Purpose: The identification of biomarkers for use during selection of embryos for intrauterine implantation can enhance in vitro fertilization (IVF) success rates. Assessing apoptotic molecular markers found in blastocoel fluid-conditioned media from human embryos with known ploidy status provides the opportunity to study patterns and processes occurring during early embryo development. Apoptosis occurs during preimplantation development and may serve to selectively eliminate aneuploid cells from the developing embryo. Therefore, apoptotic remnants such as cell-free DNA (cfDNA) may reside within the embryo’s blastocoel fluid and vary depending upon embryo ploidy status. In this study we compared cfDNA content of blastocoel fluid-conditioned media from euploid and aneuploid embryos.
Methods: Blastocoel fluid-conditioned media was collected following trophectoderm (TE) biopsy of day-5 IVF blastocysts. cfDNA in the media from 89 embryos was quantified using fluorospectrometry.
Results: cfDNA content in human blastocoel fluid-conditioned media decreased as ploidy status deviated from euploid.
Conclusions: The alterations in cfDNA content may represent levels unique to embryos that are euploid. In addition to preimplantation genetic testing for aneuploidy via analysis of trophectoderm cells, uncovering different molecular features in the blastocoel fluid that are unique to euploid embryos suggests that analysis of the fluid may provide an additional measure of embryo quality, that is marginally-invasive.
blastocoel fluid, cell-free DNA, euploidy, aneuploidy
Recurrent miscarriages are the presumed result of embryonic chromosomal abnormalities, i.e. aneuploidy, the loss or gain of chromosome(s). Moreover, approximately 30% of conceptions will result in a live birth and this low number is due to both pre- and post-implantation complications [1]. Aneuploidies are most commonly associated with advanced maternal age, since chromosomal abnormalities are often of maternal origin, caused by errors in meiotic oocyte divisions. Nevertheless, chromosomal abnormalities can also occur post-fertilization that are likely caused by errors in mitotic divisions within the developing embryo [2]. Birth outcomes suggest a small subset of aneuploid embryos form blastocysts, successfully implant and result in a live birth (i.e. a child born with Down syndrome or Turner syndrome). Interestingly, the aneuploidy rate has been demonstrated to fluctuate during the transition from oocyte to blastocyst, suggesting that some mechanism of auto-correction within the preimplantation embryo may be occurring [2,3]. However, the potential correction mechanism of aneuploidy in certain cells of the embryo during the preimplantation stages remains unknown [4].
One treatment scheme proposed for improving live birth rates is utilizing in vitro fertilization (IVF) with intracytoplasmic sperm injection (ICSI), embryo biopsy and subsequent chromosomal analysis, referred to as preimplantation genetic testing for aneuploidies or PGT-A [5]. The process of identifying a euploid embryo involves the biopsy of numerable trophectoderm cells from a day-5 or day-6 blastocyst stage embryo generated via IVF methods. Despite significant technical capabilities that clinical embryologists currently employ to identify ploidy status of embryos, IVF-generated euploid embryo implantation remains less than 60% [6].
Further complicating embryo selection, preimplantation embryos are often mosaics, whereby cells in a single embryo can differ from one another in their genetic makeup and therefore ploidy status. These differences within a single embryo have been mapped by a variety of genetic methods, including microarray analysis, which illustrate the vast differences from even neighboring cells in some mosaic embryos [7]. Considering that prevalence of mosaicism in the human embryo is estimated to be ~20%, alongside the evidence that aneuploidy rates decrease in the blastocyst stage of embryo development, the ability of an embryo to rid itself of aneuploid cells through regulated and systematic means prior to implantation is a likely hypothesis[7,8].
The best-known mechanism of selective cellular death is apoptosis. Apoptosis, known as programmed cell death, occurs during preimplantation embryo development and possibly serves as a corrective mechanism, sacrificing cells for overall embryo competence [9]. Additionally, apoptosis requires mitochondrial proteins, and both maternal age as well as aneuploid embryos have been linked to defective mitochondria [4]. Moreover, a recent study detected elevated mitochondrial DNA (mtDNA) levels in aneuploid embryos [10]. Mechanistically, if mitochondrial function is impaired, this could directly lead to reduced ability for apoptosis to occur during normal preimplantation embryo development. If one role of apoptosis is embryo self-correction via elimination of aneuploid cells, then it is possible that impaired apoptosis may contribute to aneuploid cell retention and therefore result in an embryo which is not competent for implantation.
