Purpose: The objectives of this study were to determine the prevalence of osteoporosis before transplantation and its evolution over time, the predictive factors of bone changes and the effect of treatments.
Methods: This prospective, monocentric cohort study included patients who had undergone liver transplantation between 2006 and 2015. Patients were assessed by systematic rheumatologic evaluation before transplantation (V0), biological evaluations, radiographs of the thoracolumbar spine and bone densitometry by dual-energy X-ray absorptiometry. Patients were seen during rheumatology visits at 6 months (V1) and 3 years (V2) after transplantation.
Results: 251 patients were included at V0, 202 were seen at V1 and 112 at V2. The prevalence of osteoporosis before transplantation was 26%. Radiographic evaluations revealed the existence of a vertebral fracture in 57 patients. Anti-osteoporotic treatment was introduced in 34.3% of the patients at V0, 40.6% at V1. In all of the patients, bone mineral density (BMD) was found to be significantly lower at V1 compared to V0. When compared with patients who received no treatment, patients who were treated with bisphosphonates (BP) exhibited a significant increase in BMD at lumbar spine at V1 compared to V0 and at V2 compared to V1, and at femoral neck at V2 compared to V1. Patients who received zoledronic acid exhibited a higher gain in BMD at the spine at V2 compared to V1 than those who received an oral BP.
Conclusions: Patients who underwent liver transplantation exhibited bone loss both before and after transplantation. A significant improvement was found with BP treatment.
osteoporosis, bone densitometry, bisphosphonates, liver transplantation, cirrhosis
Cirrhosis of the liver is estimated to affect 0.1 % of the global population and, in Europe, some 5000 patients receive a liver transplant each year [1].
Patients with cirrhosis often exhibit bone fragility as a consequence of hepatic osteodystrophy (HO). In these patients, bone fragility is associated with an imbalance in bone homeostasis in favor of bone resorption leading to osteoporosis. The pathophysiological process is complex. Several mechanisms have been suggested, as summarized in studies conducted by López-Larramona, et al. [2] and Collier, et al. [3], and include decreased bone formation (genetic component, vitamin D deficiency and decreased IGF1), and activation of bone resorption (hypogonadism, lack of vitamin K absorption and modulation of osteoclast activity by the RANK ligand/osteoprotegerin pathway). Other factors may contribute to bone fragility in these patients. In a study conducted at Lille University Hospital, Wibaux, et al. [4] assessed the bone status of 99 patients awaiting liver transplantation between 2006 and 2007. In that study, factors that correlated negatively with bone mineral density (BMD) were liver disease severity score, pre-transplantation glucocorticoid treatment and bone resorption markers. Other factors have been frequently reported, such as the other usual risk factors for osteoporosis.
Furthermore, in a study on solid organ transplants in general, Yu, et al. [5] reported a significantly higher risk of osteoporosis in transplant patients – as assessed by dual-energy X-ray absorptiometry (DXA) with a Hazard Ratio of 5.14 – compared with the general population. The main risk factors for post-transplantation bone fragility include the therapeutic agents administered to post-transplant patients (immunosuppressive and glucocorticoid treatments) and the persistence of pre-transplantation risk factors. As such, bone status should be evaluated in candidates for liver transplantation.
After heart and lung transplants, liver transplants are in third position for the risk of osteoporosis, but the number of liver transplants performed each year is higher compared to heart and lung transplants. In 2017, the French Biomedicine Agency recorded 1,374 liver transplants in France, i.e. more than three times the number of heart and lung transplants [6]. Bone loss has been documented as early as 3 months after liver transplantation [7,8], and the resulting bone fragility is associated with higher fracture risk leading to higher mobidity, loss of autonomy and hospitalization-related complications. Despite these data, few studies have been carried out with a large number of patients and prolonged follow-up. Also therapeutic studies evaluated the effect of anti-osteoporotic treatment in transplant patients without selection according to their risk factor and often for a period of one year only. Finally, few data are available regarding the factors that may influence the evolution of bone mineral density apart from glucocorticosteroids. The main objectives of our study were
- To assess the prevalence of osteoporosis, and more generally bone fragility, in patients with liver diseases, the influence of the associated risk factors for bone fragility before transplantation,
- To assess the changes in BMD after transplantation (6 months and 36 months)
- To determine the effects of bisphosphonates on BMD changes 6 months and 36 months after transplantation and also between 6 months and 36 months after transplantation.
Study design
We conducted a prospective, observational, single-center study. It included a cohort of patients selected on the basis of data held by Lille University Hospital’s Department of Gastroenterology, which includes information regarding all patients who receive a liver transplant at the hospital. All of the patients included in the study gave their consent before inclusion. Data were gathered from consultation records from the hospital’s Gastroenterology and Rheumatology departments. Patients underwent an initial rheumatologic evaluation (visit V0) and attended follow-up visits at 6 months (visit V1) and 3 years (visit V2) after transplantation.
Population
The study population included male and female patients over 18 years of age who had received a liver transplant between January 1 2006 and December 31 2015. The initial inclusion date was the date of the rheumatologic evaluations that was proposed in 2006 in the prospective study conducted by Wibaux, et al. evaluating bone status in patients awaiting liver transplantation [4]. The closing date was set at the end of 2015 so that all patients could be evaluated at, at least, the first follow-up visit 6 months after transplantation.
Inclusion and exclusion criteria: The patients were required to undergo a systematic rheumatologic evaluation as part of the pre-transplantation panel assessment (see below). Patients whose first rheumatologic evaluation was conducted after they had undergone liver transplantation, as well as those who had not undergone a bone densitometry assessment before transplantation, were excluded.
