Early and rapid diagnosis of highly pathogenic agent such as Ebola virus has always been problematic and challenging for the affected communities especially in the developing world. Most often, effective response to epidemic generated by such virulent pathogens implies establishment of well-structured laboratory systems or networks able to implement new diagnostic strategies and laboratory services for better containment of the infectious pathogen. If these measures are peculiar to the developed world where healthcare systems are well organized, it isn’t the case in resource limited settings where healthcare systems are very fragile and inadequately prepared not only to deal with small scale outbreaks talk less of unexpected large-scale epidemic as the most recent one in Western Africa. The unprecedented nature of the 2013-2016 Western Africa outbreaks has highlighted the need of implementing referenced health laboratory systems with established networks between laboratories within countries and even across continents in order to ensure rapid and early diagnosis, which is crucial step in outbreak control.
An Ebola diagnosis and research laboratory were implemented at Holy Spirit Hospital (HSH) Makeni during the last Ebola outbreak in Sierra Leone. A 50-meter square building was initially renovated, rehabilitated and equipped for early and rapid diagnosis of Ebola virus (EBOV) in suspected samples from patients visiting HSH or coming from surrounding EVD holding centres using molecular techniques. Later, two more units of 100 meters squares were added aiming at reinforces the capacity of HSH for better containment, management of EBOV suspected cases but also to ensure biological and immunological follow up of EVD survivors; explore other infectious diseases which may be present within local community.
Despite all the challenges, a high containment Laboratory was established at HSH for prompt management of epidemic due to highly pathogenic agent such as EBOV.
challenges, Ebola, epidemic, resource-limited-setting
Ebola virus disease (EVD) outbreak history reveals that epidemics mainly occurs in resource-limited settings [1]. Although previous Ebola virus outbreaks have enabled establishment of critical laboratory practice safeguards and diagnostic procedures for effective response [2]. Sub-Saharan Africa appears to be a theatre of most complex and deathly EVD outbreaks in the world. Commonly known to be recurrent in East and Central Africa, EVD epidemic is now officially part of Western Africa sub-region since March 2014 [1].
Western Africa outbreak presented unprecedented features with case fatality rate of approximately 70% [3]. Because it was the first time EVD outbreak appears in Western Africa. It has been so far highly challenging for WHO and many other international health partners to respond to an Ebola outbreak of such a magnitude, duration and intensity. According to WHO, more than 28 610 confirmed, probable, and suspected cases have been recorded with 11 308 deaths between December 2013 to March 2016. Sierra Leone recorded the highest burden of the epidemic with 14124 reported cases and 3956 confirmed deaths [4-6].
Weak healthcare systems, crucial lack of qualified human and infrastructural resources in the affected countries made the complexity of responding to this outbreak. From these features, we could understand that the challenges in such public health emergency highlight urgent need to establish well-structured health laboratory system with good network playing critical role in identifying the etiological agent causing outbreak and provides timely, accurate information required to guide control measures [7].
We developed “The Ebola Diagnosis and Research Laboratory initiative aiming at ensuring good laboratory service delivery during and after the outbreak while highlighting the major challenges.
Most often, in absence of public health emergency, the plate-form that will serve as laboratory no matter it level is tough and designed before in respect of international conventions or regulations. The preparedness process is observed in order to ensure effectiveness and reliability of the expected results. Once there is a sudden epidemic outbreak and the emergency state declared, the acting method usually is to use what is on hand now, making things more challenging especially in an environment where the health laboratory system and network is broken and sometime inexistent. This implies that teams working on the field must have very good ability to adapt and improvise. In such circumstances, existing buildings are often renovated for the purpose. It should be noted that despite this renovation, sometime there might still exist possible gaps between some major components such as the energy supply system of the renovated building and the quantity of energy needed by the equipment to function. This shortage can also be considered for water supply system, reagents and consumables supply chain. The sustainability of the structure could be highly tempered by these limitations. In a country like Sierra Leone with a very poor health laboratory system, which presents obvious shortage of qualified staff and basic supply at level 1 health laboratory, setting up a biosafety cabinet level 3 laboratory during an EVD epidemic outbreak, may not be an easy task. Carry out such a venture implies well-established features in order to be effective. Usually key logistic and financial requirement for intervention in emergency state are available. Even though we were able to notice that with EVD infection there is still a great concern in establishing a fluent supply chain for diagnostic reagents and some consumables. By then there was no FAD approved diagnostic test for EVD screening join to the fact that industrial firms have not yet developed a commercial interest in producing these tests. Among the major challenges encountered was identifying the skilled scientists with good biosafety knowledge ready to travel and manage laboratory activities. They have to overcome fear of contamination and stigmatization among their fellow. For example, it is said that once you have been in a country affected by EVD you are no more qualified as blood donor for five years and in some situation, you will be quarantined once back home. The other challenge was to overcome the negative influence of the mass media that relate the facts with some exaggeration most of the time. Despite all these, we understood that curiosity is also what make scientific venture.