In addition to chromosomal analysis, one method proposed to improve the implantation rates of euploid IVF embryos is to identify biological markers that are unique to implanted euploid embryos versus those that did not. An ideal biomarker is one easily obtained using non-invasive or minimally-invasive means from the early embryo at trophectoderm biopsy. Upon inspection of the IVF procedure, it is evident that the blastocoel fluid fits the criteria. During IVF, upon completion of the blastocyst biopsy for subsequent chromosomal analysis (preimplantation genetic testing for aneuploidies or PGT-A), the embryo collapses upon itself. This collapse results in the blastocoel fluid extruding into the surrounding culture medium. The blastocyst fluid-conditioned culture medium (which is generally discarded) can be frozen and subsequently assessed. This collection method, known as blastocentesis, is minimally-invasive and provides blastocoel fluid from day-5/6 embryos for study, while mitigating risk to future developmental potential of the embryo [11]. This blastocoel fluid is a recognized source of cell-free DNA (cfDNA), which may serve as a proxy for discovering ploidy status of the embryo [12-17]. Several studies have reported a limited concordance between the chromosomal status detected using cfDNA compared to PGT-A from embryonic trophectoderm biopsy [17]. Proteins, mitochondrial DNA, miRNAs, along with cfDNA have also been detected in the blastocoel fluid and the origin of these molecules may potentially be the remnants of apoptotic cells from the blastocyst [18-22]. Most recently, microRNAs, some of which were linked to apoptosis, and extracellular vesicles were found in blastocoel fluid from human embryos [23]. Therefore, if apoptosis purges the embryo of aneuploid cells in the preimplantation embryo, detection of this activity may be possible through measuring cfDNA content in the blastocoel fluid.
In the current study, we assessed cfDNA levels in blastocoel fluid-conditioned media from 89 day-5 blastocyst embryos and found higher levels of cfDNA in euploid embryos compared to aneuploid embryos. One source of the elevated cfDNA in euploid embryos may be remnants of apoptotic cells in the embryo. We hypothesize that human embryos classified as aneuploid (based on PGT-A) would have undergone less and or incomplete apoptotic cell elimination which resulted in the embryo retaining cells that harbored chromosomal abnormalities. We propose that analysis of cfDNA content in blastocoel fluid is a potential biomarker for selecting the embryo having the best chance at uterine implantation.
Research approval was granted by the Institutional Review Board (IRB) of the University of South Carolina Office of Research Compliance. Blastocoel fluid conditioned media, which is generally discarded, was collected and saved post biopsy from day-5 blastocyst stage human embryos obtained from patients undergoing IVF cycles at collaborating clinics (San Antonio, TX, Swansea, IL and Raleigh, NC). Following procedures for preimplantation genetic testing, extruded trophectoderm (TE) cells were biopsied following laser pulses between cellular junctions from the day-5 blastocyst stage embryos. The biopsied TE cells were removed by pipette and placed into buffer for PGT-A analysis via Next-Gen Sequencing (NGS) at a commercial sequencing company. Upon completion of the blastocyst biopsy, the embryo self-collapses, resulting in blastocoel fluid being extruded out into the surrounding medium. The blastocyst fluid-conditioned culture media with a volume of approximately 25μL is snap frozen prior to shipment to Greenville, SC. Biopsied embryos are cryopreserved pending outcome of the sequencing results. De-identified data including patient age, blastocyst morphology scores and ploidy status were provided by the collaborating fertility clinics.
Cell-free DNA quantitation
Cell-free DNA in the blastocoel fluid-conditioned media from 89 embryos was quantified using fluorospectrometry. An AccuBlue NextGen dsDNA Quantification Kit (Biotium) was utilized, with resulting emissions detected by a NanoDrop 3300 Fluorospectrometer (ThermoScientific) per manufacturer’s instructions. After generating a standard curve, 2mL of blastocoel fluid-conditioned media from each of the 89 embryos was quantified independently. Ploidy status (≤-2, -1, 0, +1, ≥+2) and cfDNA content were compared by analysis of variance (ANOVA) and Students’ t-test.