- Collection of data
- Initial clinical evaluation (V0)
We collected data on the demographic characteristics of our patients, including gender, age and body mass index (BMI). We also collected data on the following osteoporosis risk factors:
- Smoking history;
- Alcohol consumption (patients must have been abstinent for least 6 months before the transplant);
- History of chronic inflammatory rheumatism, chronic renal insufficiency, thyroid disease and, for women, menopausal status and early menopause (before 40 years old);
- Significant glucocorticoid therapy (above 7.5 mg per day for at least 3 months);
- History of osteoporosis or femoral neck fracture in patients and relatives;
- Personal history of non-traumatic fractures;
- Estimation of daily intake of dietary calcium using the Fardellone self-questionnaire [9];
- Physical activity score on a scale of four, from low to sustained activity.
To assess history of liver disease, we analyzed the etiologies of chronic liver diseases and liver disease severity criteria using Model for End-stage Liver Disease (MELD) and CHILD-PUGH scores at the time of the bone status evaluation. When these scores were not available, we calculated them ourselves using available values. MELD scores were calculated as X (ranging from 6 to 40) = 3.78 x ln (bilirubinemia [mg/dl]) + 11.2 x ln (INR) + 9.57 x ln (creatinine [mg/dl]) + 6.43. CHILD-PUGH scores were calculated from bilirubinemia, albuminemia, TP and presence of encephalopathy and ascites values and ranged from 5 to 15 points in three classes of increasing severity (A, B or C).
Bone mineral density (BMD) was measured using dual-energy X-ray absorptiometry. Measurements were made at the lumbar spine, total hip and femoral neck using HOLOGIC machine (HOLOGIC Discovery, HOLOGIC Inc., Waltham, MA, USA).
Plain anteroposterior and lateral radiographs of the thoracolumbar spine were performed for the purpose of diagnosing vertebral fractures. Radiographs were read by both a rheumatologist and a radiologist from the Department of Osteo-Articular Imaging and, in difficult cases, the diagnosis of vertebral fracture was obtained by consensus. Vertebral fractures were defined according to the Genant classification [10] as a loss in vertebral body height of at least 20-25%.
Patients underwent biological blood and urine tests at baseline, including:
- Serum levels of calcium and ionized calcium, phosphorus, 25(OH)D3 and 1,25(OH)2D3, parathyroid hormone, 24-hour urinary calcium, and kidney function;
At the end of this thorough assessment, a “bone strategy” was devised based on the BMD results, the presence of vertebral fracture or a past history of non-vertebral fracture, and the presence of osteoporosis related risk factors. Thus, the patients could benefit from calcium and vitamin D supplementation and anti-osteoporosis treatment. The proposed drugs were bisphosphonates (alendronate, risedronate, zoledronic acid), or teriparatide. The indication for and use of the anti-osteoporosis treatment were based on the referring physician’s expert opinion and was based on French guidelines regarding post-menopausal osteoporosis and corticosteroid-induced osteoporosis
- Clinical evaluation at visits V1 and V2 after transplantation
The patients were reviewed twice on an outpatient basis. The first evaluation (V1) took place 6 months after transplantation. The second evaluation (V2) was proposed 3 years after transplantation. Both evaluations were identical in their content.
At each visit, added risk factors for osteoporosis, immunosuppressive treatments and duration of glucocorticoid therapy were checked. We made sure that the bone treatments proposed at V0 were well established and correctly followed by interviewing patients. The patients then underwent a new BMD assessment. Biological assessment was performed at baseline only and was not repeated thereafter.
At the end of the visits, an adapted form of the treatment was proposed.
Regarding the anti-osteoporosis treatment, several courses of action were possible:
- The treatment was continued, modified (by replacing the anti-osteoporosis drug with another) or introduced (either because it had not been proposed after V0 or because patients had not started the drug even though it had been prescribed).
- No treatment was introduced, either because no treatment was indicated, or all were contraindicated.
Statistical analysis
The Chi-squared test or the exact Fisher test was used to compare qualitative parameters. The unpaired Student’s t test was used to compare continuous parameters when the Gaussian assumption was verified, and the Mann-Whitney U test was used to compare non-Gaussian continuous parameters.
The changes in BMD between V1 and V0 and between V2 and V0 were analyzed using mixed models, with “time in class” as the fixed effect. An auto-correlated error type compound symmetry was added to take into account individual short-term variations in BMD. Average BMD values were compared at each time using the generalized least squares technique with Dunnett’s correction.
Factors associated with changes in BMD (between V0 and V1 and between V0 and V2) were assessed by linear regression analyses for quantitative variables. The changes in BMD between V1 and V0 and between V2 and V0 were the variables to be explained. The associated factors to be tested were explanatory variables. Results were adjusted for confounding factors and particularly BMD at V0.
Changes in BMD between V1 and V0 and between V2 and V0 according to the use (or not) of a treatment targeting BMD or the characteristics of the population at V0 (reason for transplant, gender, use of cyclosporine) were compared using Student’s t-test for unpaired data after adjustments for confounding factors.
P values < 0.05 were considered significant. Statistical analyses were done using SAS software (SAS Institute version 9.4).
