Abstract
The aim of this research was to evaluate the possible renal and hepato-protective effects of ethanolic extract of Buchholzia coriacea seed and N-acetylcysteine, on gene expression in the liver and kidneys of Wistar rats subjected to post-treatment with sub-chronic dose of acetaminophen. Forty wistar rats were divided into eight groups of five rats in each group. Group 1 received only normal diet as control (CTRL); Group 2 received 14.28 mg/kg of acetaminophen (PM) once daily; Group 3received 200 mg/kg of extractand 14.28 mg/kg b.w of acetaminophen (PM+E1); Group 4 received 400 mg/kg b.w of extract and 14.28 mg/kg b.w of acetaminophen (PM+E2); Group 5 received 70mg/kg b.w. of N-acetylcysteine and 14.28 mg/kg b.w of acetaminophen (PM+N1); Group 6 received 150 mg/kg b.w of N-acetylcysteine and 14.28 mg/kg b.w of acetaminophen (PM+N2); Groups 7 and 8 received 200 and 400 mg/kg b.w of extract (E1 and E2) respectively. The second administration was carried out 6 hours after the first daily, and lasted for 90 days orally. The animals were sacrificed; liver and kidneys were excised for gene expression. The results showed that administration of Buchholzia coriacea extract following post-treatment with acetaminophen down-regulated the expression of fas ligand, KIM-1, interlukin-6 and prostaglandin in the kidneys. While administration of Buchholzia coriacea extract up-regulated the expression of GST, GPX-1 and CYP1A2 genes in the liver. The extract had no significant effect on COX-2 gene. In conclusion, the potential beneficial effects of the ethanolic extract of Buchhozia coriacea seeds suggested that it had a wide range of health benefits which is expressed in its ability to reduce or halt inflammation and apoptosis, boost body immune system and detoxify harmful substance. Therefore, the extract from Buchholzia coriacea seeds could serve as a potent reparative drug in the treatment of acetaminophenhepato-renal toxicity.
Keywords
N-acetylcysteine, gene expression, hepato-renal, sub-chronic, apoptosis, inflammation
Introduction
Acetaminophen is sometimes used as the pre-treatment for pain and pyrexia, it plays an important role in multimodal analgesia [1] and it is considered to possess a generally excellent safety profile except in significant overdose, with few drug interactions. In combination with opioid pain medication, acetaminophen is also used for severe pain such as cancer pain and pain after surgery, the treatment of lower back pain, fever, headache, teeth, postoperative and patient ductus arteriosus. It is typically used orally or rectally and intravenously, and its effects last between 2 and 4 hours [2]. Acetaminophen is generally safe at prescribed doses [3]. The prescribed maximum daily dose for an adult is 3 or 4 grams [4]. Abnormal doses may lead to toxicity, including liver failure.
Acetylcysteine, which is also, refers to as N -acetylcysteine (NAC), is a medication used to treat acetaminophen overdose, and to loosen thick mucus in individuals with cystic fibrosis or chronic obstructive pulmonary disease [5]. It can be taken intravenously, by mouth, or inhaled as a mist [5]. Acetylcysteine is the N- acetyl derivative of the amino acid L-cysteine, and is a precursor in the formation of the antioxidant glutathione in the body. The thiol (sulfhydryl) group confers antioxidant effects and is able to reduce free radicals. N-acetyl-L-cysteine is soluble in water and alcohol, and practically insoluble in chloroform and ether [6]. Acetylcysteine prevents the hepatic injury, mainly by restoring hepatic glutathione. It is thought to provide cysteine for the glutathione synthesis and possibly to form adduct directly with the toxic metabolite of acetaminophen, N-acetyl-p-benzoquinoneimine (NAPQI) and to thus prevent its covalent bonding to the hepatic proteins. Acetaminophen overdose leads to liver injury. CYP450 enzymes in livers, specifically isoforms such as CYP2E1, CYP3A4 and CYP1A2, metabolize acetaminophen to N-acetyl-p-benzoquinoneimine (NAPQI), a hepatotoxic metabolite [7]. NAPQI then binds to cellular proteins, specifically mitochondrial proteins, and triggers an intracellular signaling cascade ultimately resulting in liver necrosis [8,9].
Hepatotoxicity means chemical-driven liver damage. Drug-induced liver injury is a cause of acute and chronic liver disease. The liver plays the major role in transforming and clearing chemicals and is susceptible to the toxicity from these agents. Certain medicinal agents, when taken in high amount and introduced within therapeutic range may injure the organ. Other chemical agents, such as those used in laboratories and industries, natural chemicals (e.g., microcystins) and herbal remedies can also cause hepatotoxicity. Hepatotoxins are chemicals that induced liver injury. More than 900 drugs have been implicated in causing liver injury [10] and it is the most common reason for a drug to be withdrawn from the market. Hepatotoxicity and drug-induced liver injury also account for a large number of compound failures, highlighting the need for toxicity prediction models (e.g. DTI) [11], and drug screening assays, such as stem cell-derived hepatocyte-like cells, that has the capacity of detecting toxicity early in the drug development process[12]. Drug-induced liver injury is responsible for 5% of all hospital admissions and 50% of all acute liver failures [13,14]. Liver injury caused by acetaminophen overdose is the leading cause of Acute Liver Failure (ALF) in the western world, this accounts for nearly half of all of the ALF cases [15,16].
Blood is filtered by the nephron the basic structural and functional unit of the kidney, in order to regulate chemical concentrations, and thereby produce urine [17]. Nephrotoxicity is toxicity in the kidneys. It is a poisonous effect of some substances, both toxic chemicals and medications, on renal function [18]. The nephrotoxic effect of most drugs is more profound in patients already suffering from kidney failure. A decreased creatinine clearance indicates poor renal function, which may be more useful clinically when assessing patients for early kidney disease.
Buchholzia coriacea (Wonderful kola) is an evergreen shrub belonging to the family
Capparidaceae [19]. It is found in many tropical countries like Ghana, Gabon, Cameroon, Central African Republic, Congo, Angola, Nigeria, among others [20]. The ethanolic extract of Buchholzia coriacea Engler seed was revealed to have antitrypanosomal activity in mice experimentally infected with Trypanosoma brucei [19,21]. It was also discovered that the plant possess antiplasmodial [22], antibacterial [19,23], larvicidal effect [24], antiplasmodial and anti-diarrhetic properties [25], analgesic [26] and anthelminthic effects [24,27].
Recently, evident has shown that there is a gradual revival of interest in the use of medicinal plants, because of its safety and less side effect especially when compared with synthetic drugs [28].Therefore, the present study sought to evaluate the prophylactic geno-modulatory effect of Buchholzia coriacea seeds in post administration of recommended normal dose of acetaminophen for 90 days, which would further enhance its utilization as food supplement or medicine in sub-chronic dose of acetaminophen induced toxicity.