Average Cell-free DNA content is higher in euploid embryos compared to aneuploid embryos
Cell-free DNA (cfDNA) was quantified in 89 blastocoel fluid-conditioned medium samples from day-5 blastocyst embryos, characterized by ploidy status and subsequently averaged (Figure 1). Normal, euploid blastocyst stage embryos (n=45) had a mean cfDNA content of 44.9 ng/mL. Blastocyst embryos (n=18) exhibiting a single chromosomal loss had a mean cfDNA content of 37.1 ng/mL, those with 2 or more missing (n=6) had a mean cfDNA content of 32.6 ng/mL. Blastocyst embryos (n=15) exhibiting a single chromosome gain had a mean cfDNA content of 42.2 ng/mL, those with 2 or more chromosomes (n=5) had a mean cfDNA content of 30.1 ng/mL. There was a significant (p<0.05) difference in cfDNA between euploid (44.9 ng/mL) and aneuploid (36.8 ng/mL) day-5 blastocysts. ANOVA revealed a significant (p<0.05) correlation linking chromosomal status (gain/loss/euploid) and cfDNA content. Elevated cfDNA in blastocoel fluid from euploid embryos may represent the apoptotic remnants from aneuploid cells within the euploid embryo that underwent selective elimination, whereby aneuploid embryos may have been unable to selectively remove these cells via apoptosis to the same extent as euploid embryos.
We have identified a molecular faeture in blastocoel fluid-conditioned media that differs between euploid and aneuploid human embryos. Specifically, cfDNA levels are higher in fluid from euploid embryos when compared with aneuploid embryos. In addition, cfDNA content decreased as more than one chromosome was gained or lost in our pool of aneuploid embryos analyzed. One source of the cfDNA in the blastocoel fluid-conditioned media may be cellular remnants from aneuploid cells that underwent selective apoptosis early in preimplantation development. These results suggest that blastocoel fluid-conditioned media provides detectable molecular differences that vary with embryo ploidy status (Figure 1). Furthermore, the blastocoel fluid-conditioned media may retain evidence of apoptotic cell elimination of specific aneuploid cells during early preimplantation development. The extent to which apoptotic cell elimination occurred may be an indicator of future implantation success of the embryo.
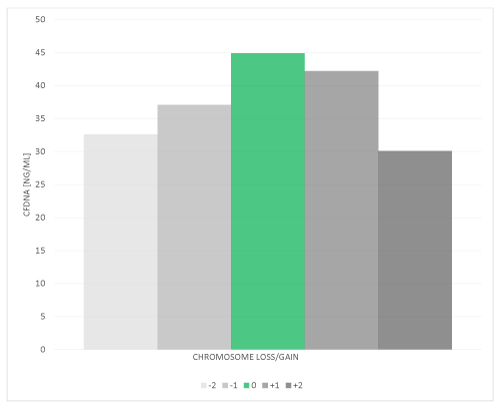
Figure 1. Elevated levels of Cell-free DNA content were detected in blastocoel fluid-conditioned media from euploid embryos as compared to aneuploid embryos. Blastocoel fluid-conditioned medium samples (N=89) from day-5 blastocyst embryos were assessed for DNA content using a fluorescent Nanodrop. cfDNA from each blastocyst stage embryo was determine by fluorospectromety and the mean is reported from 45 euploid blastocyst stage embryos (green bar), 18 blastocyst stage embryos exhibiting a single chromosomal loss, 6 blastocyst stage embryos with 2 or more missing chromosomes, 15 blastocyst stage embryos exhibiting a single chromosome gain and 5 blastocyst stage embryos with a 2 or more chromosome gain. ANOVA revealed a significant (p<0.05) difference between chromosomal status (gain/loss/euploid) and cfDNA levels
Further support for the source of the apoptotic remnants detected in the blastocoel fluid-conditioned media comes from a study by Hammond et al that identified mitochondrial DNA in spent culture media [22]. The source of the mtDNA is likely from the mitochondria of cells that underwent apoptosis during preimplantation development. Bolton at el used the chemical, reversine, to induce aneuploidies in mouse embryos and revealed apoptotic elimination of aneuploid cells from mouse preimplantation embryos [24]. This mouse study further supports the process of selective removal of aneuploid cells via apoptosis.