Descriptive analysis
Of the 507 patients initially selected (Figure 1), 79 were not known in the Department of Rheumatology and 25 were registered twice because they had a second transplant within the timeframe of the study. As such, only 403 patients were eligible for inclusion. Of those, 152 patients did not undergo a rheumatologic evaluation before their liver transplant, mainly because of the severity of the disease and the urgency of the transplant. Consequently, 251 patients were included in our study. The study was approved by the CNIL (Commission Nationale de l’Informatique et des Libertés).
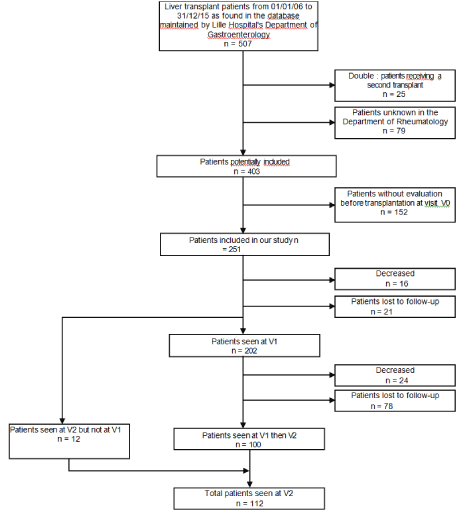
Figure 1. Flow chart
Baseline evaluation
Characteristics of the patients: 251 patients were included at V0, of which 189 (75.3 %) were men. The average age was 54.9 ± 8.8 years. The average BMI was 26.3 ± 4.4 kg/m2. The patients’ hepatitis severity scores were moderately high: the average MELD score was 13.9 ± 6.6, and the average CHILD-PUGH score was 7.8 ±2.4 or stage B.
The main reasons for transplantation were alcoholic cirrhosis (n=178, 70.9 %), hepatocellular carcinoma (n=131, 52.2 %), hepatitis C (n=36, 14.3 %) and primary or secondary biliary pathology (n=20, 8 %). Several patients had several reasons for transplantation, and this explains why the percentage is higher than 100%. In particular, patients with hepatocellular carcinoma also had alcoholic cirrhosis in 41.4 % of the cases (n=104).
The main risk factors for osteoporosis were menopause in 71% of the female patients (n=44), excessive alcohol consumption before transplantation (n=182, 72.5%), smoking history (n=125, 49.8%), low physical activity (n=232, 92.8%) and calcium intake lower than 1000 mg/day (n=134, 53.4%). 34 patients (13.6%) had received glucocorticoid therapy prior to the liver transplant. In 64.7% of the patients, treatment was prescribed for a hepatopathic condition (autoimmune or acute alcoholic hepatitis). Other cases were pulmonary disease and auto-immune diseases (hematologic, articular, cutaneous, digestive and renal).
Very few patients (n=5) had already been diagnosed with and treated for osteoporosis.
The main characteristics of the patients, including biological assessment results, are shown in Table 1.
Table 1. Characteristics of patients at inclusion (n=251)
|
Results |
Standard Deviation |
% |
Demographic characteristics: |
Men / Women (n) |
189/62 |
|
75.3/24.7 |
Age at transplant (years) |
54.9 |
± 8.8 |
|
BMI (kg/cm2) |
26.3 |
± 4.4 |
|
Risk factors for osteoporosis: |
Excessive alcohol use (n) |
182 |
|
72.5 |
Smoking (n)
|
125
81
44 |
|
49.8
32.3
17.5 |
CKD (n) |
28 |
|
11.2 |
hyperthyroidism (n) |
7 |
|
2.8 |
IRD (n) |
1 |
|
0.4 |
Prolonged immobilization (n) |
8 |
|
3.2 |
Low physical activity (n) |
232 |
|
92.8 |
Fractures (n)
|
119
87
32 |
|
47.4
34.7
12.8 |
Family femoral neck fractures (n) |
16 |
|
6.4 |
Treated osteoporosis (n) |
5 |
|
2 |
Menopause (n)
|
44
6 |
|
71
9.7 |
ERT (n) |
3 |
|
4.8 |
Glucocorticoid therapy (n) |
34 |
|
13.6 |
Calcium intake (mg/d) |
884.6 |
± 374 |
|
Biological data at baseline |
Normal range |
Average |
Median |
Standard Deviation |
25(OH)D3 (ng/ml) >30 |
13.7 |
11 |
± 9.7 |
1.25(OH)2D3 (pg/ml) 20-50 |
34.8 |
29 |
± 23.1 |
PTH (pg/ml) 15-68 |
50.3 |
37 |
± 50.6 |
Calcium (mg/l) 95-110 |
90.5 |
90 |
± 5.6 |
Phosphorus (mg/l) 30-45 |
34.7 |
34 |
± 7.2 |
Creatinine (mg/l) 5-10 |
11.5 |
9 |
± 11.9 |
Urinary calcium (mg/24h) 150-400 |
129.6 |
100 |
± 128.4 |
BMI: Body Mass Index; CKD: Chronic Kidney Disease; IRD: Inflammatory Rheumatic Diseases; ERT: Estrogen Replacement Therapy; PTH: Parathyroid Hormone.
Bone fragility: The descriptive analysis showed a reduction in bone mineral density (less than -1 SD of T-score at the hip or spine at V0) in 79% (n=198) of the patients. 26% (n=66) of the patients assessed at baseline had osteoporosis as determined by DXA.