Materials and Methods
Equipment and Laboratory Apparatus
Polymerase Chain Reaction (PCR) machine (Multi-gene optimax), Thermocycler machine, (Multigene Optimax, USA), Refrigerator, Eppendorf tube, Desiccator, Table centrifuge (Biofuge, Germany), Spectrophotometer (JENWAY, UK), Gel electrophoresis machine (USA), Micropipette, Photophoresis (USA).
Chemicals and Reagents
N-acetyl cysteine, Ethidium bromide, Tracking dye, Iso-propyl ethanol, Acetaminophen (GSK acetaminophen), Master mix (Biolabs, New England), RNA snap kits (lysis solution), Tris-Borate EDTA (Biolabs New England), Agarose gel powder (Cleaver scientific LTD, England), Primers (Inqada Biotechnical Industries (Pty) LTD., South Africa), Nuclease-free water (Life Sciences advanced technologies, England), Reverse transcriptase (Biolabs New England), Random Primer (Biolabs New England), dNTPs (Biolabs New England). All chemicals and reagents used are of high analytical grade.
Collection of Plant Materials
The seeds of Buchholzia coriacea were bought from Oja Oba Market at Ikare Akoko, Ondo State.
Identification of Sample
The seed was identified and authenticated with designated voucher-number 025 as that of Buchholzia coriacea Engler tree plant in the Department of Plant Science and Biotechnology, Adekunle Ajasin University, Akungba Akoko (AAUA), Ondo State, Nigeria and a voucher sample has been deposited in the Herbarium of the University.
Preparation of Sample
Fresh seeds of B. coriacea were washed with water. The washed seeds were carefully sliced. The sliced seeds were air-dried at room temperature for some weeks and then pulverized.
Extraction Procedure
Cold extraction method was employed. 500g of the clean, air dried and pulverized plant sample was weighed into extraction jar and 1400ml of analytical grade ethanol was added to the jar containing Buchholzia coriacea. The extraction mixture was given periodic constant agitation and left for 72 hours. The supernatant was decanted and concentrated using rotary evaporator at 40˚C and the extract was freeze-dried. The extract was packed inside an air tight sample bottle and kept at 4˚C inside the refrigerator until when needed.
Laboratory Animal
Forty (40) active male Wistar rats were purchased from the University of Ibadan, Oyo State and they were acclimatized in favorable environment for four weeks. The animals were maintained and used in accordance with the guidelines of the Committee on Care and Use of Experimental Animal Resources, Faculty of Science, Adekunle Ajasin University, Akungba Akoko, Ondo State, Nigeria.
Experimental Design
Forty male Wistar rats were divided into eight groups of five rats in each group. After acclimatization, group 1 received only normal diet as control (CRTL); Group 2 received 14.28 mg/kg of acetaminophen (PM) once daily; Group 3received 200 mg/kg of extract and 14.28 mg/kg b.w of acetaminophen (PM+E1); Group 4 received 400 mg/kg b.w of extract and 14.28 mg/kg b.w of acetaminophen (PM+E2); Group 5 received 70mg/kg b.w. of N-acetylcysteine and 14.28 mg/kg b.w of acetaminophen (PM+N1); Group 6 received 150 mg/kg b.w of N-acetylcysteine and 14.28 mg/kg b.w of acetaminophen (PM+N2); Groups 7 and 8 received 200 and 400 mg/kg b.w of extract (E1 and E2) respectively. The second administration was carried out 6 hours after the first daily, and lasted for 90 days orally.
Animal Sacrifice and Tissue Excision
At the end of the 90 days period of stable administration, animals were fasted overnight and sacrificed the next morning. Kidneys and liver tissue were excised into Eppendorf tubes containing 100ul RNA snap kit reagent across the groups. Tissues were homogenized and then stored in a refrigerator.
Gene Expression Protocol
Semi-quantitative polymerase chain reaction protocol was used to determine the relative gene expression of the following: GST, GPX-1, COX-1, IL-6, KIM-1, Prostagladins synthase, Fas ligand with slight modifications [29].
Gene Expression Analyzes
Total tissue RNA was extracted using TRIzol (Invitrogen) according to the manufacturer’s protocol. RNA pellets were resuspended in diethyl/pyrocarbonate-treated deionized water. RNA samples were analyzed by agarose gel electrophoresis and integrity was confirmed by visualization of intact 18S and 28S rRNA under UV light. Spectrophotometric study (NanoDrop, Thermo Scientific 2000c) was used to confirm the purity of total RNA and then its concentration was determined. The 1-2 μg RNA was reverse-transcribed to cDNA at 42ºC for 60 min. After enzyme inactivation (95ºC, 10 min) cDNA fragments were amplified for 35 cycles using gene-specific primers for the genes of interest. PCR products were resolved on 2% agarose gels and visualized using Uvitec gel documentation systems (Uvitec ArminTeb, Iran). Gene expression study was carried out by statistical analyzes using Uvitec Fire Reader Software (Cambridge) to compare the groups.
Results
Gene expression results of liver post-treated with sub-chronic dose of acetaminophen
The relative expression of CYP1A2 gene in the liver of rats post administered with sub-chronic dose of acetaminophen was shown in Figure 1. No statistical significant difference (p > 0.05) was observed in the relative gene expression of CYP1A2 when all the groups were compared to one another.
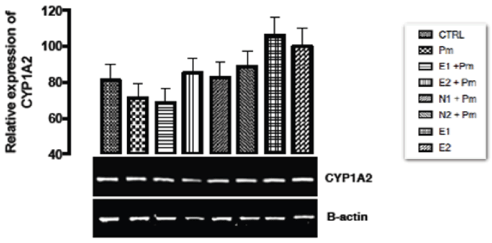
Figure 1. The effect of Buchholzia coriacea extract and N-Acetyl cysteine on the expression of CYP1A2 gene in the post-treatment (sub-chronic) stage of acetaminophen toxicity in liver of wistar rats
PM = 14.28 mg/kg body weight of acetaminophen, E1= 200 mg/kg body weight of B. coriacea, E2 = 400 mg/kg body weight of B. coriacea, N1= 70 mg/kg body weight of N-Acetyl cysteine, N2 = 150 mg/kg body weight of N-Acetyl cysteineP < 0.05, Turkey post hoc test, 95.00% CL *p < 0.033, **p < 0.002, ***p < 0.001
The relative expression of COX-2 gene in the liver of rats post administered with sub-chronic dose of acetaminophen was shown in Figure 2. From the result, statistically, no significant difference (p > 0.05) was observed in the relative gene expression of COX-2 when all the groups were compared with the basal and negative control groups.
