Background: In foot and ankle surgery, the calcaneus serves as an established harvest site for autogenous grafts. As described in current literature, the procedure is associated with a low complication rate of 0 to 13.8%. But, only small amounts of bony material are harvestable by a lateral approach that has not been standardized until now. Moreover, the current technique neither considers the material quantity nor the material quality within the calcaneus. Our study was designed to determine the calcaneal area providing the highest density values in Hounsfield units (HU) in order to harvest more material while causing less damage to the bone.
Methods: In 402 eligible sagittal foot and ankle CT scans, the calcaneus was divided into areas I, II, III and IV on the basis of anatomical landmarks. HU values were obtained per area and per measuring slice. Additional peculiarities, such as the location of the neutral triangle, were also recorded.
Results: The average density value was 284 HU for area I, 185 HU for area II, 183 HU for area III and 173 HU for area IV. Age-dependent as well as age-independent density values were significantly higher in area I than in areas II to IV (all p < 0.0001). When comparing HU values of all males (215; 53.5 %) to HU values of all females (187; 46.5 %), no significant difference was found for areas I and II (both p = 0.9995).
Conclusion: Our study revealed that area I is the best harvest site for the retrieval of cancellous autografts of the calcaneus, regarding the available material. Thus, the current lateral approach needs to be modified: a lateral-superior approach or a dorsal approach might be conceivable. Further research on consequential complications and biomechanical impacts is required.
calcaneus, cancellous autograft, calcaneal density, Hounsfield units
A variety of procedures in foot and ankle surgery, such as fore- and midfoot athrodeses and nonunion repairs, often require the use of autogenous cancellous bone [1,2].
Due to its numerous advantages, such as sharing one operative field with the primary surgery and its ease of accessibility, the calcaneus serves as an established harvest site for pedal procedures. Furthermore, calcaneal grafting is associated with a low complication rate varying from 0 to 13.8 % [1,2]. Most commonly, complications occur in the form of mild intermittent incisional pain and intermittent incisional paresthesia [2-4]. Whereas, major-complications, such as postoperative fractures of the calcaneus or persistent numbness along the lateral margin of the foot due to intraoperative violation of the sural nerve, occur rarely with an incidence of 0 to 1.4 % [2,4].
Alternative and partially favored harvest sites for the retrieval of autogenous cancellous bone are the iliac crest and the tibia [5-7].
When harvesting cancellous bone from the anterior iliac crest, discomforts associated with the procedure arise in 9.5 to 43 % of all cases; graft retrievals from the posterior iliac crest are related to a complication rate of 2 to 47 % [8-11]. Both formerly aforementioned alternatives are associated with enduring and intense conditions of pain at the harvest site. Moreover, local paresthesia caused by intraoperative injury of the lateral femoral cutaneous nerve occurs regularly. Nevertheless, for most surgeries, the iliac crest remains the favored donor site when a cancellous autograft is required, since a vast amount of high quality bone can be easily harvested [8-14].
Complication rates following tibial grafting are comparable to those after cancellous autograft harvest from the calcaneus. Discomforts occur with an incidence of 1 to 15 % [6,14] with the proximal tibial metaphysis being the donor site. When retrieval from the distal tibial metaphysis is performed, complications develop in 0 to 13 % of cases [[4,5,16,17]. After tibial bone graft harvest, especially minor-complications arise in the form of mild pain while palpating or kneeling, paresthesia or wound margin erythemas; major-complications such as fractures are less common with an incidence of 0 to 4 % [4-6,16-19].
Owing to prospective [1] and retrospective [2] studies evaluating complication rates after calcaneal grafting and due to surgeries executed both clinical-experimentally [20,21] and on cadaveric feet [3], an agreement was obtained on performing a lateral approach for harvesting autogenous cancellous bone from the calcaneus. This technique protects structures and joints and thus causes low complication rates. Nevertheless, until now it was neither possible to standardize this harvesting technique completely nor to define one specific harvest site within the calcaneus. Moreover, the current approach neither considers the quality of the material nor the quantity of trabecular bone being harvestable from the donor bone [1-7,20,21].