Fracture history-related low trauma fractures were found in 12.8% of the patients (n=32). The total number of fractures observed was 41 (6 vertebral fractures, 1 pelvic fracture and 34 peripheral fractures, including wrist, leg, ankle, metacarpal, ribs, shoulder and femoral neck). 6 patients had a history of multiple fractures, with a maximum of 5 fractures per patient. Standard thoracolumbar spine X ray examinations revealed at least one vertebral fracture in 57 patients (22.7%). In those patients in whom at least one vertebral fracture was found during the X-ray evaluation, 32 (56.1%) had osteoporosis at at least one site, as determined by DXA scan
Treatments by bisphosphonates and vitamin D: At V0, osteoporosis therapy was initiated in 86 (34.3 %) of the 251 patients. The treatments of choice were alendronate (n=55, 64%) and zoledronic acid (n=18, 22%) (Table 2). Vitamin D supplementation was proposed to 86.9 % of the patients according to vitamin D status assessed at baseline (n=218).
Table 2. Osteoporosis treatment during follow-up: newly and continuation treatment
|
V0
n=251 |
% |
V1
n=202 |
% |
V2
n=112 |
% |
Total |
86 |
34.3 |
82 |
40.6 |
46 |
41.1 |
BP: Alendronate |
55 |
64 |
46 |
56.1 |
28 |
60.9 |
Risedronate |
12 |
13 |
20 |
24.4 |
8 |
17.4 |
Zol Ac. |
18 |
22 |
15 |
18.3 |
10 |
21.7 |
Teriparatide |
1 |
1 |
1 |
1.2 |
0 |
0 |
BP: Bisphosphonates; Zol Ac: Zoledronic Acid.
Therapeutics
Post-transplantation treatment: All the patients received glucocorticoids following the transplant: intravenous bolus of 500mg of solumedrol on the day and the day after the transplant, then 20mg/day of prednisone until liver parameters normalized, then a progressive decrease of 5 mg/week until cessation. In case of autoimmune disease, corticosteroid therapy was maintained between 5 and 10 mg/day. The average duration of treatment was 8.2 ± 7.8 months. After the transplant (V1), 213 patients (84.8 %) received glucocorticoid treatment. Glucocorticoid treatment was continued in 11.7% of the patients after V2.
Immunosuppressive therapy at V1 included mycophenolate mofetil (MMF) in 74.5% of the cases (n=150 out of 202 patients), cyclosporine in 46.6% of the cases (n=94) and tacrolimus in 32% of the cases (n=65). All of the patients received a double-agent therapy. The main combination was MMF + cyclosporine in 54.5% (n=110) of the patients, followed by MMF + tacrolimus in 33.2% (n=67) of the patients.
At V2, out of 112 patients, 75% were still being treated with MMF (n=84), 42% with cyclosporine (n=47) and 28.6% with tacrolimus (n=32). Other immunosuppressive drugs were rare. The main combination was still MMF + cyclosporine in 42% (n=47) of the patients, followed by MMF + tacrolimus in 28.6% (n=32) and MMF + everolimus in 17.9% (n=20).
Treatments by bisphosphonates and vitamin D: At V1, 82/202 patients (40.6 %) were treated or were advised to begin a treatment by bisphosphonates. The treatment of choice was alendronate (n=46, 56.1%). A new prescription was initiated at V1 for 22 patients. The reasons of this new prescription were: significant bone loss between V1 and V0 associated with low BMD at V1, occurrence of fracture or ongoing glucocorticoid therapy. The treatments initiated at V1 were: alendronate (n=1), risedronate (n=15) and zoledronic acid (n=6) (Table 2)
At V2, 46 of the 82 patients requiring anti-osteoporosis treatment at V1 had followed this recommendation: alendronate (n=28), risedronate (n=8) and zoledronic acid (n=10)
Bone fragility
Changes in BMD in the overall population: During the follow-up, a decrease in bone mineral density (osteopenia or osteoporosis) was observed as follows: 53% (n=107) at V1 and 55% (n=62) at V2 for osteopenia, 29% (n=59) at V1 and 27% (n=30) at V2 for densitometric osteoporosis (Table 3).
Table 3. Bone mineral density values at V0, V1 and V2
|
V0
Hip |
Neck |
Spine |
V1
Hip |
Neck |
Spine |
V2
Hip |
Neck |
Spine |
BMD (g/cm2) |
0.91 |
0.76 |
0.96 |
0.88 |
0.72 |
0.95 |
0.88 |
0.73 |
0.99 |
|
± 0.2 |
± 0.1 |
± 0.9 |
± 0.2 |
± 0.1 |
± 0.2 |
± 0.2 |
± 0.1 |
± 0.2 |
BMD T-score (ds) |
-0.87 |
-1.54 |
-1.12 |
-1.07 |
-1.72 |
-1.18 |
-0.98 |
-1.61 |
-0.85 |
|
± 1.1 |
± 1.1 |
± 1.5 |
± 1 |
± 1.1 |
± 1.4 |
± 1.1 |
± 1.1 |
± 1.4 |
At total hip a decrease in BMD was observed between V0 and V1 (delta = 0.033 ± 0.005 g/cm2 or -3.63 ± 0.55%, p < 0.001). BMD increases between V1 and V2: delta = + 0.0429 ± 0.006 g/cm2 or 4.88 ± 0.68% (p<0.0001). Between V0 and V1, BMD at femoral neck decreased by 0.0366 ± 0.005 g/cm2 or -4.82 ± 0.66% (p <0.0001), and increased by + 0.0154 ± 0.006 g/cm2 or 2.14 ±0.83% (p=0.0224) between V1 and V2.