Setting up molecular and immunology diagnostic laboratory
By November 2014, a specific preliminary mission was carried out for the identification of the Laboratory site, and the hospital organisation for handling Ebola-suspected and confirmed cases. Because of this mission, an independent building of about 50 meters square was renovated in Holy Spirit Hospital Makeni, Bombali District in the Northern region of Sierra Leone, to perform early diagnosis of EVD in suspected cases. Because of the invasive nature of this outbreak, we could glimpse that the ability of ensuring biosecurity and safety for staff during the operation will be a major challenge. Reason why we identified well-trained skilled scientists with advanced knowledge on managing biosafety level 3 cabinet laboratory, molecular and immunology techniques. We constituted a mixed team of both European and Africa scientists. Prior their trip to Sierra Leone, these scientists went through a one-month intensive training on how to handle highly pathogenic agents like Ebola virus. They were also though on how to behave in such a critic environment.
By January 2015, we arrived at the laboratory site. Few days later, all equipment, materials and reagents also arrived, and we proceeded to their installation and assessment. Upon validation of this assessment, the emergency phase in our setting was officially launched by February 2015. This phase consisted at using molecular techniques for rapid and early detection of EBOV in suspected cases from patients visiting HSH or from surrounding EVD holding centres.
We implemented the system for EVD diagnosis in suspected sample using molecular techniques in respect to the international standards. We properly designed the flow of the work at the Lab identifying each workstation to perform the analyses. All the standards operational procedures (SOPs) needed for sample processing and the manipulations were written and implemented as we moved forward with the analyses. Although the SOPs validation process was truncated under such circumstances due to time constraints.
Routine molecular diagnosis of EBOV in suspected cases
We used the Real Time Polymerase chain reaction (RT-PCR) technique for the detection of Ebola viral genetic material in the suspected sample. Manual ARN extraction was done after sample inactivation; we used reagents of a commercial Kit (PureLink® Viral RNA/DNA Kits, Invitrogen life technologies). EBOV genetic material was quantified using a commercial kit (RealStar® Ebolavirus RT-PCR Kit 1.0 of Altona Diagnostic), according to the manufacturer’s instructions. Internal control, positive control and PCR grade water all provides by the kit were used in each test run.
We settled in the laboratory site in the week to 25 January 2015; due to the fact that case incidence continues to rapidly decline in Sierra Leone as WHO reports revealed (WHO, 2015), we received only 16 suspected samples with an average of about 2 suspected samples per month from February to November 2015. Ebola viral genetic material was not detected in all the 16 samples. These samples were from patients visiting HSH or coming from surrounding EVD holding centres.
By September 2015 we started the enlargement phase of the laboratory as part of the post EBOV or EVD strategic plan. By November 2015, two more units of about 100 meters square were added: the immunology unit for immunology and serological analyses and the blood bank unit for blood supply in order to strengthen the capacity of HSH. As part of the post outbreak activities, we performed hepatitis B (HBV) and hepatitis C (HCV) makers’ detection on a set of well-defined samples in order to have a picture of these infections in our working environment. This was especially made to insert the serological diagnosis routine of HBV and HCV using ELISA at HSH since there was no provision for before.
Technology and knowledge transfer during an Ebola outbreak
As we mentioned earlier, lack of qualified human resources is an obstacle to good service delivery within health care facilities. Reports reveal that Sierra Leone is a country with high illiteracy rate [8,9]. Despite all the efforts the government and stakeholders have been putting together to rebuild the educational system that was also seriously destroyed by the civil war, the system is still very weak. Identifying skilled local health care workers with whom we could work during the emergency was another major challenge we faced. The country was facing the epidemic for the first time, health staff have never come across such an infection and they were unable discriminate between EVD symptoms and malaria or typhoid fever symptoms or any other infection. Because of the proximity with infectious people, many of them contracted the disease and die. Fear of contamination became a major concern to overcome because many healthcare staff lost their life while trying to save other lives.
