Breast cancer patients treated with cyclin-dependent kinase inhibitors may need concomitant antithrombotic drugs for therapy and prevention purposes. Optimal management of drug-drug interactions is necessary in this setting. We describe the case of a patient who developed a cerebrovascular event during the treatment with palbociclib. Consultation to guidelines and interdisciplinary involvement was used to avoid dangerous interactions with direct oral anticoagulants and statins.
apixaban, atorvastatin, breast cancer, cyclin-dependent kinase inhibitor, palbociclib, thromboembolic events
AUC: Area Under the Curve; Cmax: Maximal (peak) plasmatic concentration; CT: Computed tomography; CYP: Cytochrome P450; FDA: US Food and Drug Administration.
Palbociclib, ribociclib and abemaciclib are third generation cyclin-dependent kinase 4 and 6 inhibitors approved and widely used for the treatment of breast cancer with positive hormone receptors in combination with hormone therapy [1]. A substantial part of candidate patients for treatment with these drugs are elderly, and some of them have comorbidities or other factors that increase the risk of thromboembolic events. Systematic reviews and meta-analyses show an increased risk of thromboembolic events, including pulmonary embolisms [2] and venous thromboembolisms [3] in patients who received treatment with cyclin-dependent kinase inhibitors. This risk has been also described in studies of real-world practice [4]. There are multiple potential pharmacokinetic interactions between cyclin-dependent kinase inhibitors and several drugs used in the prevention and treatment of thromboembolic and cardiovascular events, including some anticoagulants, platelet inhibitors and lipid-lowering compounds [5].
We describe the case of a patient with metastatic breast cancer on treatment with palbociclib who needed therapy for preventing embolic and thrombotic complications and was the subject of multidisciplinary consultations between different medical specialties in order to optimize her drug therapy.
A 74-year lady had a medical history of hypertension, diabetes mellitus, Parkinson disease and atrial fibrillation. She was diagnosed with breast cancer in 2003, and treated with surgery, adjuvant chemotherapy and radiotherapy. Widespread bone metastases were diagnosed in September 2020, and she was started on therapy with letrozole (2,5 mg/d) plus palbociclib (125 mg/d for 3 weeks every 4 weeks). She was on treatment with apixaban for the prevention of embolic events from her atrial fibrillation. In January 2021 a stroke code was activated as she had a sudden weakness of the left arm and leg on wakening. During admission at the Stroke Unit there was a progressive improvement towards disappearance of the neurological focality. Imaging studies with angioCT scan, perfusion CT scan and cranial magnetic resonance did not reveal acute lesions. A point-of-care doppler ultrasound (Philips CX50) showed significant carotid stenosis (Figure 1). The diagnosis was of transient ischemic attack. Revascularization was discarded, and she continued anticoagulant therapy and started high doses of atorvastatin (80 mg/d). Palbociclib was temporarily suspended because of the significant interaction with atorvastatin, but it was resumed a few weeks later when an interdisciplinary evaluation between Neurology and Medical Oncology gave priority to palbociclib over atorvastatin at that time. Apixaban was also changed to edoxaban after an interdisciplinary evaluation between Hematology and Medical Oncology. In June 2021 she continues on treatment with palbociclib and edoxaban without new thrombotic, bleeding or cardiovascular events.