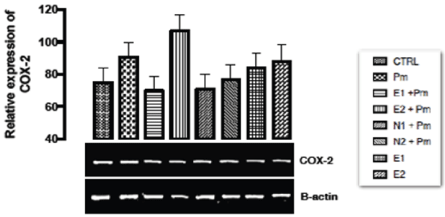
Figure 2. The effect of Buchholzia coriacea extract and N-Acetyl cysteine on the expression of COX-2 gene in the post-treatment (sub-chronic) stage of acetaminophen toxicity in liver of wistar rats
PM = 14.28 mg/kg body weight of acetaminophen, E1 = 200 mg/kg body weight of B. coriacea, E2 = 400 mg/kg body weight of B. coriacea, N1 = 70 mg/kg body weight of N-Acetyl cysteine, N2 = 150 mg/kg body weight of N-Acetyl cysteine.
P < 0.05, Turkey post hoc test, 95.00% CL *p < 0.033, **p < 0.002, ***p < 0.001
The relative expression of GST gene in the liver of rats administered with sub-chronic dose of acetaminophen was shown in Figure 3. Statistically, only E1 group showed significant (p < 0.033) upregulation in the expression of the gene. No significant difference (p > 0.05) was observed in the expression of the gene in the remaining groups when compared with the negative and basal control groups.
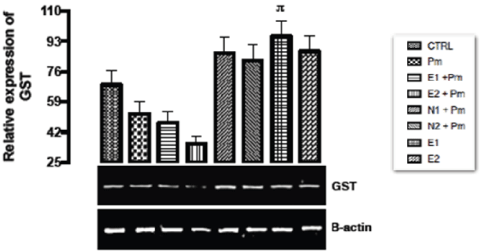
Figure 3. The effect of Buchholzia coriacea extract and N-Acetyl cysteine on the expression of GST gene in the post-treatment (sub-chronic) stage of acetaminophen toxicity in liver of wistar rats
PM = 14.28 mg/kg body weight of acetaminophen, E1 = 200 mg/kg body weight of B. coriacea, E2 = 400 mg/kg body weight of B. coriacea, N1 = 70 mg/kg body weight of N-Acetyl cysteine, N2 = 150 mg/kg body weight of N-Acetyl cysteine.
P < 0.05, Turkey post hoc test, 95.00% CL *p < 0.033, **p < 0.002, ***p < 0.001
The relative expression of Fas/FasL gene in the liver of rats post administered with sub-chronic dose of acetaminophen was shown in Figure 4. From the result, statistically, significant difference of repression (p < 0.001) was observed in the relative gene expression of Fas/FasL when E1+PM group was compared with basal and negative control groups. The remaining groups showed no significant difference in the expression of Fas/FasL gene when compared with the basal and negative control groups.
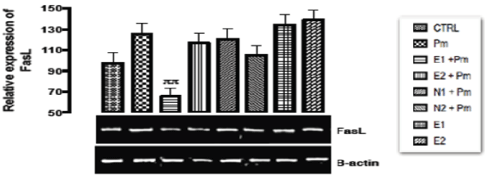
Figure 4. The effect of Buchholzia coriacea extract and N-Acetyl cysteine on the expression of FasL gene in the post-treatment (sub-chronic) stage of acetaminophen toxicity in liver of wistar rats
PM = 14.28 mg/kg body weight of acetaminophen, E1 = 200 mg/kg body weight of B. coriacea, E2 = 400 mg/kg body weight of B. coriacea, N1 = 70 mg/kg body weight of N-Acetyl cysteine, N2 = 150 mg/kg body weight of N-Acetyl cysteine.
P < 0.05, Turkey post hoc test, 95.00% CL *p < 0.033, **p < 0.002, ***p < 0.001
From the relative expression of the GPx-1 gene in the liver of rats administered with sub-chronic dose of acetaminophen as shown in Figure 5. There was significant upregulation (p < 0.001) expression of GPX-1 gene when all the groups were compared with basal and negative control groups. Acetaminophen treated only group (negative control) did not show any significant difference from the basal control group.
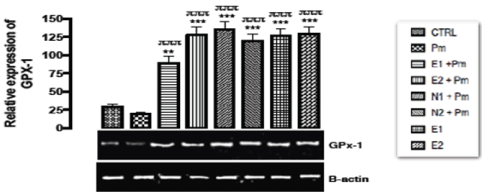
Figure 5. The effect of Buchholzia coriacea extract and N-Acetyl cysteine on the expression of GPX-1 gene in the post-treatment (sub-chronic) stage of acetaminophen toxicity in liver of wistar rats
PM = 14.28 mg/kg body weight of acetaminophen, E1 = 200 mg/kg body weight of B. coriacea, E2 = 400 mg/kg body weight of B. coriacea, N1 = 70 mg/kg body weight of N-Acetyl cysteine, N2 = 150 mg/kg body weight of N-Acetyl cysteine.
P < 0.05, Turkey post hoc test, 95.00% CL *p < 0.033, **p < 0.002, ***p < 0.001
Gene expression results of kidney post-treated with sub-chronic dose of acetaminophen
The relative expression of CYP1A2 gene in the kidney of rats post administered with sub-chronic dose of acetaminophen was shown in Figure 6. There was a statistically significant difference (p < 0.001) between the group treated with 70mg/kg of NAC and 14.28mg/kg of acetaminophen compared to the basal and negative control groups. There was no significant difference (p > 0.05) in the expression of the gene when the remaining groups were compared with basal and negative control groups.
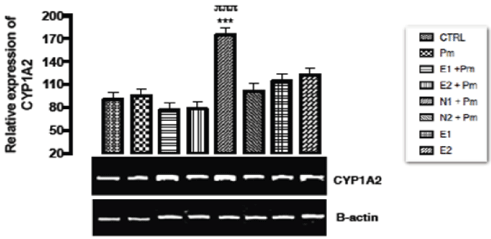
Figure 6. The effect of Buchholzia coriacea extract and N-Acetyl cysteine on the expression of CYP1A2 gene in the post-treatment (sub-chronic) stage of acetaminophen toxicity in kidney of wistar rats
PM = 14.28 mg/kg body weight of acetaminophen, E1 = 200 mg/kg body weight of B. coriacea, E2 = 400 mg/kg body weight of B. coriacea, N1 = 70 mg/kg body weight of N-Acetyl cysteine, N2 = 150 mg/kg body weight of N-Acetyl cysteine.
P < 0.05, Turkey post hoc test, 95.00% CL *p < 0.033, **p < 0.002, ***p < 0.001
The relative expression of GST gene in the kidney of rats post administered with sub-chronic dose of acetaminophen was shown in Figure 7. There was significant upregulation (p < 0.001) in the level of GST gene expressed when the group treated with 200mg/kg of extract only was compared to the basal and acetaminophen treated only groups. Significant down regulation (p < 0.001) was also observed in the level of GST gene expressed when the group treated with 400 mg/kg of extract and 14.28 mg/kg of acetaminophen was compared with the basal group and the group treated with acetaminophen only. There was no statistical significant difference observed in the other groups when compared to the basal group and the group treated with acetaminophen only.