Our study was designed to identify the intracalcaneal region which contains the highest bone density by performing a computed tomography supported measurement in Hounsfield units (HU). This was undertaken for the purpose of enhancing the amount of retrievable trabecular bone during calcaneal cancellous autograft harvest. Simultaneously, we aim to reduce the current complication rate by harvesting the same quantity of bone, while causing less damage to the calcaneus.
A retrospective study was conducted on pre-existing, consecutive foot and ankle CT scans which were recorded between January 2009 and March 2016.
All patients agreed to the use of their anonymized data for research purposes by having undergone the preceding CT examination. Therefore, obtaining a separate vote by our local ethics committee was not required.
Inclusion criteria consisted of sagittal CT scans recorded electively by a Siemens Definition or Siemens Definition AS CT scanner, equally including left and right feet as well as patients of any sex and age. If several foot and ankle CT scans per patient existed, either the latest image was used or the image providing the best quality in terms of completeness and sagittality was chosen.
Exclusion criteria included CT scans resulting from a whole body computed tomography due to polytrauma diagnostics or CT images showing the calcaneus only partially. Equally, the presence of calcaneal fractures, osteosynthesis material or residual drill holes after external pin insertion as well as previous surgeries about the calcaneus and pedal disuse osteopenia described in the radiological report lead to exclusion.
In order to determine the calcaneal region providing the highest CT density, the calcaneus was divided into areas I, II, III and IV. For this purpose, lines A, B and C were defined on the basis of anatomical landmarks by utilizing sagittal CT scans (Figure 1):
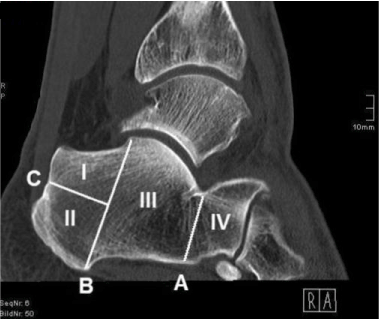
Figure 1.Sagittal CT scan of calcaneus (Division into areas I, II, III and IV through lines A, B and C).
Line A: Superiorly, line A intersects the posterior facet of the calcaneus at its lowest point and forms a parallel to line B. Hence, line A divides the dorsal area III from the anterior area IV and thus basically the calcaneal corpus from the anterior process of the calcaneus.
Line B: Superiorly, line B intersects the posterior talar articular surface of the calcaneus at its highest point and proceeds through the medial process of the calcaneal tuber, inferiorly. Hence, line B divides the posterior process of the calcaneus from the calcaneal corpus.
Line C: Posteriorly, line C intersects the dorsal wall of the calcaneus by passing through a notch being located directly superior to the calcaneal tuber’s compact bone. Anteriorly, line C has its terminal point in line B with both lines being perpendicular to one another. The calcaneal region formed superiorly to line C is defined as area I, the region located inferiorly is named area II.
For measuring Hounsfield units of the calcaneus in sagittal CT scans, the radiological program IMPAX EE (IMPAX EE R20 XV SU4; Agfa Health Care N.V., Mortsel, Belgium), containing the function ROI-tool, was used. Since this function prohibits a slice-across measurement, five density values per area were obtained in previously defined measuring slices (Figure 2):
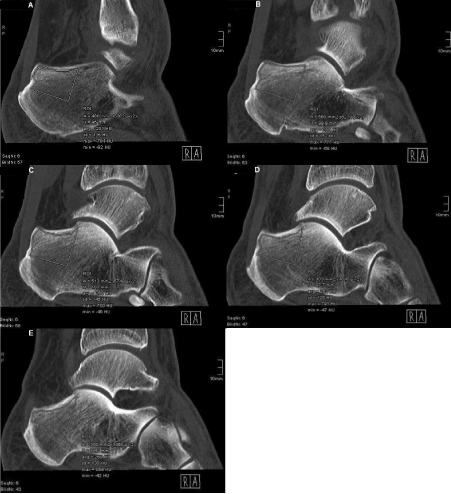
Figure 2. Detection of Hounsfield units using the example of area I
Important density values: ‘avg’ and ‘max’
A: Measuring field in lateral measuring slice
B: Measuring field in lateral-central measuring slice
C: Measuring field in central measuring slice
D: Measuring field in central-medial measuring slice
E: Measuring field in medial measuring slice (Superiorly, exclusion of compact bone from measuring field)
Lateral slice: The lateral slice is the most lateral CT layer not containing any compact bone of the lateral calcaneal wall within the cancellous bone.
