 patients with 2019 novel coronavirus pneumonia. Zhonghua jie he he hu xi za zhi= Zhonghua jiehe he huxi zazhi= Chinese Journal of Tuberculosis and Respiratory Diseases 43: E005-E006.
- Li Lq, Huang T, Wang Yq, Wang Zp, Liang Y, et al. (2020) COVID-19 patients' clinical characteristics, discharge rate, and fatality rate of meta-analysis. Journal of Medical Virology 92: 577-583.
- Mo P, Xing Y, Xiao Y, Deng L, Zhao Q, et al. (2020) Clinical characteristics of refractory COVID-19 pneumonia in Wuhan, China. Clinical Infectious Diseases 270.
- Gao Y, Li T, Han M, Li X, Wu D, et al. (2020) Diagnostic utility of clinical laboratory data determinations for patients with the severe COVID-19. J Med Virol 92: 791-796. [Crossref]
- Zini G, Bellesi S, Ramundo F, d'Onofrio G (2020) Morphological anomalies of circulating blood cells in COVID-19. American Journal of Hematology 95: 870-872.
- Henry BM, De Oliveira MHS, Benoit S, Plebani M, Lippi G (2020) Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clinical Chemistry and Laboratory Medicine (CCLM) 58: 1021-1028.
- Yufei Y, Mingli L, Xuejiao L, Xuemei D, Yiming J, et al. (2020) Utility of the neutrophil-to-lymphocyte ratio and C-reactive protein level for coronavirus disease 2019 (COVID-19) Scandinavian. Journal of Clinical and Laboratory Investigation 80: 536-540. [Crossref]
- Bal T, Dogan S, Cabalak M, Dirican E (2021) Lymphocyte-to-C-reactive protein ratio may serve as an effective biomarker to determine COVID-19 disease severity. Turkish Journal of Biochemistry 46: 23-28.
- Feng X, Li S, Sun Q, Zhu J, Chen B, et al. (2020) Immune-inflammatory parameters in COVID-19 cases: a systematic review and meta-analysis. Frontiers in Medicine 7: 301.
- Ullah W, Basyal B, Tariq S, Almas T, Saeed R, et al. (2020) Lymphocyte-to-C-Reactive Protein Ratio: A Novel Predictor of Adverse Outcomes in COVID-19. Journal of Clinical Medicine Research 12: 415.
- Liu Y-P, Li G-M, He J, Liu Y, Li M, et al. (2020) Combined use of the neutrophil-to-lymphocyte ratio and CRP to predict 7-day disease severity in 84 hospitalized patients with COVID-19 pneumonia: a retrospective cohort study. Annals of Translational Medicine 8: 635.
- Bacha S, Sghaier A, Habibech S, Cheikhrouhou S, Racil H, et al. (2017) Combined C-reactive protein and Neutrophil to Lymphocyte ratio use predict survival innon-small-cell lung cancer. Tunis Med 95: 229-235. [Crossref]
- Noguchi D, Kuriyama N, Nakagawa Y, Maeda K, Shinkai T, et al. (2021) The prognostic impact of lymphocyte-to-C-reactive protein score in patients undergoing surgical resection for intrahepatic cholangiocarcinoma: A comparative study of major representative inflammatory/immunonutritional markers. Plos one 16: e0245946. [Crossref]
- Lagunas-Rangel FA (2020) Neutrophil-to-lymphocyte ratio and lymphocyte-to-C-reactive protein ratio in patients with severe coronavirus disease 2019 (COVID-19): a meta-analysis. Journal of Medical Virology 92: 1733-1734. [Crossref]
Editorial Information
Editor-in-Chief
Terry Lichtor
Tsuyoshi Hirata
Shinya Mizuno
Giacomo Corrado
Article Type
Research Article
Publication history
Received: July 11, 2021
Accepted: August 02, 2021
Published: August 05, 2021
Copyright
©2021 Rahman MUA. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation
Rahman M U A, Prabhu KS, Raza A, Elmi M, Mahmood N, et al. (2021) Biological and radiological biomarkers in SARS-CoV-2 infected healthcare workers in governmental hospitals in Qatar. J Transl Sci 7: DOI: 10.15761/JTS.1000464.