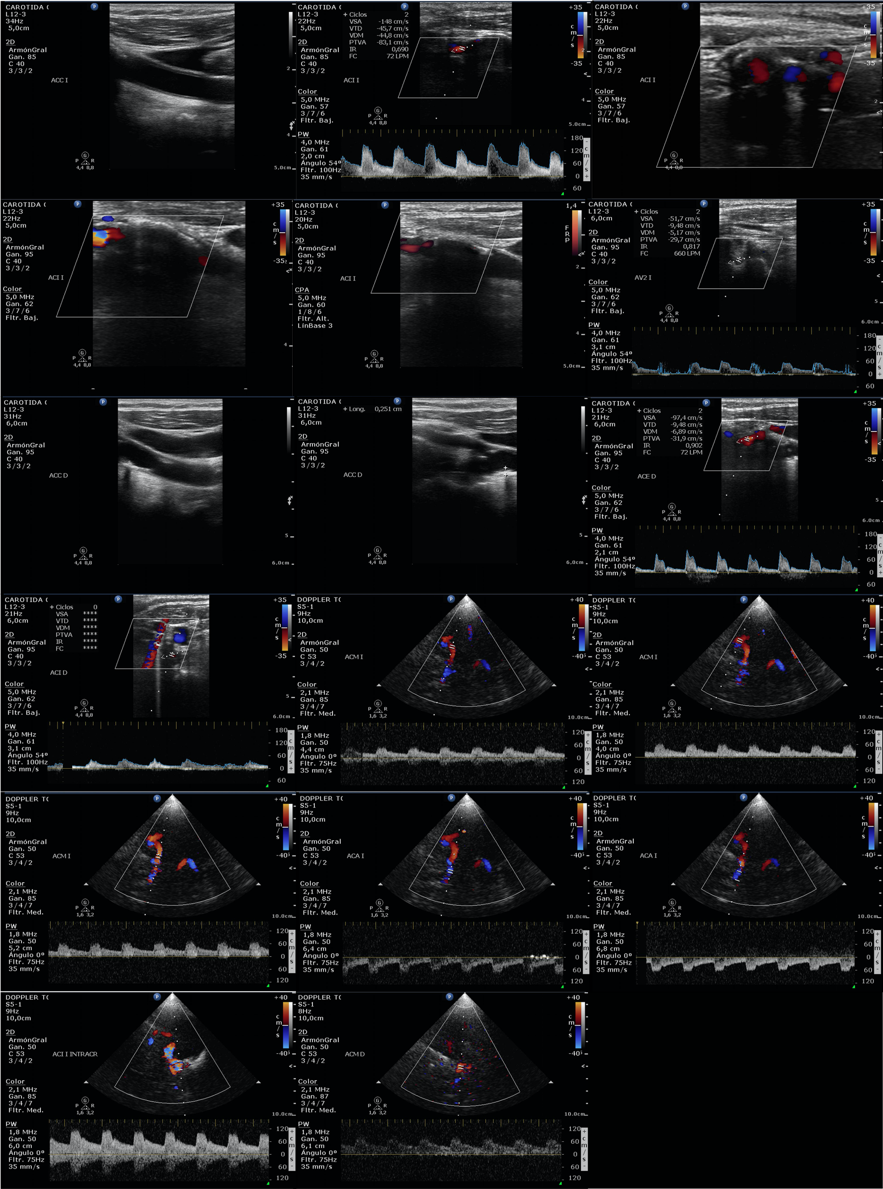
Figure 1. Point-of-care cervical and transcranial doppler ultrasound showing significant stenosis in the right and left internal carotid arteries
Direct oral anticoagulants like apixaban are increasingly used for the prevention and treatment of thromboembolic events in patients with cancer, and potential interactions with anticancer agents is among the concerning points of some of these drugs [6]. A recent review of the FDA Adverse Event Report System regarding thromboembolic events in patients on treatment with cyclin-dependent kinase inhibitors reported that more than one hundred of such patients were taking direct oral anticoagulants but was unable to distinguish if it was before and/or after the thromboembolic events [7]. 57 out of 424 patients treated with cyclin-dependent kinase inhibitors in a hospital series were already on treatment with anticoagulants (5 with direct oral anticoagulants) with none of them experiencing venous thromboembolic events [8].
Published case reports on the use of direct oral anticoagulants during the treatment with cyclin-dependent kinase inhibitors are scarce. One patient on palbociclib was started on rivaroxaban after a diagnosis of deep vein thrombosis [9]. She had no problems with the direct oral anticoagulant but developed a saddle-type pulmonary embolism two weeks later. A Consensus on the Management of Concomitant Medication in Patients on Treatment with Palbociclib or Ribociclib indicates that apixaban and rivaroxaban should be avoided in combination with such drugs [5]. Acenocoumarol, warfarin, argatroban and bivalirudin are considered safe options. Dabigatran and edoxaban display a minor statin interaction effect, and caution instead of avoidance is suggested by this consensus. A comprehensive review on the subject advices to avoid concomitant use of apixaban or rivaroxaban if ribociclib is used at 600 mg/d, and monitoring toxicity of direct oral anticoagulants when used with abemaciclib, palbociclib or a dose of 400 mg/d of ribociclib [10]. There is little information on the treatment of thromboembolic events in patients taking cyclin-dependent kinase inhibitors, but low molecular weight heparin was the most common treatment after venous thromboembolic events that developed during the treatment with abemaciclib in the pivotal MONARCH 2 and MONARCH 3 randomized trials, with no interruption of this drug or reduction in the dose [11]. As patient survival is relatively long in metastatic breast cancer patients treated with cyclin-dependent kinase inhibitors [1], some clinicians will consider to switch to direct oral anticoagulants in these patients.