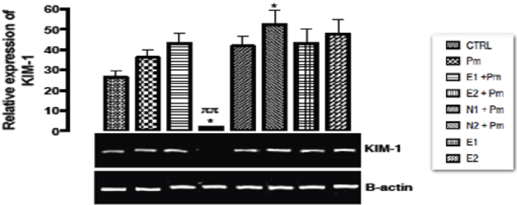
Figure 7. The effect of Buchholzia coriacea extract and N-Acetyl cysteine on the expression of KIM-1 gene in the post-treatment (sub-chronic) stage of acetaminophen toxicity in kidney of wistar rats
PM = 14.28 mg/kg body weight of acetaminophen, E1 = 200 mg/kg body weight of B. coriacea, E2 = 400 mg/kg body weight of B. coriacea, N1 = 70 mg/kg body weight of N-Acetyl cysteine, N2 = 150 mg/kg body weight of N-Acetyl cysteine.
P < 0.05, Turkey post hoc test, 95.00% CL *p < 0.033, **p < 0.002, ***p < 0.001
From the relative expression of the GPx-1 gene in the kidney of rats administered with sub-chronic dose of acetaminophen was shown in Figure 8. No significant difference (p > 0.05) was observed when all the groups were compared with basal and negative control groups.
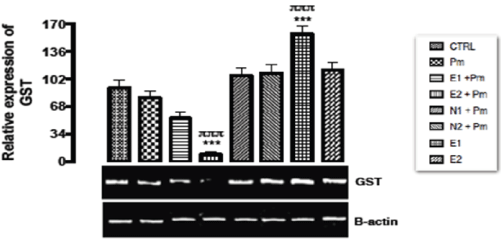
Figure 8. The effect of Buchholzia coriacea extract and N-Acetyl cysteine on the expression of GST gene in the post-treatment (sub-chronic) stage of acetaminophen toxicity in kidney of wistar rats
PM = 14.28 mg/kg body weight of acetaminophen, E1 = 200 mg/kg body weight of B. coriacea, E2 = 400 mg/kg body weight of B. coriacea, N1 = 70 mg/kg body weight of N-Acetyl cysteine, N2 = 150 mg/kg body weight of N-Acetyl cysteine.
P < 0.05, Turkey post hoc test, 95.00% CL *p < 0.033, **p < 0.002, ***p < 0.001
The relative expression of KIM-1 gene in the kidney of rats post administered with sub-chronic dose of acetaminophen was shown in Figure 9. There was significant repression (p < 0.033) observed in the expression of KIM-1 gene when the group treated with 400 mg/kg of extract and 14.28 mg/kg of acetaminophen was compared to the basal control group and the group treated with acetaminophen only. There was significant upregulation (p < 0.033) in N2+PM group when compared with the basal and negative control groups. There was no significant difference (p > 0.05) in the other groups when compared with basal and negative control groups.

Figure 9. The effect of Buchholzia coriacea extract and N-Acetyl cysteine on the expression of GPX-1 gene in the post-treatment (sub-chronic) stage of acetaminophen toxicity in kidney of wistar rats
PM = 14.28 mg/kg body weight of acetaminophen, E1 = 200 mg/kg body weight of B. coriacea, E2 = 400 mg/kg body weight of B. coriacea, N1 = 70 mg/kg body weight of N-Acetyl cysteine, N2 = 150 mg/kg body weight of N-Acetyl cysteine.
P < 0.05, Turkey post hoc test, 95.00% CL *p < 0.033, **p < 0.002, ***p < 0.001
The relative expression of IL-6 gene in the kidney of rats post administered with sub-chronic dose of acetaminophen was shown in Figure 10. There was significant down regulation (p < 0.002) in E1+PM and E2+PM groups when compared with basal control group. Significant upregulation (p < 0.001) was observed in N1+PM group when compared with basal control group. The other groups did not show any significant difference (p > 0.05) from the basal and negative control groups.
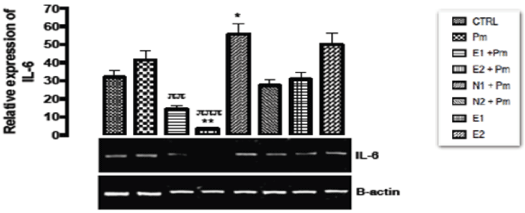
Figure 10. The effect of Buchholzia coriacea extract and N-Acetyl cysteine on the expression of IL-6 gene in the post-treatment (sub-chronic) stage of acetaminophen toxicity in kidney of wistar rats
PM= 14.28 mg/kg body weight of acetaminophen, E1 = 200 mg/kg body weight of B. coriacea, E2 = 400 mg/kg body weight of B. coriacea, N1 = 70 mg/kg body weight of N-Acetyl cysteine, N2 = 150 mg/kg body weight of N-Acetyl cysteine.
P < 0.05, Turkey post hoc test, 95.00% CL *p < 0.033, **p < 0.002, ***p < 0.001
The relative expression of Fas/FasL gene in the kidney of rats post administered with sub-chronic dose of acetaminophen was shown in Figure 11. There was a statistical significant (p < 0.002) down-regulation in the level of the gene expressed in the group treated with 400 mg/kg of extract and 14.28 mg/kg of acetaminophen group when compared with the basal and negative control groups. The remaining other groups showed no significant difference (p > 0.05) from the basal and negative control groups.
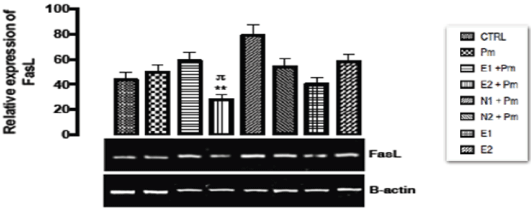
Figure 11. The effect of Buchholzia coriacea extract and N-Acetyl cysteine on the expression of FasL gene in the post-treatment (sub-chronic) stage of acetaminophen toxicity in kidney of wistar rats
PM = 14.28 mg/kg body weight of acetaminophen, E1 = 200 mg/kg body weight of B. coriacea, E2 = 400 mg/kg body weight of B. coriacea, N1 = 70 mg/kg body weight of N-Acetyl cysteine, N2 = 150 mg/kg body weight of N-Acetyl cysteine.
P < 0.05, Turkey post hoc test, 95.00% CL *p < 0.033, **p < 0.002, ***p < 0.001
The relative expression of prostaglandin synthase gene in the kidney of rats post administered with sub-chronic dose of acetaminophen was shown in Figure 12. There was statistical significant (p < 0.003) down regulation observed in the level of the gene expressed when the group treated with 400 mg/kg of extract and14.28 mg/kg of acetaminophen was compared with basal control group and negative control group. The remaining other groups showed no significant difference (p > 0.05) from the basal and negative control groups.