In order to ensure knowledge and technology transfer in the due time, we trained local health staff to learn and gain experience to run all the molecular biology and immunology methodologies and tools used at the Lab. The Holy Spirit hospital administrative board nonetheless help us to identify the local health staff namely laboratory technicians with whom we worked. The inclusion criteria for the selection of trainees was to be a health worker with laboratory technician background. At day one of the training, that is during the first contact, about 20 laboratory technicians were presented to us. We did a baseline assessment related to basic knowledge on laboratory features. At the end of this assessment, we identified 12 health staff from three different institutions. That is 60% of the initial 20 lab technicians.
Training courses were scheduled into two phases over a period of six months: Three months theoretical and three months practical. We had three (3) hours class lecturing per day from Monday to Friday every morning. The majors’ axes of the courses were general information on operating biosafety level 3 cabinet laboratory, molecular biology and immunology methodologies for infectious disease diagnosis. We did two other major assessments along the training. The first was done just after the theoretical before going into the practical and the second at the end of the practical session. We defined by ourselves grade interval as follow: 0-5 is weak; 6-8 is good and 9-10 very good.
Practical training at laboratory was carried out to reach the basic technology and skills to operate in the High Containment Laboratory. In particular, the participants were trained to acquire the following practical knowledge:
Work in safety condition under the BSL3 Cabinet (glove-box);
Extract viral DNA/RNA from biological samples;
Amplify and quantify viral genetic materials for diagnostic purposes;
Measure serum antibodies for diagnostic and epidemiological purposes.
During practical training, all participants worked by themselves in teams of two, with a regular monitoring of respect of the procedures and reliability in the quality of the result generated by them. Only technical staffs succeeding this practical assessment were admitted for the regular laboratory activity. The other participants were enrolled according to their abilities in the other aspects of the laboratory chain like blood collection, field activities etc... Among the 12 health staffs we had throughout the training, 03 performed very good, 05 were good and 02 weak. We had 02 Lab technicians who stop the training along the way because overwhelmed with their work in their job site.
Laboratory analyses toward capacity building
We had to step directly into scientific and field research activities in order to achieve our goal because of the EVD incidence decline. We decided to carry out Epidemiological Survey and Serological Analyses among EVD Survivors and their community contacts. We aimed at understanding the diffusion or circulation of Ebola virus within local communities through antibodies detection. These activities enabled us to better capacitate the local lab technicians on laboratory service delivery in emergency state. Serology analyses were also performed using the Enzyme ImmunoSorbent Assay (ELISA) techniques (commercial kit AE320520-1, Alpha Diagnostic International [ADI], Texas, USA) to identify anti-EBOV antibodies among EVD survivors and their community contact (that is household contact of the survivors or household contact of EVD infected person who has died). Here also we followed the manufacturer instructions.
The preliminary data of this project showed that the overall circulation rate of EVD virus among asymptomatic Community Contacts in the area of Sanda Loko Chiefdom was about 11.4% [9]. All these activities were performed along with the routine activities of the laboratory.
Confirmation Serological analyses among EVD Survivors and their community contacts
Fear, anxiety, stress and instability of electricity supply (although not measurable) are factors that could have had a negative impact on the reliability of analyses performed during the emergency. Reason while we decided to confirm the result obtained at that time. We randomly selected samples for this purpose: 15 survivors (positive control group), 15 community contacts with anti-EBOV antibodies and 15 community contacts not presenting anti-EBOV antibodies. We first performed the detection of IgG against the EBOV nucleoprotein (NP) using the new set of a commercial kit for Enzyme-Linked Immunosorbent assay (AE320520-1, Alpha Diagnostic International [ADI], Texas, USA). All the analyses were done following the manufacturer instruction. Threshold index to discriminate positive and negative antibody reactivity to EBOV NP was calculated following manufacturer’s instructions.
Achievement 1:
- A high containment laboratory was implemented in HSH for early and rapid detection of Ebola virus genetic material in suspected samples using real time polymerase chain reaction (RT- PCR) technique.
- The capacity of HSH has been reinforced for better containment, management of EBOV suspected cases; also, to ensure biological and immunological follow up of EVD survivors, and to explore other infectious diseases like HBV and HCV.
Achievement 2:
- The experience we had enabled us to show that is possible to transfer knowledge even in critical event. We observed a significant evolution of performances among the local health staff we trained. About 30% of these trainees were readily skilled for laboratory management of suspected samples for EVD. (Figure 1).
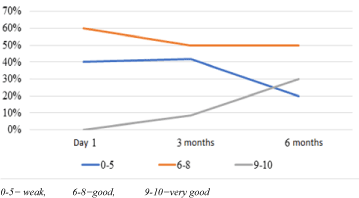
Figure 1. Evolution of performance of the trained local lab technicians.