The potential for interaction between cyclin-dependent kinase inhibitors and lipid-lowering statins is more frequent, since the use of these drugs is common. A real-world study from a longitudinal claims database described that more than 20% of an extensive series of patients treated with palbociclib were also on treatment with lipid-lowering agents [12]. An experimental study showed that standard doses of palbociclib during 2 months moderately increased the AUC and Cmax of atorvastatin (40 mg/d) and the metabolite atorvastatin lactone between 1.25 fold and 2-fold, with elevation in the risk of statin-induced muscular toxicity [13]. Obviously, a greater dose of atorvastatin increases the risk. The dose of atorvastatin used in our patient was 80 mg/d, according to the results of trials for reduction of vascular events [14]. Several cases of severe and sometimes fatal rhabdomyolisis and/or liver injury have been described in patients on concomitant treatment with some cyclin-dependent kinase inhibitors and atorvastatin or simvastatin [15-19, Table 1). A Consensus on the Management of Concomitant Medication in Patients on Treatment with Palbociclib or Ribociclib stated that simvastatin and atorvastatin should be avoided in patients on treatment with such drugs [5]. Pitavastatin is regarded as a safe option statin with low risk of interaction. Fluvastatin, pravastatin and rosuvastatin have a minor interaction effect with palbociclib and ribociclib and caution instead of avoidance is suggested by this consensus. A review by other authors includes abemaciclib with the same recommendations [20]. Nevertheless, there is a major difference since abemaciclib does not have a clinically meaningful effect on pharmacokinetics of CYP3A4 substrates (like statins) in patients with cancer [21]. This is recognized in the online cyclingtoool platform on pharmacokinetics and interactions of cyclin-dependent kinase inhibitors developed by the SOLTI group (www.cyclibtool.org) We must also consider that the risk of statin-related rhabdomyolysis is increased in elderly patients. The Young International Society of Geriatric Oncology gives a general recommendation to close monitoring of symptoms or dose reduction of concomitant sensitive substrates (as some statins) in patients on treatment with cyclin-dependent kinase inhibitors [22].
Table 1. Published severe adverse events (liver/muscle) with use of CDKI plus statins
CDKI: Cyclin-dependent kinase 4 and 6 inhibitors
The potential interaction between cyclin-dependent kinase inhibitors and some anticoagulants and lipid-lowering agents must be considered in terms of patient safety. There have been consensus developments on this subject with practical and useful information. An interdisciplinary approach is important in order to prioritize and optimize the use of concomitant drugs in this setting.
All the authors contributed equally to this manuscript.
JJI received honoraria from Pfizer for focus discussions.
The patient signed informed consent to participate in a study on cardiovascular complications in long-term survivors of breast cancer (ILL-CAR-2018-1) that was approved by the Ethical Committee of our region. She also gave authorization for the publication of these clinical findings.
- Ibrahim EM, Eldahna WM, Abulkhair O, Al-Mansour MM, Al-Foheidi MO, et al. (2018) CDK 4/6 inhibitors in hormone receptor-positive advanced breast cancer: A meta-analysis. Trends Cancer Res Chemother 1: 1-8
- Xie N, Qin T, Ren W, Yao H, Yu Y, et al. (2020) Efficacy and safety of cyclin-dependent kinases 4 and 6 inhibitors in HR+/HER2- advanced breast cancer. Cancer Manag Res 12: 4241-4250. [Crossref]
- Thein KZ, Htut TW, Ball S, Swarup S, Sultan A, et al. (2020) Venous thromboembolism risk in patients with hormone receptor-positive Her2-negative metastatic breast cancer treted with combined CDK4/6 inhibitors plus endocrine therapy alone: a systematic review and meta-analysis of randomized controlled trials. Breast Cancer Res Treat 183: 479-487. [Crossref]
- West MT, Smith CE, Kaempf A, Kohs TCL, Amirsoltani R, et al. (2021) CDK 4/6 inhibitors are associated with a high incidence of thrombotic events in women with breast cancer in real-world practice. Eur J Haematol 106: 634-642.