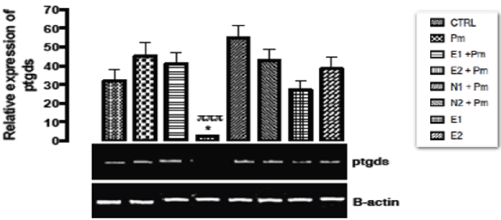
Figure 12. The effect of Buchholzia coriacea extract and N-Acetyl cysteine on the expression of Ptgds gene in the post-treatment (sub-chronic) stage of acetaminophen toxicity in kidney of wistar rats
PM = 14.28 mg/kg body weight of acetaminophen, E1 = 200 mg/kg body weight of B. coriacea, E2 = 400 mg/kg body weight of B. coriacea, N1 = 70 mg/kg body weight of N-Acetyl cysteine, N2 = 150 mg/kg body weight of N-Acetyl cysteine.
P < 0.05, Turkey post hoc test, 95.00% CL *p < 0.033, **p < 0.002, ***p < 0.001
Discussion
The liver and the kidney whose primary functions are to metabolize xenobiotic and excrete the metabolic wastes suffer debilitating effects from pharmaceuticals [30]. Acetaminophen is an antipyretic drug use in treating headaches, inflammation and as an adjuvant in fever treatment. Acetaminophen overdose has been confirmed to affect kidney and liver [31]. Hence, the development of drugs that can be used to treat acetaminophen toxicity has drawn attention.
Natural drug development from plants has drawn the attention of researchers and drug manufacturing companies. This is because phytochemicals from plants are less expensive, have less retention time and more bioavailability when compared to their synthetic counterparts [32]. The extract of B. coriacea has been used traditionally to treat some conditions. Therefore, extract of B. coriacea has been used in this research to treat acetaminophen toxicity with N-acetylcysteine as the standard drug. The effect of the plant extract and N-acetylcysteine on some genes expressed in the liver and kidney were investigated to determine the biochemical effects of B. coriacea in sub-chronic post-treatment administration of acetaminophen.
From the relative expression of the GPx-1 gene in the liver of rats administered with sub-chronic dose of acetaminophen was shown in Figure 5. Significant up regulation (p < 0.001) was observed in the relative gene expression of GPX-1 when all the groups were compared with basal and negative controls. Acetaminophen metabolism leads to the formation of free radicals, and since GPX-1 functions in protection against free radicals. Gupta and co-workers [33] reported on the effect of Phytosome curcumin against acetaminophen-induced liver toxicity. It was stated that Phytosome curcumin has a strong protective effect against acetaminophen-induced acute hepatic damage in mice, and the hepatoprotective effect of Phytosome curcumin may be explained by increasing levels of antioxidant enzymes and decreasing the lipid peroxidation and liver enzyme on acetaminophen-induced damage in mice [33]. Since phytochemicals (flavonoid) from plants have been described as free radicals scavengers [34], therefore, it can be concluded from our result that the Buchholzia coriacea extract might possess antioxidants that could scavenge free radicals or prevent damage induced by sub-chronic dose of acetaminophen in the liver of rats.
From the relative expression of the GPx-1 gene in the kidney of rats administered with sub-chronic dose of acetaminophen was shown in Figure 9. No significant difference (p < 0.05) was observed when all the groups were compared with basal and negative control groups. Pre-treatment with doses of extract followed by acetaminophen caused no significant effect on the expression of the gene in the kidney. Administration of the extract alone increased glutathione peroxidase which involves in the detoxification of hydrogen peroxide and protects cells from oxidative stress. Xin [35] concluded in his study that, normal expression of GPX-1 is essential and over expression of GPX-1 is beneficial to protect mice against acute oxidative stress. The expression of GPX-1 that showed no statistical significant difference in the kidney in this study was in relation with what Ehsan, et al. [36] reported in their study that no significant difference was found when the group treated with curcumin was compared with basal control. Similarly, in the group treated with 25mg/kg curcumin and acetaminophen also maintained the activity of glutathione peroxidase at normal levels despite the presence of acetaminophen.
The relative expression of GST gene in the liver of rats administered with sub-chronic dose of acetaminophen was shown in Figure 3. Acetaminophen, which was used to induce liver damage, is an antipyretic drug at normal dose but at toxic dose, it causes the formation of free radicals and induction of ROS. GST functions in conjugation of GSH to the NAPQI (accumulated toxicants produced from acetaminophen metabolism) in order to block the induction of ROS and free radicals. This explained the repression in the level of the gene in negative control group (as the toxicity increases, GST activities depreciate progressively due to depletion in glutathione). But statistically, administration of extract alone at dose of 200 mg/kg b.w showed higher expression of the gene compared to acetaminophen only treated group.
According to a research work by Hayes, et al. [37] and Singh [38] on the biological roles of glutathione-S-transferases on xenobiotics and the cytoprotective regulatory function of GST respectively, it was illustrated that increasing level of cytosolic GSTP1 is able to block the JNk pathways thus reducing cancer generation and hepatocytes necrosis and apoptosis. The bio-transformation role of GST has also enabled the conversion of accumulated xenobiotics metabolites to harmless compound for excretion by facilitating their conjugation with glutathione (GSH). Therefore, from the result above the ethanolic extract of Buchholzia coriacea might possess antioxidant activity which aids the scavenging of free radicals hence aiding in excretion of acetaminophen toxic metabolites (NAPQI). However, administration of extract and acetaminophen did not show up regulation of GST gene in the liver.
The relative expression of GST gene in the kidney of rats post administered with sub-chronic dose of acetaminophen was shown in Figure 8. There was statistical significant difference (p < 0.001) observed in the level of GST gene expressed when the group treated with 200mg/kg of extract only was compared to the basal and acetaminophen only groups. The up-regulation observed in the level of GST gene expressed in the group above agreed with the results of a study by Soliman, et al. [39] which showed that curcumin treatment caused significant up-regulation of GST gene. Significant down regulation (p < 0.001) was also observed in the level of GST gene expressed when the group treated with 400mg/kg of extract and 14.28mg/kg of acetaminophen was compared to the basal group and the group treated with acetaminophen only. The repression in the level of GST gene expressed here was in support of the findings of Soliman et al. [39], who reported that there was significant down regulation of GST gene when curcumin was used with acetaminophen. It was discovered in this study that the GST gene levels were repressed in the groups pre-treated with extract and then followed by acetaminophen; this might imply that the extract was unable to elicit the ability to detoxify acetaminophen via phase II biotransformation processes. However, the extract alone upregulated GST gene and may be used to induce antioxidant enzyme i.e upregulation of GST gene leads to increase in GSH level, a reductant needed to detoxify acetaminophen toxicity.