Our results support the notion that blastocoel fluid-conditioned media contains the molecular remnants of apoptotic cells selectively sacrificed by the preimplantation embryo, therefore cfDNA in blastocoel fluid may not be the best indicator of ploidy status as suggested by others [25]. This study poses that the level of apoptotic remnants (i.e. cfDNA) may represent a molecular indicator of embryo ploidy statues, and future, embryo implantation potential. We propose that selective self-sacrificing of aneuploid cells via apoptosis within the pre-implantation embryo is a natural process, and if carried out to the necessary extent, will yield a euploid embryo, or potentially an aneuploidy embryo, competent for uterine implantation. Therefore, assessing the apoptotic process in preimplantation embryos, we argue, resides in the blastocoel fluid-conditioned media.
Ethics approval and consent to participate
The study itself is conducted as Not Human Research (since the fluid samples were all de-identified) set forth by the Code of Federal Regulations (45 CFR 46) and was exempt from IRB review. The collection of blastocoel-fluid conditioned media was conducted under informed patient consent. The informed consent for treatment (American Society for Reproductive Medicine-Society for Assisted Reproductive Technology consent template) was modified to include that any unused biological material may be used for current or future research. All patients signed a consent form allowing PGT-A on TE cells as well.
Facility: Vios Fertility Institute. All methods were performed in accordance with the relevant guidelines and regulations.
Facility: University of Texas Health Sciences Center San Antonio. All methods were performed in accordance with the relevant guidelines and regulations.
Facility: Atlantic Reproductive Medicine Specialists. All methods were performed in accordance with the relevant guidelines and regulations.
University of South Carolina Magellan Scholar funds supported JB and offset some costs for experiments.
RJC and WER designed the experiments. SZ, TAC, RDR and JDW carried out sample collection, pooled patient data records and contributed to revision of the manuscript. AL and JB helped carry out the experiments and aid in data analysis. AL led figure construction and helped with manuscript writing. WER and RJC led the data analysis, interpretation of results and manuscript writing.
The authors declare no competing interests.
- Macklon NS, Geraedts JP, Fauser BC (2002) Conception to ongoing pregnancy: the 'black box' of early pregnancy loss. Hum Reprod Update 8: 333-343. [Crossref]
- Fragouli E, Alfarawati S, Spath K, Jaroudi S, Sarasa J, et al. (2013) The origin and impact of embryonic aneuploidy. Hum Genet 132: 1001-1013. [Crossref]
- Robberecht C, Vanneste E, Pexsters A, D'Hooghe T, Voet T, et al. (2010) Somatic genomic variations in early human prenatal development. Curr Genomics 11: 397-401.
- Daughtry BL, Chavez SL (2016) Chromosomal instability in mammalian pre-implantation embryos: potential causes, detection methods, and clinical consequences. Cell Tissue Res 363: 201-225.
- Brezina PR, Anchan R, Kearns WG (2016) Preimplantation genetic testing for aneuploidy: what technology should you use and what are the differences? J Assist Reprod Genet 33: 823-832.
- Fragouli E, Munne S, Wells D (2019) The cytogenetic constitution of human blastocysts: insights from comprehensive chromosome screening strategies. Hum Reprod Update 25: 15-33.
- Mertzanidou A, Wilton L, Cheng J, Spits C, Vanneste E, et al. (2013) Microarray analysis reveals abnormal chromosomal complements in over 70% of 14 normally developing human embryos. Hum Reprod 28: 256-264.
- Schattman GL (2018) Chromosomal mosaicism in human preimplantation embryos: another fact that cannot be ignored. Fertil Steril 109: 54-55. [Crossref]
- Brill A, Torchinsky A, Carp H, Toder V (1999) The role of apoptosis in normal and abnormal embryonic development. J Assist Reprod Genet 16: 512-519.