At lumbar spine BMD did not change between V0 and V1 (p = 0.476). Between V1 and V2, BMD increased at lumbar spine by + 0.0649 ± 0.007 g/cm2 or 6.83 ±0.74% (p<0.0001). At lumbar site, the difference in BMD between V0 and V2 was significant: between the 2 visits, BMD increased by + 0.0706 ± 0.007 g/cm2 or 7.35 ± 0.73% (p<0.0001). At total hip, the difference in BMD between V0 and V2 was not significant (p=0.1849). At femoral neck, between V0 and V2, BMD decreased by + 0.0212 ± 0.006 g/cm2 or -2.79 ± 0.79% (p=0.0008).
Vertebral and non-vertebral fractures in the overall population: At V1, 6 months after transplantation, new symptomatic vertebral fragility fractures were found in 13 of the 202 patients (6.44%). Of those 13 patients, 7 (53.8%) had been receiving an anti-osteoporosis treatment that was introduced at V0. Overall, 37 vertebral fractures were found. Moreover, 4 patients had new peripheral fractures. The sites of those fractures were the upper extremity of the humerus (1 patient), the wrist (2 patients) and the ankle (1 patient).
At V2, 3 years after transplantation, new symptomatic vertebral fragility fractures were found in 3 of the 112 patients (2.68%). Of those 3 patients, 2 (66.7%) had been receiving an anti-osteoporosis treatment that was introduced at V0. The third patient presented a theoretical indication for treatment, but treatment was not started due to severe renal impairment. Overall, 6 fractures were found. Two patients had new vertebral fractures at V1 and at V2. There were no new peripheral fractures (between V1 and V2).
In the overall study population
Factors influencing changes in BMD between V0 and V1 (Table 4):
Table 4. Independent factors associated with changes in BMD
|
r value |
P value |
Between V0 and V1: |
At femoral neck: |
|
|
BMI |
0.36 |
<0.001 |
Biliary disease (yes/no) |
NA |
<0.001 |
MELD |
0.31 |
0.002 |
CHILD |
0.35 |
<0.001 |
Glucocorticosteroid duration |
0.38 |
<0.001 |
At lumbar spine: |
|
|
Transplant reason: alcohol (yes/no) |
NA |
0.01 |
CHILD |
-0.27 |
0.008 |
Treatment: zoledronic acid V0 (yes/no) |
NA |
0.003 |
Between V0 and V2: |
At femoral neck: |
IS treatment: Cyclosporine (yes/no) |
NA |
0.001 |
At total hip: |
|
|
Gender (male/female) |
NA |
002 |
IS treatment: Cyclosporine |
0.40 |
0.0006 |
At lumbar spine: |
CHILD |
0.38 |
0.002 |
VF radiograph V0 (yes/no) |
NA |
0.009 |
BMI: Body Mass Index; MELD and CHILD: Liver disease severity scores; IS: Immunosuppressant; VF: Vertebral fractures; r represents the coefficient of correlation.
At femoral neck, lower BMIs at baseline and post-transplantation glucocorticoid therapy were associated with loss of BMD (p<0.01 for both).
Changes in BMD correlated with hepatitis severity scores at femoral neck and lumbar spine: higher MELD and CHILD scores were associated with greater decreases in BMD (p=0.002 and p<0.001 respectively).
Where reason for transplantation was concerned, "biliary pathology" for the neck and "alcohol" for the spine, were associated with greater changes in BMD compared to the other reasons for transplantation (p<0.001 and p=0.01 respectively).
The introduction of anti-osteoporosis treatment using zoledronic acid alone was associated with a lesser reduction in BMD at the spine compared to bisphosphonates administered by oral route (p=0.003).
None of the results at total hip were significant.
Factors influencing changes in BMD between V0 and V2:
At total hip and femoral neck, the introduction of immunosuppressive therapy using cyclosporine was associated with greater bone loss compared to other immunosuppressive drugs (p=0.0060 and p=0.001 respectively).
At lumbar spine, high CHILD scores and the presence of vertebral fractures at V0 were associated with a significant reduction in BMD (p=0.02 and p=0.009 respectively). Male sex was also associated with significant decreases in BMD at total hip compared to female sex (p=0.02). No other factors were associated with changes in BMD between V0 and V2 (Table 4).
Effect of treatment by bisphosphonates: comparison of patients with and without anti-osteoporosis therapy:
Between V0 and V1, the use of bisphosphonates was associated with lesser loss of BMD at lumbar spine compared to no therapy (difference=0.021 ± 0.06 g/cm2, 2.11 ± 6.33%, p=0.04). Between V1 and V2, BMD gains at the spine were significantly higher in treated patients (difference=0.049 ± 0.05 g/cm2, 3.1 ± 3.16%, p=0.001). The findings were the same at femoral neck (difference=0.038 ± 0.13 g/cm2, 0.5 ± 0.09%, p<0.0001) (Table 5).