Lateral-central slice: The lateral-central slice is located equidistantly between the lateral and the central slice.
Central slice: The central slice is centered at equal distance to the lateral and the medial slice.
Central-medial slice: The central-medial slice is located equidistantly between the central and the medial slice.
Medial slice: The medial slice is the most medial CT layer not containing any compact bone of the medial calcaneal wall within the cancellous bone.
HU values were obtained successively in the aforementioned five measuring slices and measurements were performed constantly from lateral to medial for each individual area. While manually marking every single measuring field through the ROI-tool, care was taken to ensure congruence with the previously defined form of the corresponding area (Figure 1). Moreover, the field was not allowed to contain any compact bone. This was warranted by prohibiting the Hounsfield units within the measuring field from exceeding a value of 1000 (‘max’ in program). Inclusions of compact bone, calcaneal cysts or epiphyseal plates were excluded from the measurement by omitting these structures while marking. The average density value in Hounsfield units (‘avg’ in program) per measuring field and per area was measured and tabulated. Thus, 20 density values per calcaneus were obtained. Visual peculiarities, such as the location of the neutral triangle, were also recorded.
Statistical analyses of repeated measures, regarding the mean density values for the four areas, were performed fitting two linear mixed models. The first model included the factors age and area as fixed effects without modeling an interaction term; the second model contained the factors age, sex and area with all interaction terms. For multiple testing, p-values were adjusted with the tukey method or based on the multivariate t distribution. Adjustments were constrained by the particular comparisons. All analyses were executed by using R (version 3.4.0 for Windows 7). Confidence levels were set to 95 % and p-values less than 0.05 were considered significant.
A total of 402 CT scans were analyzed with an average patients’ age of 40.4 ± 19.2 years. The patient population consisted of 215 (53.5 %) men and 187 (46.5 %) women. Density values of 174 (43.3 %) left and 228 (56.7 %) right calcanei were obtained. The neutral triangle was located in area III or in the transition area between areas III and IV in 91.6 % of patients.
Independently of age and sex, the mean density value was 283.90 ± 58.77 HU for area I, 184.94 ± 48.54 HU for area II, 182.53 ± 50.86 HU for area III and 173.49 ± 52.84 HU for area IV. In the corresponding model including the factors age and area only, all differences between the four areas were statistically significant to one another (all p < 0.0001); merely for the comparison between areas II and III no significant difference could be demonstrated (p = 0.6389) (Figure 3).
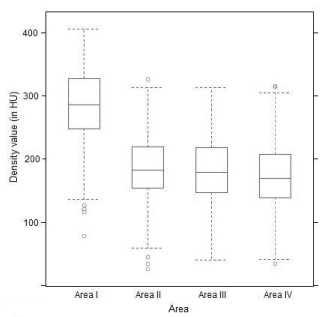
Figure 3. Mean density values (Density values per area; age- and sex-independent).
Comparing density values of measuring slices, we found higher HU values medially than laterally for areas I, II and III, when analyzed independently of age and sex. Regarding area I, the density values of the measuring slices were of an approximately campanulate shape, presenting the highest HU values in the central slice with the medial density exceeding the lateral one. Whereas, in areas II and III the density values of the measuring slices continuously increased from lateral to medial with a less steep rise in area II than in area III. In area IV, the density distribution did not follow the aforementioned pattern: Density values of the measuring slices formed a U-shape, providing the lowest HU values in the central slice and the highest HU values in the lateral slice (Figure 4).