The relative expression of CYP1A2 gene in the liver of rats post administered with sub-chronic dose of acetaminophen was shown in Figure 1. No statistical significant difference was observed in the relative gene expression of CYP1A2 when all the groups were compared to one another. Acetaminophen which undergoes metabolic breakdown by CYP450s (DMEs) at normal dose to elicit its analgesic effect causes an increase in the expression of CYP450s at high doses/toxicity level. The explanation for the no significant effect of the acetaminophen treated group on the expression of CYP1A2 in this study was not known despite the long time exposure.
The relative expression of CYP1A2 gene in the kidney of rats post administered with sub-chronic dose of acetaminophen was shown in Figure 6. There was significant up regulation (p < 0.001) in the group treated with 70mg/kg of NAC and 14.28mg/kg of acetaminophen compared to the basal control group and other groups. There was no statistical significant difference in the expression of the gene among the remaining groups. In this study, sub-chronic administration of acetaminophen led to no significant up-regulation of CYP1A2 gene that induces the oxidation of the drug (acetaminophen) to NAPQI which counter what Gill, et al. [40] reported that CYP1A2 level decrease in response to acetaminophen toxicity. This may imply that the extract was able to protect kidney from possible acetaminophen toxicity. This study had shown that there was no statistical significant difference observed in the expression of CYP 1A2 gene in all groups except the group treated with70mg/kg of NAC and 14.28mg/kg of acetaminophen.
Figure 1 above, expression of CYP1A2 gene was unaffected in groups treated with ethanolic extract of Buchholzia coriacea and acetaminophen indicating that the rats treated under these groups are free from acetaminophen induced toxicity at sub-chronic studies, and also to regulate continuous production of NAPQI at the dose. However, over expression of CYP1A2 may mean to metabolize the extract alone into useful metabolic product in the kidneys. From this study it can be deduced that the ethanolic extract of B.coriacea might possessed some secondary metabolites that could aid in the modulation of the metabolic enzyme gene (CYP1A2) of the rat induced with acetaminophen normal dose at sub-chronic level. Therefore, regulates the activities of the enzyme (CYP1A2) and possibly reduces over production of NAPQI.
The relative expression of COX-2 gene in the liver of rats post administered with sub-chronic dose of acetaminophen was shown in Figure 2. From the result, statistically, no significant difference was observed in the relative gene expression of COX-2 when all the groups were compared to the basal and negative control groups. Acetaminophen which was used to induce liver damage is an analgesic drug at normal dose but at toxic dose, it causes the formation of free radicals and induction of ROS from accumulation of NAPQI produced thus causing inflammation and hepatotoxicity. Aggarwal and Shishisoda [41] investigated therapeutic potentials of curcumin as an anti-carcinogenic and anti-inflammatory compound using Wistar albino male rats. It was proposed that curcumin inhibits the expression of the enzyme, cyclo-oxygenase-2 via interference with activation of the transcriptional factor NF-kB and is a potential hepato-protectant. Thus it can be deduced from our result that the ethanolic extract of Buchholzia coriacea might possess anti-inflammatory properties which might have modulated COX-2 by not up regulating its expression through interference with activation of the transcriptional factor NF-kB, and is a potential hepato-protectant.
Amoras, et al. [42] reported the correlation of hepatocellular diseases and Fas gene expression, explained that the decrease in mRNA expression of Fas and FasL gene was correlated with lower levels of inflammation and diseases progression which was associated with decreased levels of ALT and AST followed by a decrease in tissue cirrhosis. This means that the fas gene down regulated during DNA damage functions to induce cell cycle arrest and at subchronic state it channeled damage hepatocellular cells to apoptosis (potential cell death) in order to inhibit hepatocellular carcinoma but incomplete apoptosis preferentially give way to cancer formation which may occur at over expression of fas gene, over expression of this Fas/FasL gene will also lead to hepatocellular apoptosis the prominent features of all kinds of hepatocellular diseases. A report by Tanaka, et al. [43] showed that over expression of Fas/Fas ligand initiate apoptosis to healthy body cells which might also destroy the body immunity; however the level of the gene was significantly down regulated when the rat was post treated with acetaminophen. This work has justified that administration of ethanolic extract of Buchholzia coriacea at doses of 200 and 400 mg/kg alongside with post treatment of acetaminophen (14.29mg/kg) was able to repress the expression of Fas/FasL gene. This modulation of FasL gene expression could therefore serve as another therapeutic potential justifying the ethanolic extract of Buchholzia coriacea as a natural ameliorating agent to combat acetaminophen induced apoptosis at sub-chronic dose. Therefore it can be deduced from the above that the ethanolic extract of B.coriacea might possess anti-apoptotic effect that could mitigate and/or prevent apoptosis.
The relative expression of KIM-1 gene in the kidney of rats post administered with sub-chronic dose of acetaminophen was shown in Figure 7. Report by Bonventre [44] indicated that up regulation of KIM-1 gene was closely correlated with renal tubular damage. Waanders, et al. [45] also reported that KIM-1 gene is not present under normal conditions but increases in proximal tubular membrane tissue cells after an injury that serves as a compensatory mechanism. Similarly, Adil, et al. [46] also observed significantly increased KIM-1 expression in acetaminophen induced renal toxicity, and treatment with naringin (40 and 80 mg/kg,) showed significant amelioration (p < 0.05) in the alterations induced in renal KIM-1 expression as compared to acetaminophen treated rats. Due to KIM-1 predictive value as a biomarker with high sensitivity and specificity, FDA has approved the use of KIM-1 to monitor drug-induced nephropathy [47]. In this study, at 400 mg/kg dose of extract and14.28 mg/kg acetaminophen, KIM-1 gene was repressed significantly (p < 0.033). This indicated the protective potentials of the extract in possible toxicity of the drug. However, the group treated with 150mg/kg NAC and 14.28mg/kg acetaminophen, significant up regulation (p < 0.033) was observed in the level of KIM-1 gene expression when compared to basal control group. This antidote of acetaminophen toxicity may be toxic at this dose because of the long period of administration.