Table 5. Changes in BMD with or without (“not”) osteoporosis treatment
|
V0-V1
Treated
(n=82) |
P |
Not
(n=120) |
P |
Delta Treated / Not |
P |
V1-V2
Treated
(n=46) |
P |
Not
(n=66) |
P |
Delta Treated / Not |
P |
Hip
(g/cm2) |
-0.030 ± 0.13 |
<0.0001 |
-0.035 ± 0.13 |
<0.0001 |
0.065 ± 0.004 |
0.556 |
0.058 ± 0.12 |
<0.0001 |
0.033 ± 0.15 |
0.018 |
0.025 ± 0.10 |
0.110 |
Neck
(g/cm2) |
-0.025 ± 0.10 |
0.001 |
-0.043 ± 0.12 |
<0.0001 |
0.018 ± 0.06 |
0.066 |
0.036 ± 0.10 |
<0.0001 |
-0.002 ± 0.13 |
0.847 |
0.038 ± 0.13
0.5% |
<0.0001 |
Spine
(g/cm2) |
0.009 ± 0.14 |
0.369 |
-0.012 ± 0.14 |
0.026 |
0.021 ± 0.006
2.11% |
0.043 |
0.087 ± 0.14 |
<0.0001 |
0.038 ± 0.14 |
<0.0001 |
0.049 ± 0.05
3.1% |
0.001 |
Regarding bisphosphonates, we noted a greater improvement in BMD between V0 and V1 in patients treated with zoledronic acid compared to oral bisphosphonates. The difference was significant only at the spine (0.054 ± 0.07 g/cm2, p=0.024) (Table 6).
Table 6. Comparison of changes in BMD according to the route of administration of treatment
|
V0-V1
BP OR
(n=66) |
P value |
BP IF
(n=15) |
P value |
Delta |
P |
V1-V2
BP OR
(n=36) |
P value |
BP IF
(n=10) |
P value |
Delta |
P |
Hip
(g/cm2) |
-0.033 ± 0.13 |
<0.0001 |
-0.023 ± 0.12 |
0.310 |
0.01 ± 0.02 |
0.43 |
0.058 ± 0.12 |
<0.0001 |
0.052 ±0.15 |
0.070 |
-0.006 ± 0.01 |
0.22 |
Neck
(g/cm2) |
-0.023 ± 0.09 |
0.005 |
-0.035 ± 0.09 |
0.053 |
0.058 ± 0.08 |
0.08 |
0.039 ± 0.10 |
0.001 |
0.011 ± 0.13 |
0.541 |
-0.028 ± 0.05 |
0.071 |
Spine
(g/cm2) |
-0.004 ± 0.15 |
0.686 |
0.05 ± 0.12 |
0.071 |
0.054 ± 0.07 |
0.024 |
0.084 ± 0.15 |
<0.0001 |
0.115 ± 0.14 |
0.006 |
0.031 ± 0.03 |
0.085 |
OR: oral route; IF: infusion.
In our study, the prevalence of osteoporosis according to WHO criteria was 26% among patients awaiting liver transplantation. At 6 months and 3 years post-transplantation, the prevalence in our population was 29% and 27% respectively. Analyses of changes in BMD revealed an initial bone loss at 6 month post-transplantation and an increase in BMD between 6 month and 3 years post-transplantation.
In the reviews conducted by Collier and al. in 2002 [6] (and updated in 2007 [3]), and more recently by Lan, et al. [11], the prevalence of osteoporosis in patients with cirrhosis was reported to vary between 12 and 55%. Our results fall in the lower part of this range. As was the case in the study conducted by Wibaux et al. [4], a reduction in BMD was found in more than 70% of the patients awaiting transplantation, and the prevalence of osteoporotic fractures was a little higher (35.5%). The differences could possibly be explained by the fact that patients now receive transplants more quickly and earlier in the course of liver disease.
The BMD change profiles reported in this study were comparable to those reported by Monegal et al. [8],[8] i.e., a major loss in the first 6 months post-transplantation, followed by an increase trending towards baseline values. This was histologically demonstrated using bone biopsies taken just after transplantation and 6 months later. The authors reported an uncoupling of bone resorption and bone formation, with the isolated activation of bone formation at 6 months. Hamburg et al. [12] followed up 66 patients receiving no treatment for up to 15 years post-transplantation. Over the first 2 years, the results are practically the same as ours. Thus, for Hamburg et al. [13], over a prolonged follow-up, and despite the low number of patients, BMD at the spine stabilized but decreased slowly at the hip. We should probably pay particular attention to the values measured at the hip.
Regarding risk factors for osteoporosis during the follow-up: Six months after transplantation, we found several factors that influence changes in BMD, including BMI, MELD and CHILD liver disease severity scores, biliary disease (for femoral neck BMD) and alcoholic cirrhosis (for lumbar spine BMD). Guichelaar et al. [13] also reported an association between low BMI and BMD loss after transplantation. Monegal et al. [8] reported an association between a reduction in BMD and a high CHILD score. We found no data in the literature on post-transplantation MELD scores. Cirrhosis stage at the time of transplantation is therefore crucial before the transplant and then for bone monitoring. In the study conducted by Hamburg, et al. [12] regarding the influence of biliary disease, the authors reported a decrease in Z-score at spine, total hip and femoral neck associated with the presence of cholestasis. Bjoro, et al. [14] found the same association when patients were compared with a control group. Our results were similar only at femoral neck. In the meta-analysis conducted by Bang, et al. [15] regarding the influence of alcoholic cirrhosis, the authors reported that the relative risks of vertebral fractures and osteoporosis were higher in patients with alcoholic cirrhosis. In our study, alcoholic cirrhosis was associated with a decrease in BMD at lumbar spine. Furthermore, alcohol is often associated with under-nutrition, hypogonadism and low vitamin D level, which could also explained the findings.
Regarding post-transplantation therapy, we found an association between glucocorticoid therapy or immunosuppressive treatment using cyclosporine and acute bone loss.
