Abstract
Purpose: West Syndrome (WS) is a rare epileptic condition which specifically affects young infants, with a potentially severe outcome. Its pathophysiology remains unclear, which hinders progress in developing targeted medications. Here, we sought to determine whether WS patients have a specific cerebrospinal fluid (CSF) metabolic profile which could help to characterize the alterations involved.
Method: CSF samples were collected during the 2010-2016 period from WS patients (n=9). The control group (n=9) included normally developing and seizure-free children who underwent a lumbar puncture (LP). Targeted and untargeted CSF metabolomics analyses were performed by Liquid Chromatography coupled to High Resolution Mass Spectrometry (LC-HRMS). The metabolic patterns were analyzed by multivariate analysis based on multiple machine-learning methods (i.e. biosigner algorithm including the use of training, test sets and bootstraps) and univariate statistical analysis.
Results: Biosigner strategy revealed a significant model discriminating WS and controls from 2 metabolites including serine. The model correctly predicted diagnosis for 83% of subjects. Serine levels were also statistically different between the two groups by univariate analysis (p=0.0023).
Conclusion: This is the first metabolomics study in WS. Our results suggest a pivotal need of serine in this disorder. The symptoms observed in WS are consistent with alterations of the serine metabolism pathway. We provide new data concerning this severe epileptic syndrome and highlight the potential value of metabolomics studies in pediatric neurological disorders, even when patients are scarce.
Key words
West Syndrome, epilepsy, cerebrospinal fluid, biomarkers, metabolomics, serine
Introduction
West Syndrome (WS) refers to a subset of infantile spasm that can either be secondary to a neurological disorder or of unknown cause. Though it was identified in the 19th century, our understanding of its pathophysiology remains relatively poor and robust biomarkers are needed. Using proteomics approach, Greene et al. highlighted some potential biomarkers in animal models. However, this proteomics profile is not specific and such a technically challenging approach precludes its use in routine practice. Metabolomics is a powerful strategy to ascertain metabolic signatures from small molecules in biological fluids. In this study, we used for the first time a metabolomics approach, based on mass spectrometry to explore WS. This may provide an opportunity to improve the management and therapy of this disease.
Methods
Patient recruitment and data selection
The patients were recruited in the pediatric neurology unit of the Tours University Hospital (July 2010 - May 2016). The inclusion criterion was a diagnosis of WS, based on the ILAE definition [1]. CSF samples were collected before introduction of antiepileptic drugs. The control group included normally developing and seizure-free children, who initially presented with various symptoms, requiring a lumbar puncture (LP) during their investigation. The following elements were collected: sex, age at epilepsy onset, psychomotor development, developmental regression, WS etiology, ongoing treatment, age at LP, fasting or non-fasting state at LP. Ethical consent was obtained from both parents.
Sample preparation and LC-HRMS analysis
We prepared the samples and quality control samples (QC) for metabolomics by standard methods using LC-HRMS. Analysis of LC-HRMS was performed using an UPLC Ultimate 3000 system (Dionex), coupled to a Q-Exactive Mass Spectrometer (Thermo Fisher Scientific, Bremen, Germany), and operated in the positive (ESI+) and negative (ESI-) electrospray ionization modes, as previously published [2].
Data preprocessing
We performed both untargeted and targeted analysis. SIEVER software (Thermo Fisher Scientific) was used to process raw data for untargeted analysis and metabolites were identified from our own library for targeted analysis. We only kept metabolites having a coefficient of variation < 30% in the QC samples in the final dataset.
Multivariate data analysis
Clustering of QC and patients’ samples was assessed using principal component analysis (PCA) to evaluate outliers. OPLS-DA was first performed using SIMCA® version 13.0 (Umetrics, Umeå, Sweden) to provide the Score Scatter Plot and the Loading Score Plot to visualize both, the discrimination of samples and the most discriminant variables.
We also used the biosigner algorithm in R [3] to assess as a new strategy for discovering significant molecular signatures. This data-mining algorithm is independently wrapped around different machine-learning approaches, i.e. partial least-squares discriminant analysis (PLS-DA), random forest (RF), and support vector machines (SVM). This strategy aims at finding the smallest pattern that provides a significant model after the combination of sampling (bootstrap, n=1000), ranking of relevant metabolites and the evaluation of performance after permutation within the test set and the half-interval search. The final training of the model is based on all samples from the dataset and the selected features. First, the dataset is split into training and testing datasets (by boot-strapping, controlling class proportions), then a model is built on the training set and performance of prediction is evaluated on the test set.
Univariate analysis
The univariate analysis of metabolites was based on fold-change (FC) values and the threshold of significance with the volcano plot and the non-parametric Wilcoxon test using Metaboanalyst, version 2.1. We highlighted only metabolites with p < 0.05 and FC > 1.5.