The relative expression of IL-6 gene in the kidney of rats post administered with sub-chronic dose of acetaminophen was shown in Figure 10. Although acetaminophen is classified as an antipyretic drug, it does not have any significant anti-inflammatory activity [48]. A significant down-regulation (p < 0.002) in the level of IL-6 gene was observed in the groups treated with 200 mg/kg extract and 14.28 mg/kg acetaminophen, and 400 mg/kg extract and 14.28 mg/kg acetaminophen when compared to basal control and negative control. Based on the report by Ibiam and co-workers [49], ethanolic extract of B. coriacea was proven to have anti-inflammatory potential which could be attributed to phytochemical components acting individually or collectively as seen in the group treated with 200 mg/kg extract only. Treatment with the extract of B. coriacea would serve as a potent anti-inflammatory agent and could be a good reparative drug in treating kidney injury since Ranganatha, et al. [50] discovered in their study that fibrosis was associated with increased expression of IL-6 and the concentration of IL-6 is elevated in glomeruli, which positively associated with disease activity [51,52]. Grigoryev, et al. [53] had shown a close correlation between IL-6 expression and Acute Kidney Injury (AKI). In ischemic AKI animal model, it was found that IL-6 transcription and signaling are elevated locally and systemically after 60 min bilateral kidney ischemia. And their finding indicated that IL-6 signaling connected local and systemic inflammation and can be employed as a biomarker and therapeutic target in ischemic AKI. However, there was significant up-regulation (p < 0.001) in the level of IL-6 gene in the group treated with 70 mg/kg of NAC and 14.28mg/kg acetaminophen when compared to negative control group. The reason for this up regulation was not known; hence the extract could be a better reparative drug than the standard commercial drug available for the treatment of acetaminophen toxicity.
The relative expression of prosta gland in synthase gene in the kidney of rats post administered with sub-chronic dose of acetaminophen was shown in Figure 12. There was significant (p < 0.003) down regulation in the level of the gene expressed when extract and acetaminophen were administered together. This study has showed that the extract taken with acetaminophen could modulate the activity of inflammation according to Jurenka [54] who reported that curcumin modulate the inflammatory response by down-regulating the activity of prostaglandin synthase.
Conclusion
This study showed that Buchholzia coriacea demonstrated antioxidant and protective ability against pro-oxidant, necrotic and inflammatory potentials of acetaminophen. Moreover, Buchholzia coriacea could be studied for its possible antigenotoxic and biosafety before being considered for clinical trials as potent cytoprotective agent in the treatment of acetaminophen induced hepato-renal toxicities.
Acknowledgements
Appreciation to all Staff at the Centre for Bio-computing and Drug Development Unit (CBDD), Department of Biochemistry, Faculty of Science, Adekunle Ajasin University, Akungba Akoko, Ondo State, Nigeria, for their assistance during this research work.
References
- Mattia C, Coluzzi F (2009) What anesthesiologists should know about acetaminophen (paracetamol). Mineral Anestesiol 75: 644.
- Hochhauser D (2014) Cancer and its Management. John Wiley & Sons 119.
- Russell FM, Shann F, Curtis N, Mulholland K (2003) Evidence on the use of acetaminophen in febrile children. Bulletin of the World Health Organization 81: 367-372.
- Murnion B (2010) Combination analgesics in adults. Australian Prescriber 33: 113-115.
- Talbott, Shawn M (2012) A Guide to Understanding Dietary Supplements. Routledge 469.
- Kupchik YM, Moussawi K, Tang XC, Wang X, Kalivas BC, et al. (2012) The effect of N- acetylcysteine in the nucleus accumbens on neurotransmission and relapse to cocaine. Biological Psychiatry 71:978-986. [Crossref]
- Lancaster EM, Hiatt JR, Zarrinpar A (2015) Acetaminophen hepatotoxicity: an updated review. Arch toxicol 89: 193-199. [Crossref]
- Jaeschke H, McGill MR, Ramachandran A (2012) Oxidant stress, mitochondria, and cell death mechanisms in drug-induced liver injury: lessons learned from acetaminophen hepatotoxicity. Drug Metab Rev 44: 88-106. [Crossref]
- Bhushan B, Edwards G, Desai A, Michalopoulos GK, Apte U (2016) Liver-specific deletion of integrin-linked kinase in mice attenuates hepatotoxicity and improves liver regeneration after acetaminophen overdose. Gene Expr 17: 35-45. [Crossref]
- Friedman SE, Grendell J, McQuaid H, Kenneth R (2010) Current diagnosis and treatment in gastroenterology. NewYork Lang Medical Books/McGraw-Hill 664-669.
- Dixit VA, Bharatam PV (2019) Toxic Metabolite Formation from Troglitazone (TGZ). Chem Res Toxicol 24: 1113-1122. [Crossref]
- Greenhough S, Hay DC (2012) Stem Cell-Based Toxicity Screening; Recent Advances in Hepatocyte Generation. Pharmacological Med 26:85-89.
- Mcnally K, Audhya A, Oegema K, Mcnally FJ (2006) Katanin controls mitotic and meiotic spindle length. The J Cell Biol 175: 881-891. [Crossref]
- Ostapowicz G, Fontana RJ, Schiødt FV (2008) Results of a prospective study of acute liver failure at 17 tertiary care centers in the United Status. Ann Intern Med 12: 947-954.
- Lee WM (2012) Acute liver failure. Semin Respir Crit Care Med 33: 36-45.
- Bhushan B, Poudel S, Manley Jr MW, Roy N, Apte U (2017) Inhibition of glycogen synthase kinase 3 accelerated liver regeneration after acetaminophen-induced hepatotoxicity in mice. Am J Pathol 187: 543-552. [Crossref]
- Jameson JL, Loscalzo, J (2010) Harrison’s Nephrology and Acid-base Disorders. McGraw-Hill Professional 3-10.
- Galley HF (2007) Can acute renal failure be prevented. JR Coll Surg Edinb 45: 44-50.
- Gbagbeke KO, Naiho AO, Okonkwo BC, Omoirri MA, Emojevwe V, et al. (2018) Hepatic, Pancreatic, and Renal Histo-Morphologic Alterations in Administration of Aqueous and Ethanol Seed Extract of Buchholzia coriacea in Alloxan-Induced Diabetic Rats. Asian Journal of Medicine and Health 1-11.
- Okoye TC (2012) Anti-diabetic Effects of Methanol Extract of the Seeds of Buchholzia coriacea and its Synergistic Effects with Metformin. Asian Journal of Biomedical and Pharmaceutical Sciences 2: 32.
- Nweze NE, Fakae NE, Asuzu IU (2008) Trypanocidal activity of the ethanolic extract of Buchholzia coriacea seed. Nigerian Veterinary Journal 29.
- Okoli BJ, Okere OS, Adeyemo SO (2010) The antiplasmodial activity of Buchholziacoriacea. Journal of medical and applied Biosciences 2: 21-29.
- Mbata TI, Duru CM, Onwumelu HA (2009) Antibacterial activity of crude seed extracts of Buchholzia coriacea E. on some pathogenicbacteria. Journal of Developmental Biology and Tissue Engineering 1: 1-5.
- Adediwura F, Omonike O, Olamide A, Oluwatosin E (2011) Larvicidal effect of the petroleum ether, chloroform fractions and methanol extract of Buchholzia coriacea Engl. Seed. International Journal of Pharmaceutical Sciences and Research 2: 1736-1739.