Nonetheless, skilled worked force development remains a major concern in limited setting no matter the circumstances.
Achievement 3:
- We observed significant difference (p=0.001) between antibody index we had in April (Ab Index NP1) and those we had in November 2015 (Ab Index Np2). We noted that two samples among the 15 community contacts who presented anti-EBOV antibodies in April did not showed anti-EBOV antibodies in November (Figure 2 and Table 1).
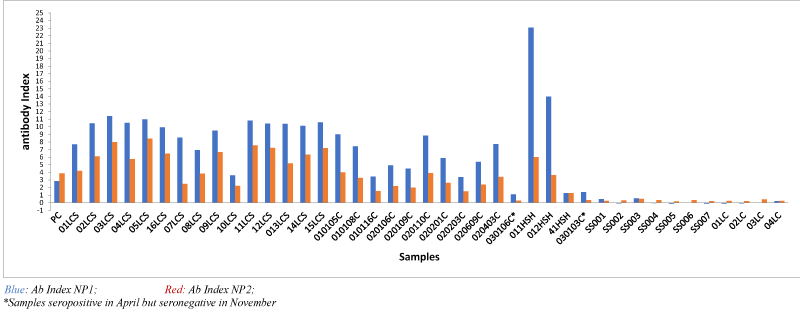
Figure 2. Comparison between Ab index Np1 and Ab index Np2.
Table 1: Comparison between Ab index Np1 and Ab index Np2
Variable |
Samples |
Minimum |
Maximum |
Mean |
Ecart-type |
Ab Index Np1 |
45 |
-0,132 |
23,074 |
5,950 |
5,217 |
Ab Index Np2 |
45 |
0,177 |
8,454 |
3,154 |
2,678 |
p=0.001
- We can’t assume that these differences are only due to fear and anxiety or stress but also the stability of the antibody proteins may have been tempered due to fluctuation in energy supply.
Limited settings capability to ensure effective response to highly infectious outbreak epidemics relies on major improvements in the laboratory healthcare system. Thus, addressing issue of quality infrastructures, limited availability of well trained and experienced staff, adequate supply chain management for logistics and availability of approved diagnostic tools represent crucial elements for preventing such outbreaks, as they have direct impact on epidemic control measures. Unlike Tara et al. [2] findings, our experience reveals an urgent need for a country like Sierra Leone, to revise its infrastructural and organizational reforms to ensure access, quality, safety and continuity of care across health conditions, across different locations and over time. These reforms will surely enhance prevention of similar tragic event.
We express our gratitude to the Italian Episcopal Conference (Camillian Task Force), the Italian Cooperation (Dokita ONG) and the Italian Geographical Society (Centro Relazioni con l’Africa).
View supplementary data
- Outbreaks Chronology: Ebola Virus Disease, accessed in April 2017. Available from: https://www.cdc.gov/vhf/ebola/outbreaks/history/chronology.html
- Sealy TK, Erickson BR, Taboy CH, Ströher U, Towner JS, et al. (2016) Laboratory Response to Ebola — West Africa and United States. MMWR Suppl 65: 44-49. [Crossref]
- WHO (2014) Affected Countries Ebola Virus Disease Outbreak Response Plan in West Africa Period: July – December 2014 launched by WHO on 31 July 2014.
- WHO (2014) Ebola response roadmap situation report, 17 December 2014. Available from: http://apps.who.int/iris/bitstream/10665/145679/1/roadmapsitrep_17Dec2014_eng.pdf
- WHO (2016) World Ebola Situation Report - 30 March 2016. Available from: http://apps.who.int/ebola/current-situation/ebola-situation-report-30-march-2016.
- United Nations Development Programme (UNDP) (2013) Human development report. The rise of the south: human progress in a diverse world. New York: UNDP; 2013. Available from: http://hdr.undp.org/sites/default/files/reports/14/hdr2013_ en_complete.pdf
- Chua KB, Gubler DJ (2013) Perspectives of public health laboratories in emerging infectious diseases. Emerg Microbes Infect 2: e37. [Crossref]
- About Sierra Leone, accessed 15 June 2017. Available from: http://www.sl.undp.org/content/sierraleone/en/home/countryinfo.html
- Mafopa NG, Russo G, Wadoum REG, Iwerima E, Batwala V, et al. (2017) Seroprevalence of Ebola Virus Infection in Bombali District, Sierra Leone. J Public Health Afr 8: 732. [Crossref]