Results
CSF collection
Nine WS patients (six girls/three boys) were included. The clinical data are summarized in Table 1. The clinical features of the control group were as follows:
Table 1. Clinical features of the West Syndrome subjects (F: female, M: male, m: month, w: week, d: day, div: diversified food)
|
Patient Number
|
Sex |
Age at onset |
Age at LP |
Fast at LP |
Diet |
Psychomotor development |
Psychomotor Regression |
WS etiology |
Antiepileptic treatment |
1 |
M |
1 m 3 w |
2 m 1 w |
no |
milk |
delayed |
yes |
Unkown cause |
Vigabatrin (200mg/kg/d for 13 d)
Carbamazepine (10mg/kg/d for 2 d)
Biotin (5mg/kg/d for 2 d)
Folinic acid (2.5mg/kg/d for 2 d) |
2 |
M |
1 m 3 w |
2 m |
no |
milk |
delayed |
yes |
Unkown cause |
Phenobarbital (5mg/kg/d for 4 d) |
3 |
F |
9 m |
10 m |
no |
div |
normal |
no |
Unkown cause |
Vigabatrin (90mg/kg/d for 4 d) |
4 |
F |
6 m 2 w |
6 m 3 w |
yes |
div |
normal |
yes |
Unkown cause |
Vigabatrin (60mg/kg/d for 2 d) |
5 |
F |
6 m |
9 m |
no |
div |
delayed |
yes |
Tuberous sclerosis |
none |
6 |
F |
8 m |
8 m 6w |
yes |
div |
delayed |
no |
Unkown cause |
Vigabatrin (60mg/kg/d for 11 d) |
7 |
F |
5 m |
11 m 6w |
yes |
div |
delayed |
yes |
Unkown cause |
Vigabatrin (150mg/kg/d for 6 d) |
8 |
F |
6 m |
6 m |
no |
milk |
delayed |
no |
2p duplication |
Pyridoxine (80mg/kg/d for 2 d) |
9 |
M |
6 m |
6 m |
yes |
milk |
delayed |
yes |
Unkown cause |
Vigabatrin (50mg/kg/d for 6 d) |
2021 Copyright OAT. All rights reserv
patient 1: 14 years and 1-month-old girl, limb Pain;
patient 2: 4 years and 11-month-old girl, ataxia
patient 3: 10 years and 4-month-old boy, headache and dizziness
patient 4: 14 years and 6-month-old girl, severe chronic back pain
patient 5: 9 years and 1-month-old girl, headache and ataxia
patient 6: 9 years and 4-month-old girl paresthesia
patient 7: 15 years and 6-month-old girl, right hemiparesis
patient 8: 15-year-old girl, fainting
patient 9: 12 years and 10 month-old-girl, diplopia
LC-HRMS analysis
Untargeted analysis revealed 291 and 82 frames for ESI+ and ESI- mode acquisitions, respectively and targeted analysis led to the identification of 62 metabolites.
Multivariate analysis
PCA showed a tight cluster for the QC and for subjects within each group (data not shown). The Figure 1A shows the Score Scatter Plot built from OPLS-DA (p=0.0001) performed using the 10 most relevant metabolites (Figure 1B) such as serine, tryptophan, histidine, 3-methylhistidine, glutamic acid, citrate, vitamin B5 and unidentified metabolites. Biosigner analysis revealed a significant signature for WS patients from 2 metabolites: serine and an unknown metabolite X363 from ESI –. The percentage of correctly predicted patients was 83% in the test set after 1000 bootstraps.
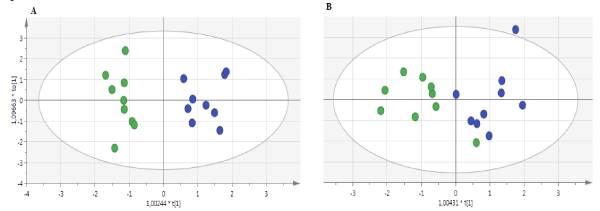
Figure 1. OPLS-DA obtained from CSF samples analyzed by metabolomics based on LC-HRMS (blue dots: controls, n=9 and green dots: WS patients; n=9) from 10 metabolites. A) Score Scatter Plot. X-axis and Y-axis represent score vectors summarizing all the variables entering the analysis: t1 and to1 B) Loading Score Plot. Variables near each other are positively correlated; variables opposite to each other are negatively correlated
Univariate analysis
Volcano plot revealed 8 metabolites with p-value < 0.05 and FC >1.5: serine (p=0.0023), 3-methylhistidine (p=0.024), histidine (p=0.0152), vitamin B5 (p=0.0152), succinate (p=0.03) and 3 unidentified metabolites including X363 (p=0.005, data not shown).
Discussion
This is the first CSF metabolomics study in WS patients. One of its strengths lies in the combination of several statistical approaches, i.e. the biosigner machine-learning strategy and univariate analysis, based on the volcano plot. Despite the small cohort size, PCA findings within each group of subjects revealed no bias due to fasting or treatment, and robust multivariate analysis provided an excellent model to discriminate WS and controls. We could not select matched controls due to ethical issues, but this preliminary study enabled to show that metabolomics strategy may be used in epilepsy.