In fact, the introduction of cyclosporine was associated with significant bone loss at total hip and femoral neck. Monegal, et al. [16] studied the effect of cyclosporine on 18 transplanted patients. The decrease in femoral neck BMD was greater than with tacrolimus, but these patients also received a higher cumulative dose of glucocorticoid and for longer durations. In our study, the concomitant use of glucocorticoid treatment did not explain these results. Indeed, in the patients receiving cyclosporine, the average duration of glucocorticoid therapy was 5.76 ± 6.9 months, i.e., lower than the average for our general population. In basic research, cyclosporine, which is a calcineurin inhibitor, causes a decrease in osteoclastogenesis [17]. In clinical trials, the results are often discordant, but the studies were conducted for kidney transplants. Besides, Yeo, et al. [18] attribute an anabolic or catabolic role to calcineurin inhibitor according to the dose used (dose<1micromol/l in vitro, or <35 nanomol/l in vivo) .
In our study, bone loss was also associated with prolonged glucocorticoid therapy. In a study conducted by Guichelaar, et al. [14], the authors found that the shorter the period of glucocorticoid therapy, the more the increase in post-transplantation BMD was significant. Canalis, et al. [19] reported that fracture risk was related to the dose and duration of glucocorticoid therapy and decreased after treatment was discontinued. In our study, only 11.7% of the patients received long-term glucocorticoid therapy after their transplant. This probably limited its adverse effect on bone.
In our study, the introduction of osteoporosis therapy is associated with a maintain of BMD at lumbar spine in the first 6 months. At femoral neck osteoporosis treatment slowed bone loss at 6 months. BMD increases thereafter at all the sites for patients who were receiving osteoporosis treatment. Although we did not calculate a priori the number of patients necessary to assess an effect of bisphosphonates for preventing bone loss in this usual care study, our results appear relevant. In the meta-analysis conducted by Katsuri, et al. [20], the authors reported that bisphosphonates, without distinction, significantly improved lumbar spine BMD by 0.03 g/cm2 12 months after the transplant compared to the untreated control group. Our results are consistent with this finding.
Zoledronic acid slowed down the decrease in lumbar spine BMD compared with oral therapy. Our results are consistent with the literature. In a study conducted by Crawford, et al. [21], 62 liver-transplant patients received, in a double-blind protocol, either zoledronic acid infusions of 4 mg (n = 32), or saline (n = 30) given within 7 days of transplantation and again at 1, 3, 6 and 9 months after transplantation. All patients received supplementation with calcium carbonate (600 mg/d) and ergocalciferol (1000 U/d). Compared to the untreated patients, the treated patients showed a significant improvement in lumbar spine, hip and femoral neck BMD of about 4% 3 months after transplantation, and 2% 1 year after transplantation. As with previous findings, Bodingbauer, et al. [22], in a study involving 96 patients, reported an improvement in femoral neck BMD at 6 months (8 infusions of 4mg zoledronic acid during the first 12 months after liver transplantation, calcium (1000 mg/d) and vitamin D (800 IU/d)). Retrospective studies have also reported an improvement in BMD in post-liver-transplant patients treated with zoledronic acid compared to alendronate [23]. The results of these various studies have been summarized in the meta-analysis of Athanasios, et al. [24]. But owing to the lack of head-to-head comparative studies, we cannot recommended one bisphosphonate over the others.
In the presence of osteoporosis diagnosed on the basis of BMD values measured by DXA, in particular between 6 months and 3 years after transplantation, we found an improvement in BMD in more than 80% of the treated patients. However, in our study, initiation of and persistence with osteoporosis therapy were low. The need for patient therapeutic education and close collaboration between rheumatologists and hepatogastroenterologists is therefore crucial. Moreover, the administration of zoledronic acid by injection (5mg once a year) might improve adherence.
Our study does have several strengths because it included a significant number of patients with a 3-year follow-up after the transplant. Also we assessed the risk factors influencing the evolution of BMD. Particularly we studied the impact of certain liver diseases on the BMD changes (negative impact of biliary diseases). However, there are several limitations of our study. Firstly, the single-center design of our study – which was conducted at a university hospital – limited recruitment. As such, we could not extrapolate our results. However, in most cases, the transplanted patients were followed up in an expert center. Another limitation was due to the significant loss in patient numbers during the follow-up, which limited the statistical power of our study. This was mainly due to the duration of the study, and to how patients were invited to visits, which meant an increase in the number of visits for patients who were already being followed up on a regular basis in the Department of Hepatology. Of the 251 patients included at V0, less than half were followed up until 3 years after transplantation.
In conclusion, our study demonstrates that osteoporosis is found in a high proportion of patients awaiting liver transplantation and at 3 years after transplantation. More specifically, we showed that maximum bone loss was attained in the first 6 months, followed by a progressive improvement thereafter. Our data suggest that bone status should be assessed in all the patients with cirrhosis, then followed up more closely after transplantation. Finally, we showed that BMD improves in liver-transplant patients treated with bisphosphonates – especially for infusions of zoledronic acid – in the first 6 months after transplantation.
Agathe Grandjean, Sébastien Dharancy, Guillaume Lassailly, Bernard Cortet and Isabelle Legroux-Gérot declare that they have no conflict of interest.