- Fragouli E, Spath K, Alfarawati S, Kaper F, Craig A, et al. (2015) Wells D Altered levels of mitochondrial DNA are associated with female age, aneuploidy, and provide an independent measure of embryonic implantation potential. PLoS Genet 11: e1005241.
- Gianaroli L, Magli MC, Pomante A, Crivello AM, Cafueri G, et al. (2014) Blastocentesis: a source of DNA for preimplantation genetic testing. results from a pilot study. Fertil Steril 102: 1692-1699. [Crossref]
- Capalbo A, Romanelli V, Patassini C, Poli M, Girardi L, et al. (2018) Diagnostic efficacy of blastocoel fluid and spent media as sources of DNA for preimplantation genetic testing in standard clinical conditions. Fertil Steril 110: 870-879.
- Tšuiko O, Zhigalina DI, Jatsenko T, Skryabin NA, Kanbekova OR, et al. (2018) Karyotype of the blastocoel fluid demonstrates low concordance with both trophectoderm and inner cell mass. Fertil Steril 109: 1127-1134. [Crossref]
- Li P, Song Z, Yao Y, Huang T, Mao R, et al. (2018) Preimplantation genetic screening with spent culture medium/blastocoel fluid for in vitro fertilization. Sci Rep 8: 9275-018-27367-4.
- Xu J, Fang R, Chen L, Chen D, Xiao JP, et al. (2016) Noninvasive chromosome screening of human embryos by genome sequencing of embryo culture medium for in vitro fertilization. Proc Natl Acad Sci USA 113: 11907-11912.
- Magli MC, Albanese C, Crippa A, Tabanelli C, Ferraretti AP, et al. (2019) Deoxyribonucleic acid detection in blastocoelic fluid: a new predictor of embryo ploidy and viable pregnancy. Fertil Steril 111: 77-85.
- Ho JR, Arrach N, Rhodes-Long K, Ahmady A, Ingles S, et al. (2018) Pushing the limits of detection: investigation of cell-free DNA for aneuploidy screening in embryos. Fertil Steril 110: 467-475.
- Capalbo A, Ubaldi FM, Cimadomo D, Noli L, Khalaf Y, et al. (2016) MicroRNAs in spent blastocyst culture medium are derived from trophectoderm cells and can be explored for human embryo reproductive competence assessment. Fertil Steril 105: 225-235.
- Palini S, Galluzzi L, De Stefani S, Bianchi M, Wells D, et al. (2013) Genomic DNA in human blastocoele fluid. Reprod Biomed Online 26: 603-610.
- Poli M, Ori A, Child T, Jaroudi S, Spath K, et al. (2015) Characterization and quantification of proteins secreted by single human embryos prior to implantation. EMBO Mol Med 7: 1465-1479.
- Tobler K, Zhao Y, Ross R, Benner A, Xu X, et al. (2014) Blastocoel fluid (bf) harbors embryonic DNA that may result from the marginalization of aneuploid cells during embryogenesis. Fertil Steril 102: e205.
- Hammond ER, McGillivray BC, Wicker SM, Peek JC, Shelling AN, et al. (2017) Characterizing nuclear and mitochondrial DNA in spent embryo culture media: genetic contamination identified. Fertil Steril 107: 220-228.
- Battaglia R, Palini S, Vento ME, La Ferlita A, Lo Faro MJ, et al. (2019) Identification of extracellular vesicles and characterization of miRNA expression profiles in human blastocoel fluid. Sci Rep 9: 84-018-36452-7.
- Bolton H, Graham SJ, Van der Aa N, Kumar P, Theunis K, et al. (2016) Mouse model of chromosome mosaicism reveals lineage-specific depletion of aneuploid cells and normal developmental potential. Nat Commun 7: 11165.
- Vera-Rodriguez M, Diez-Juan A, Jimenez-Almazan J, Martinez S, Navarro R, et al. (2018) Origin and composition of cell-free DNA in spent medium from human embryo culture during preimplantation development. Hum Reprod 33: 745-756.