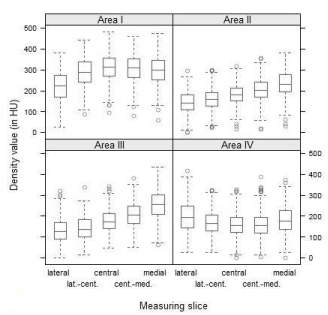
Figure. 4 Density distributions (Lateral to medial density distribution per area and per measuring slice; age- and sex-independent).
Further, we performed age- and sex-dependent analyses on our data set using a model with three-way interaction of age, sex and area. Since linear and exact testings revealed the same results, the following findings refer to the linear statistical model for simplification.
When comparing density values of all included men to density values of all women included in our study, no significant difference was detected for areas I and II (both p = 0.9995); whereas, in areas III ( p = 0.0003) and IV (p = 0.0014), densities were significantly higher in men than in women. Both for the males and for the females, area I contained the significantly highest HU values compared to all other areas (all six p < 0.0001). The latter result matches our findings for the patient population. Moreover, no significant difference was found between areas II and III (p = 0.2530) as well as between areas II and IV (p = 0.6859) for the men. For the women, density values of areas III and IV did not show significance when contrasted to one another (p = 0.0626).
With increasing age, all areas experienced a loss of bone substance represented by lower density values. When compared to areas II, III and IV, area I either lost an approximately identical or a greater amount of bone substance; the latter process was more distinct in women than in men. Nevertheless, with increasing age area I still contained the highest density values of all four areas (Figure 5).
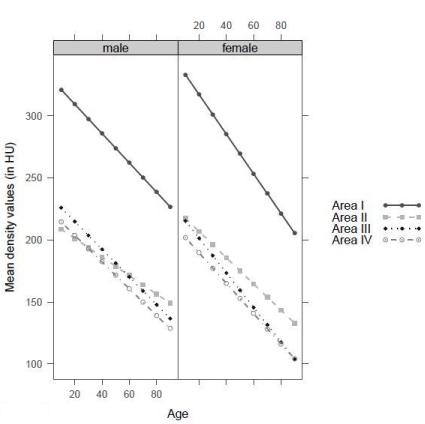
Figure. 5 Density values per area with increasing age (With increasing age, loss of density in all areas).
Since first performed in the early 1990s, the current technique for harvesting cancellous trephine autografts of the calcaneus has experienced only minimal modifications. These were undertaken in order to minimize occurring complications. But, until now, no standardized approach was obtained for the procedure [1-4].
There is a legitimate interest in improving the current technique, since the calcaneus is a suitable donor bone in foot and ankle surgery due to its ease of accessibility and low complication rate [1-3]. Furthermore, patient satisfaction rates after calcaneal grafting exceed those after grafting from alternative sites, especially from the iliac crest [4,10]. But, at present, the calcaneus offers only small amounts of harvestable cancellous bone ranging from 3 to 5 cm3 [20].
To the best of our knowledge, no research study has been published, investigating area-bound density values of the calcaneus in Hounsfield units by use of computed tomography. Moreover, no reference values for Hounsfield units of the calcaneus have been determined yet. A comparison of our findings to pre-existing reference values is therefore not possible at this point. Nonetheless, a recent investigation by Barwick et al. [22] on derived bone densities in the diabetic foot presented average HU values of the calcaneus which are comparable to our findings, despite the underlying disease in the patient population.
There are some limitations to our study: First of all, the density value (in HU) cannot be equated with the actual bone mineral density (in mg/cm3). But, it is well known that the higher the actual bone mineral density of a tissue, the higher the corresponding Hounsfield units in the computed tomography [23]. Thus, it can definitely be said that area I contains a significantly higher bone mineral density in proportion to areas II, III and IV. It is not necessary to have notice of the specific bone mineral density value, because our study compares intracalcaneal areas and not donor bones with one another.
Secondly, despite previous selection, the possible inclusion of altered bone substance in the form of sclerosis or osteopenia while measuring might have biased our results. In case of such a modification, the entire bone and thus each of the areas I to IV would be affected, causing therefore no change to the areas’ density ratios.