- Adam R, Karam V, Delvart V, O'Grady J, Mirza D, et al. (2012) Evolution of indications and results of liver transplantation in Europe. A report from the European Liver Transplant Registry (ELTR). J Hepatol 57: 675-688. [Crossref]
- López-Larramona G, Lucendo AJ, González-Castillo S, Tenias JM (2011) Hepatic osteodystrophy: An important matter for consideration in chronic liver disease. World J Hepatol 3: 300-307. [Crossref]
- Collier J (2007) Bone disorders in chronic liver disease. Hepatology 46: 1271-1278. [Crossref]
- Wibaux C, Legroux-Gerot I, Dharancy S, Boleslawski E, Declerck N, et al. (2011) Assessing bone status in patients awaiting liver transplantation. Jt Bone Spine Rev Rhum 78: 387-391. [Crossref]
- Yu TM, Lin CL, Chang SN, Sung F, Huang S, et al. (2014) Osteoporosis and fractures after solid organ transplantation: a nationwide population-based cohort study. Mayo Clin Proc 89: 888-895. [Crossref]
- https://www.agence-biomedecine.fr/annexes/bilan2017/donnees/organes/02-organes/synthese.htm
- Crosbie O, Freaney R, McKenna M, Curry MP, Hegarty JE, et al. (1999) Predicting bone loss following orthotopic liver transplantation. Gut 44:430 [Crossref]
- Monegal A, Navasa M, Guañabens N, et al. (2001) Bone disease after liver transplantation: a long-term prospective study of bone mass changes, hormonal status and histomorphometric characteristics. Osteoporos Int J 12: 484-492. [Crossref]
- Fardellone P, Sebert JL, Bouraya M, Bonidan O, Leclercq G, et al. (1991) Evaluation of the calcium content of diet by frequential self-questionnaire. Rev Rhum Mal Osteoartic 58: 99-103. [Crossref]
- Genant HK, Wu CY, van Kuijk C, Nevitt MC (1993) Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res 8: 1137-1148. [Crossref]
- Lan G, Xie X, Peng L, Liu L, Song L, et al. (2015) Current Status of Research on Osteoporosis after Solid Organ Transplantation: Pathogenesis and Management. BioMed Res Int 2015: 413169. [Crossref]
- Hamburg SM, Piers DA, van den Berg AP, Slooff MJ, Haagsma EB (2001) Bone mineral density in the long term after liver transplantation. Osteoporos Int J 11: 600-606. [Crossref]
- Guichelaar MMJ, Kendall R, Malinchoc M, Hay JE (2006) Bone mineral density before and after OLT: long-term follow-up and predictive factors. Liver Transplant 12: 1390-1402. [Crossref]
- Bjøro K, Brandsaeter B, Wiencke K, Bjøro T, Godang K, et al. (2003) Secondary osteoporosis in liver transplant recipients: a longitudinal study in patients with and without cholestatic liver disease. Scand J Gastroenterol 38: 320-327. [Crossref]
- Bang CS, Shin IS, Lee SW, Kim JB, Baik GH, et al. (2015) Osteoporosis and bone fractures in alcoholic liver disease: A meta-analysis. World J Gastroenterol 21: 4038. [Crossref]
- Monegal A, Navasa M, Guañabens N, Peris P, Pons F, et al. (2001) Bone mass and mineral metabolism in liver transplant patients treated with FK506 or cyclosporine A. Calcif Tissue Int 68: 83-86. [Crossref]
- Sun L, Peng Y, Zaidi N, Zhu L, Iqbal J, et al. (2007) Evidence that calcineurin is required for the genesis of bone-resorbing osteoclasts. Am J Physiol Renal Physiol 292: F285-F291. [Crossref]
- Yeo H, Beck LH, McDonald JM, Zayzafoon M (2007) Cyclosporin A elicits dose-dependent biphasic effects on osteoblast differentiation and bone formation. Bone 40: 1502-1516. [Crossref]
- Canalis E, Mazziotti G, Giustina A, Bilezikian JP (2010) Glucocorticoid-induced osteoporosis: pathophysiology and therapy. Osteoporos Int 18: 1319-1328. [Crossref]
- Kasturi KS, Chennareddygari S, Mummadi RR (2010) Effect of bisphosphonates on bone mineral density in liver transplant patients: a meta-analysis and systematic review of randomized controlled trials. Transpl Int 23: 200-207. [Crossref]
- Crawford BAL, Kam C, Pavlovic J, Byth K, Handelsman DJ, et al. (2006) Zoledronic acid prevents bone loss after liver transplantation: a randomized, double-blind, placebo-controlled trial. Ann Intern Med 144: 239-248. [Crossref]
- Bodingbauer M, Wekerle T, Pakrah B, Roschger P, Peck-Radosavljevic M, et al. (2007) Prophylactic Bisphosphonate Treatment Prevents Bone Fractures After Liver Transplantation. Am J Transplant 7: 1763-1769. [Crossref]
- Shane E, Cohen A, Stein EM, McMahon DJ, Zhang C, et al. (2012) Zoledronic Acid Versus Alendronate for the Prevention of Bone Loss after Heart or Liver Transplantation. J Clin Endocrinol Metab 97: 4481-4490. [Crossref]
- Anastasilakis AD, Tsourdi E, Makras P, Polyzos SA, Meier C, et al. (2019) Bone disease following solid organ transplantation: A narrative review and recommenadtions for management from European Calcified Tissue Society. Bone 127: 401-418. [Crossref]
Editorial Information
Editor-in-Chief
Dr. Abdullah H. A. Almalki
Section Head of Nephrology, Department of Medicine, KAMC, Saudi Arabia
Article Type
Research Article
Publication history
Received date: July 05, 2021
Accepted date: July 13, 2021
Published date: July 16, 2021
Copyright
©2021 Grandjean A. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation
Grandjean A, Dharancy S, Lassailly G, Cortet B, Legroux-Gérot I (2021) Changes in bone mineral density in liver transplant patients and effects of bisphosphonates. Trends in Transplant 14(4): DOI: 10.15761/TiT.1000306