A specific CSF metabolome in WS patients
OPLS-DA revealed an excellent model to discriminate WS and controls from only 10 metabolites. Surprisingly, the highly restrictive biosigner analysis also provided a significant model with an accuracy superior to 80%, from only 2 metabolites. This new method, based on different types of recent machine-learning strategies, including bootstrap resampling, is considered the most robust for highlighting discriminant compounds and judging the fitness of a model.
Discriminant role of serine
The most prominently affected metabolite was serine. Elevated serine levels have already been observed in animal models. In humans, in situ elevation of serine levels is associated with intractable seizures. Thus, serine homeostasis appears to play an important role in cerebral neurotransmission and epileptogenesis. Both glutamate and serine elevation are required to activate NMDARs, representing a key factor in synaptic plasticity and excitatory transmission [4]. It has been shown in a rat model of chronic epilepsy that altered D-serine levels modify NMDAR subunit composition, altering long-term potentiation and subsequent memory functions [5]. Serine also participates in the regulation of the mTOR (mammalian target of rapamycin) pathway, implicated in synaptic plasticity, neuronal repair mechanisms, and memory [6,7]. Inhibition of the mTOR pathway is considered to be a promising anticonvulsant strategy [7]. The ketogenic diet, which increases serine levels in CSF, also has a beneficial effect in severe epileptic patients [8].
We also observed that the concentration of tryptophan tended to be higher in WS patients. This is consistent with the findings of serine, as tryptophan results from the irreversible condensation of indole and serine. Tryptophan degradation secondarily leads to the production of nicotinamide adenine dinucleotide (NAD+), known as the kynurenine pathway. Our results further emphasize the involvement of this pathway in epilepsy [9,10]. However, its involvement in the pathophysiology of WS is still hypothetical and requires further specific studies.
Other biochemical pathways involved in WS
Citrate and succinate involved in the tricarboxylic cycle were also identified. Citrate is highly linked to epileptogenic process as its transport is altered in patients carrying the SLC13A5 gene mutation, in which epilepsy is a key feature.
Modulation of the histidine-carnosine pathway was also highlighted. This data is consistent with a previous report showing that histidine and carnosine both exhibit anticonvulsant effects in the rat PTZ kindling seizures model and penicillin-induced epileptiform activity in rats.
In conclusion, this is the first study to address the potential use of metabolomics in WS. We showed a discriminant profile and our data shed light on the pathways involved in WS pathophysiology, especially concerning serine. The next step will be to validate these results on an independent cohort, comparing unknown cause WS patients to other epileptic children.
Declarations
Authorship / Contributor ship / Acknowledgements: All authors listed have contributed sufficiently to the project to be included as authors, have approved the manuscript and this submission.
Funding information: To the best of our knowledge, no conflict of interest, financial or other, exists.
Competing interest: We don’t have any competing interest to declare.
References
- Berg AT, Berkovic SF, Brodie MJ, Buchhalter J, Cross JH, et al. (2010) Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE Commission on Classification and Terminology, 2005-2009. Epilepsia 51: 676-685.
- Blasco H, Corcia P, Pradat PF, Bocca C, Gordon PH, et al. (2013) Metabolomics in cerebrospinal fluid of patients with amyotrophic lateral sclerosis: an untargeted approach via high-resolution mass spectrometry. J Proteome Res 12: 3746-54.
- Rinaudo P, Boudah S, Junot C, Thévenot EA (2016) biosigner: A New Method for the Discovery of Significant Molecular Signatures from Omics Data. Front Mol Biosci 3: 26. [Crossref]
- Martineau M, Parpura V, Mothet JP (2014) Cell-type specific mechanisms of D-serine uptake and release in the brain. Front Synaptic Neurosci 6:12.
- Klatte K, Kirschstein T, Otte D, Pothmann L, Muller L, et al. (2013) Impaired D-serine-mediated cotransmission mediates cognitive dysfunction in epilepsy. J Neurosci 33: 13066-80.
- Bockaert J, Marin P (2015) mTOR in Brain Physiology and Pathologies. Physiol Rev 95: 1157-1187. [Crossref]
- McDaniel SS, Rensing NR, Thio LL, Yamada KA, Wong M (2011) The ketogenic diet inhibits the mammalian target of rapamycin (mTOR) pathway. Epilepsia 52: e7-11.
- Dahlin M, Elfving A, Ungerstedt U, Amark P (2005) The ketogenic diet influences the levels of excitatory and inhibitory amino acids in the CSF in children with refractory epilepsy. Epilepsy Res 64: 115-125.
- Moroni F (1999) Tryptophan metabolism and brain function: focus on kynurenine and other indole metabolites. Eur J Pharmacol 375: 87-100.
- Yamamoto H (1991) Studies on CSF tryptophan metabolism in infantile spasms. Pediatr Neurol 7: 411-414.