2021 Copyright OAT. All rights reserv
Another weak point of our implemented research remains that measuring density values in Hounsfield units means detecting the amount of calcified bone. Hence, this allows an evaluation of the bone quantity within the calcaneus; an evaluation regarding the quality of the bony material is not possible.
Based on anatomical considerations, we generally expect areas III and IV to be high risk harvest sites appearing therefore less suitable for calcaneal grafting than areas I and II. Both area III and area IV may contain the neutral triangle offering a poor quantity of material when retrieving a cancellous graft. Additionally, penetrating the neutral triangle with a tool endangers the intraosseus blood supply. This might lead to the formation of necroses due to the calcaneal watershed phenomenon [24]. Especially a calcaneal graft harvest performed from area IV involves the danger of violating the lateral neurovascular bundle or the lateral ligaments and the danger of penetrating adjacent articular surfaces causing consequential instability [24-27]. In our findings, the inclusion of the neutral triangle might have biased our results for area III in the form of lowering average Hounsfield units for this area. But, regarding the aforementioned conditions, no further considerations have to be bestowed upon area III.
The results of our study demonstrate that, when analyzed independently of age and sex, area I contain
ns the highest density values with the highest HU values being located in the central slice. Simultaneously, area I experiences the greatest loss of bone substance, but nevertheless, still provides the highest density values with increasing age and therefore compares favorably to areas II, III and IV when harvesting cancellous autografts of the calcaneus. Due to these findings, modifications of the established lateral approach to the calcaneus, which is currently performed in area II and in the transition area between areas I and II, respectively, should be considered.
We suggest performing either a lateral but more superior approach to the calcaneus, as already used for the harvest of corticocancellous segmental grafts [1,28], or a dorsal approach as described for the reduction of calcaneal fractures [24]. With the latter approach, density distributions of the calcaneus from lateral to medial can be taken into account, thus allowing a maximum harvest of cancellous bone.
Nevertheless, to what extent this iatrogenic loss of substance affects biomechanical properties and therefore causes complications, remains a subject to future studies; an area-bound determination of the actual bone mineral density and the material quality of the calcaneus ought to be investigated in further research.
In foot and ankle surgery, the calcaneus serves as an easily accessible donor bone for the harvest of autogenous cancellous grafts. The procedure causes low complication rates (0 to 13.8 %), but provides only small amounts of bony material [1,2,4,20]. Our study demonstrates that, independently of age and sex, area I compares favorably to areas II, III and IV regarding highest density values in Hounsfield units. Thus, harvesting calcaneal cancellous autografts from area I might be associated with an enhanced material quantity.
Previous studies on calcaneal grafting and on the reduction of calcaneal fractures proved that performing alternative approaches, such as a lateral-superior or a dorsal one, is possible [1,28,29].
We consider a standardized technique for the harvest of cancellous trephine autografts of the calcaneus in favor of area I. But, detailed investigations on potential complications and area-bound determinations of the available bone quality are yet to come.
On behalf of all authors, the corresponding author states that there is no conflict of interest.
- Mahan KT (1994) Calcaneal donor bone grafts. J Am Podiatr Med Assoc 84: 1-9.
- O'Malley MJ, Sayres SC, Saleem O (2014) Morbidity and complications following percutaneous calcaneal autograft bone harvest. Foot Ankle Int 35: 30-37. [Crossref]
- Biddinger KR, Komenda GA, Schon LC, Myerson MS (1998) A new modified technique for harvest of calcaneal bone grafts in surgery on the foot and ankle. Foot Ankle Int 19: 322-326.
- Raikin SM, Brislin K (2005) Local bone graft harvested from the distal tibia or calcaneus for surgery of the foot and ankle. Foot Ankle Int 26: 449-453. [Crossref]
- Mendicino RW, Leonheart E, Shromoff P (1996) Techniques for harvesting autogenous bone grafts of the lower extremity. J Foot Ankle Surg 35: 428-435.
- Miller CP, Chiodo CP (2016) Autologous bone graft in foot and ankle surgery. Foot Ankle Clin 21: 825-837.