- Anowi FC, Ike C, Ezeokafor E, Ebere CN (2012) The phytochemical, antispamodic and antidiarrhoea properties of the methanol extract of the leaves of Buchholzia coriacea family Capparaceae. Int J Curr Pharm Res 4: 52-55.
- Ezeja MI, Ezeigbo II, Madubuike KG (2011) Analgesic activity of the methanolic seed extract of Buchholzia coriacea. Research Journal of Pharmaceutical, Biological and Chemical Sciences 2: 187-193.
- Nweze NE, Asuzu IU (2006) The anthelmintic effects of Buchholzia coriacea seed. Nigerian Veterinary Journal 27: 60-65.
- Bakebain DA, Giami SY (2004) Proximate composition of full fat fluted pumpkin (Telfairiaoccidentalis) seed flour. J Sci food Agric 59: 321-325.
- Burgmann H, Burgmann G, Widmer F, Sigler WV, Zeyer J (2003) mRNA extraction and reverse transcription-PCR protocol for detection of nif H gene extpression by Azotobacter vinelandii in Soil. Appl Environ Microbiol 69: 1928-1935. [Crossref]
- Le-Tao (2013) First Aid for the USMLE Step 1. New York: McGraw-Hill Medical 35: 45-49.
- Ghanem CI, Pérez MJ, Manautou JE, Mottino AD (2016) Acetaminophen from liver to brain: new insights into drug pharmacological action and toxicity. Pharmacol Res 109: 119-131. [Crossref]
- Ikpeazu OV, Otuokere IE, Igwe KK (2017) Preliminary Studies on the Secondary Metabolites of Buchholzia coriacea (Wonderful Kola) Seed Ethanol Extract by GC-MS Analysis. Intern J Res Eng Appl 7: 17-26.
- Gupta SC, Patchva S, Aggarwal BB (2013) Therapeutic roles of curcumin. Lessons learned from clinical trials. AAPSJ 15: 195-218. [Crossref]
- Murni NS, Qarmar UA, Siti ZMS, Alhassan MA, Sugannya M, et al. (2017) Antioxidant and antidiabetic effects of flavonoids: A structure-activity relationship based study. Biomed Res Int 1-14. [Crossref]
- Xin GL (2001) Glutathione peroxidase-1 gene knockout on body antioxidant defense in mice. Bio Factors 14: 93-99. [Crossref]
- Ehsan K, Mohammad-Reza P, Ramin M, Katayoun J, Ali N, et al. (2010) Curcumin protects rats against acetaminophen-induced hepatorenal damages and shows synergistic activity with N-acetyl cysteine. Eur J Pharmacol 628: 274-281. [Crossref]
- Hayes JD, Flanagan JU, Jowsey IR (2005) Glutathione transferases and its action. Annual Review of Pharmacology and Toxicology 45: 51-88.
- Singh S (2015) Cytoprotective and regulatory functions of glutathione S-transferases in cancer cell proliferation and cell death. Cancer Chemother Pharmacol 75: 1-15. [Crossref]
- Soliman MM, Nassan MA, Ismail TA (2014) Immunohistochemical and molecular study on the protective effect of curcumin against hepatic toxicity induced by acetaminophen in wistar rats. BMC complement Altern Med 14: 457. [Crossref]
- Gill P, Sudeepa B, McCullough1 S, Letzig1 L, Prasun JM, et al. (2017) MicroRNA regulation of CYP 1A2, CYP3A4 and CYP2E1 expression in acetaminophen toxicity. Sci Rep 7: 1-2. [Crossref]
- Aggarwal BB, Shishioda S (2006) Molecular targets of dietary agents for prevention and therapy of cancer. Biochem Pharmacol 71: 1397-1421. [Crossref]
- Amoras ESG, Samara TMG, Felipe BF, Santana BB, Ishak G, et al. (2016) Intrahepatic mRNA Expression of FAS/, FASL, and FOXP3 Genes is associated with hepatic viral infections and fibrosis. Plos ONE 11: 1-6. [Crossref]
- Tanaka M, Suda T, Takahashi T, Nagata S (2008) Expression of the functional soluble form of human fas ligand in activated lymphocytes. EMBO J 14: 1129-1135. [Crossref]
- Bonventre JV (2009) Kidney injury molecule-1 (KIM-1): A urinary bio marker and much more. Nephrol Dial Transplant 24: 3265-326.
- Waanders F, Timmeren MM, Stegeman CA, Bakker SJ, van-Goor H (2010) Kidney injury molecule-1 in renal disease. J Pathol 220: 7-16. [Crossref]
- Adil SF, Alabbad S, Kuniyil M, Khan M, Alwarthan A, et al. (2015) Vanadia supported on nickel manganese oxide nanocatalysts for the catalytic oxidation of aromatic alcohols. Nanoscale Res Lett 10: 1-9. [Crossref]
- Dieterle F, Perentes E, Cordier A, Roth DR, Verdes P, et al. (2010 ) Urinary clusterin, cystatin C, beta2-microglobulin and total protein as markers to detect drug-induced kidney injury. Nat Biotechnol 28: 463-469. [Crossref]
- McKay Gerard A, Walters T, Matthew R (2013) "Non-Opioid Analgesics". Lecture Notes Clinical Pharmacology and Therapeutics (9thed.). Hoboken: Wiley. 453: 221-234.
- Ibiam UA, Alum EU, Orji OU, Aja P, Maduabuchi EN, et al. (2018) Anti- inflammatory effects of buchholzia coriaceaethanol leaf-extract and fractions in freund’s adjuvant-induced rheumatoid arthritis albino rats. Indo american journal of pharmaceutical sciences 5: 6341-6357.
- Ranganath LR, Jarvis JC, Gallagher JA (2013) Recent advances in management of alkaptonuria. J Clinical Pathol 66: 143- 148. [Crossref]
- Cash H, Relle M, Menke J, Brochhausen C, Jones SA, et al. (2010) Interleukin 6 (IL-6) deficiency delays lupus nephritis in MRL-Faslpr mice: the IL-6 pathway as a new therapeutic target in treatment of autoimmune kidney disease in systemic lupus erythematosus. J Rheumatol 37: 60-70. [Crossref]
- Abdel GSM, Ezzeldin N, El-Boshy ME (2015) The role of serum IL-17 and IL-6 as biomarkers of disease activity and predictors of remission in patients with lupus nephritis. Cytokine 76: 280-287. [Crossref]
- Grigoryev DN, Liu M, Hassoun HT, Cheadle C, Barnes KC, et al. (2008) The local and systemic inflammatory transcriptome after acute kidney injury. J Am Soc Nephrol 19: 547-558. [Crossref]
- Jurenka JS (2009) Anti-inflammatory properties of curcumin, a major constituent of Curcumalonga: a review of preclinical and clinical research. Altern Med Rev 14: 141-153. [Crossref]