- Bellet M, Ahmad F, Villanueva R, Valdivia C, Palomino-Doza J, et al. (2019) Palbociclib and ribociclib in breast cancer: consensus workshop on the management of concomitant medication. Ther Adv Med Oncol 11: 1758835919833867. [Crossref]
- García-Escobar L, Brozos Vázquez E, Gutierrez Abad D, Martínez-Marín V, Pachón V, et al. (2021) Direct oral anticoagulants for the treatment and prevention of venous thromboembolism in patients with cancer: current evidence. Clin Transl Oncol 23: 1034-1046. [Crossref]
- Raschi E, Fusaroli M, Ardizzoni A, Poluzzi E, De Ponti F (2021) Thromboembolic events with cyclin-dependent kinase 4/6 inhibitors in the FDA Adverse Reporting System. Cancers (Basel) 13: 1758. [Crossref]
- Gervaso L, Montero AJ, Jia X, Khorana AA (2020) Venous thromboembolism in breast cancer patients receiving cyclin-dependent kinase inhibitors. J Thromb Haemost 18: 162-168. [Crossref]
- Levy O, Ptashkin E, Yitzhak S, Tamar K, Noam N, et al. (2019) Fatal palbociclib-related pneumonitis. Arch Clin Med Case Rep 3: 162-166.
- Gatti M, Raschi E, Poluzzi E, Martignani C, Salvagni S, et al. (2020) The complex management of atrial fibrillation and cancer in the COVID-19 era: Drug interactions, thromboembolic risk and proarrythmia. Curr Heart Fail Rep 17: 365-383. [Crossref]
- Rugo HS, Huober J, García-Saenz J, Masuda N, Sohn JH, et al. (2021) Management of abemaciclib-associated adverse events in patients with hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: Safety analysis of MONARCH 2 and MONARCH 3. Oncologist 26: e522. [Crossref]
- Beachler DC, De Luise C, Jamal-Allial A, Yin R, Taylor DH, et al. (2021) Real-world safety of palbociclib in breast cancer patients in the United States: a new user cohort study. BMC Cancer 21: 97. [Crossref]
- Li S, Yu Y, Jin Z, Dai Y, Lin H, et al. (2019) Prediction of pharmacokinetic drug-drug interactions causing atorvastatin-induced rhabdomyolysis using physiologically based pharmacokinetic modelling. Biomed Pharmacother 119: 109416. [Crossref]
- Szarek M, Amarenco P, Callahan A, et al. Atorvastatin reduces first and subsequent vascular events across vascular territories: The SPARCL Trial. J Am Coll Cardiol 75: 2110-2118. [Crossref]
- Gopalan PK, Villegas AG, Cao C, Pinder-Schenck M, Chiappori A, et al. (2018) CDK 4/6 inhibition stabilizes disease in patients with p16- null non-small lung cancer and is synergistic with mTOR inhibition. Oncotarget 9: 37352-37366. [Crossref]
- Nelson KL, Stenehjem D, Driscoll M, Gilcrease GW (2017) Fatal statin-induced rhabdomyolysis by possible interaction with palbociclib. Front Oncol 7: 150. [Crossref]
- Nersesjan V, Hansen K, Krag T, Duno M, Jeppesen TD (2019) Palbociclib in combination with simvastatin induce severe rhabdomyolysis: a case report. BMC Neurology 19: 247.
- Streicher C, Daulange A, Madranges N, Vayre L (2021) Severe rhabdomyolysis induced by possible drug-drug interaction between ribociclib and simvastatin. J Oncol Pharm Pract 27: 722-726. [Crossref]
- Atallah R, Parker NA, Hamouche K, Truong QV, Dingwall QV (2020) Palbociclib-induced liver failure. Kans J Med 13: 81-82. [Crossref]
- Roncato R, Angelini J, Pani A, et al. CDK 4/6 inhibitors in breast cancer treatment: Potential interactions with drug, gene, and pathophysiological conditions. Int J Mol Sci 21: 6350. [Crossref]
- Turner PK, Hall SD, Chapman SC, Rehmel JL, Royalty JE, et al. (2020) Abemaciclib does not have a clinically meaningful effect on pharmacokinetics of CYP1A2, CYP2C9, and CYP3A4 substrates in patients with cancer. Drug Metab Dispos 58: 796-803. [Crossref]
- Battisti NML, De Glas N, Sedrak MS, Loh KP, Liposits G, et al. (2018) Use of cyclin-dependent kinase 4/6 (CDK4/6) inhibitors in older patients with ER-positive HER2-negative breast cancer: Young International Society of Geriatric Oncology review paper. Ther Adv Med Oncol 10: 1758835918809610. [Crossref]