- Myeroff C, Archdeacon M (2011) Autogenous bone graft: Donor sites and techniques. J Bone Joint Surg Am 93: 2227-2236.
- Ahlmann E, Patzakis M, Roidis N, Shepherd L, Holtom P (2002) Comparison of anterior and posterior iliac crest bone grafts in terms of harvest-site morbidity and functional outcomes. J Bone Joint Surg Am 84: 716-720. [Crossref]
- Banwart JC, Asher MA, Hassanein RS (1995) Iliac crest bone graft harvest donor site morbidity. A statistical evaluation. Spine 20: 1055-1060.
- DeOrio JK, Farber DC (2005) Morbidity associated with anterior iliac crest bone grafting in foot and ankle surgery. Foot Ankle Int 26: 147-151.
- Younger EM, Chapman MW (1989) Morbidity at bone graft donor sites. J Orthop Trauma 3: 192-195.
- Chiodo CP, Hahne J, Wilson MG, Glowacki J (2010) Histological differences in iliac and tibial bone graft. Foot Ankle Int 31: 418-422.
- Hyer CF, Berlet GC, Bussewitz BW et al. (2013) Quantitative assessment of the yield of osteoblastic connective tissue progenitors in bone marrow aspirate from the iliac crest, tibia, and calcaneus. J Bone Joint Surg Am 95: 1312-1316.
- Kuhn DA, Moreland MS (1986) Complications following iliac crest bone grafting. Clin Orthop Relat Res 209: 224-226.
- Chen YC, Chen CH, Chen PL (2006) Donor site morbidity after harvesting of proximal tibia bone. Head Neck 28: 496-500.
- Fitzgibbons TC, Hawks MA, McMullen ST, Inda DJ (2011) Bone grafting in surgery about the foot and ankle: Indications and techniques. J Am Acad Orthop Surg 19: 112-120.
- O'Malley DF, Conti SF (1996) Results of distal tibial bone grafting in hindfoot arthrodeses. Foot Ankle Int 17: 374-377.
- O'Keeffe RM, Riemer BL, Butterfield SL (1991) Harvesting of autogenous cancellous bone graft from the proximal tibial metaphysis. A review of 230 cases. J Orthop Trauma 5: 469-474. [Crossref]
- Whitehouse MR, Lankester BJA, Winson IG, Hepple S (2006) Bone graft harvest from the proximal tibia in foot and ankle arthrodesis surgery. Foot Ankle Int 27: 913-916.
- DiDomenico LA, Haro AA III (2006) Percutaneous harvest of calcaneal bone graft. J Foot Ankle Surg 45: 131-133.
- Roukis T (2006) A simple technique for harvesting autogenous bone grafts from the calcaneus. Foot Ankle Int 27: 998-999. [Crossref]
- Barwick A, Tessier J, Mirow J, de Jonge XJ, Chuter V (2017) Computed tomography derived bone density measurement in the diabetic foot. J Foot Ankle Res 10:11
- Hounsfield GN (1973) Computerized transverse axial scanning (tomography): Part I. Description of system. Br J Radiol 46: 1016-1022.
- Andermahr J, Helling HJ, Rehm KE, Koebke Z (1999) The vascularization of the os calcaneum and the clinical consequences. Clin Orthop Relat Res 363: 212-218.
- Hall RL, Shereff MJ (1993) Anatomy of the calcaneus. Clin Orthop Relat Res 290: 27-35.
- Lutz M, Benedetto KP, Kunzel KH (1993) Operative approach to the calcaneus: A comparison of classical and modified techniques with regard to arterial vascularization. Clin Anat 6: 275-279.
- Netter FH (1997) Atlas der Anatomie des Menschen. Stuttgart, New York; Thieme 484-497, 500-513.
- Feeney S, Rees S, Tagoe M (2007) Tricortical calcaneal bone graft and management of the donor site. J Foot Ankle Surg 46: 80-85.
- Essex-Lopresti P (1952) The mechanism, reduction technique, and results in fractures of the os calcis. Br J Surg 39: 395-419.





