Background: Cardiovascular disease (CVD) is a major cause of the morbidity and mortality in patients with type 2 diabetes mellitus (T2DM).
Objectives: The aim of this study was to assess the characteristics of the clinical background and coronary artery lesions in patients with T2DM with silent myocardial ischemia (SMI), symptomatic stable angina pectoris (SSAP), and acute coronary syndrome (ACS) without previous history of CVD.
Methods: One hundred two consecutive patients with SMI, 172 with SSAP, and 122 with ACS with T2DM without any previous histories of CVD were evaluated.
Results: The patients with ACS or SSAP had a lot of or a few points that they should correct, such as a poor control of and/or being untreated for T2DM, hypertension, and dyslipidemia, no internal use of statins, and renal dysfunction, as compared to those with SMI with T2DM. Those points may contribute to the development of atherosclerosis, such as multi-vessel complex coronary lesions and/or the formation of unstable plaque developing into the onset of ACS in those patients.
Conclusions: Thus, an intensified multifocal intervention for common conditions for hypertension, and dyslipidemia, and the maintenance of the renal function may be as important as the control of T2DM to prevent or slow CVD associated with more multi-vessel complex coronary lesions probably progressing to ACS in patients with T2DM. Moreover, the detection and treatment of more single-vessel simple coronary lesions before more multi-vessel complex coronary lesions by the screening tests for CVD may be one of the reasonable optional therapies for patients with T2DM with SMI, even though they have no previous histories of CVD, because that the prognosis of patients with T2DM with SMI is worse than that in those without SMI.
acute coronary syndrome, complex coronary lesion, diabetes mellitus, multi-vessel disease, patients characteristics, silent myocardial ischemia, simple coronary lesion, single vessel disease, stable angina pectoris
The number of patients with type 2 diabetes mellitus (T2DM) continues to increase all over the world, and the annual economic loss due to T2DM reaches 548 billion dollars [1,2]. Cardiovascular disease (CVD), especially coronary artery disease (CAD), is a major cause of the morbidity and mortality in patients with T2DM, and about 40% of the patients with T2DM die CVD [2]. In 2013, the Evidence-based Practice Guideline for the Treatment for Diabetes in Japan 2013 recommends screening tests for CVD, and it is desirable that those examinations should be performed in patients with T2DM once a year [3]. Thus, we have started the screening tests for CVD of the patients with T2DM in hour hospitals in 2014, and recently reported that the those screening tests for CVD could detect 19% of patients with silent myocardial ischemia (SMI) in patients with asymptomatic T2DM without a previous history of CVD [4]. Furthermore, the factors, including a longer history of T2DM and co-existence of a family history of CVD, were demonstrated as independent risk factors of SMI by a multivariate analysis (Odds ratio 1.060 and 4.000, respectively) [4]. However, the characteristics of the clinical background and coronary artery lesions in patients with T2DM without a previous history of CVD are still not well known. Thus, the aim of this study was to assess them in patients with T2DM with silent myocardial ischemia (SMI), symptomatic stable angina pectoris (SSAP), and acute coronary syndrome (ACS) without a previous history of CVD.
Study population and laboratory analysis
This study was approved by the institutional review committee and ethics review board of our hospitals. From March 2014 to January 2017, 102 consecutive patients with SMI [4] (SMI group), 172 with SSAP (SSAP group), and 122 with ACS (ACS group) with T2DM who were admitted to our hospitals without any previous history of CVD, including CAD, cerebral infarctions, arteriosclerosis obliterans, and heart failure (HF), were retrospectively evaluated. Patients that underwent hemodialysis were excluded. A ‘Silent’ was defined as a patient without any symptoms even with the existence of myocardial ischemia detected by exercise stress testing, 201Tl-schintigraphy, and/or fractionated flow reserve (FFR) measurements [5], as previously reported [4]. All patients had their history recorded including the disease duration of T2DM and underwent a physical examination and laboratory analysis. All patients were treated with optimal medical therapies (OMTs) and/or revascularization with percutaneous coronary intervention (PCI) and/or a coronary artery bypass grafting (CABG). The inpatient hospitalization days and medical expense, including the CVD screening tests in all patients, were also evaluated.
Evaluation and/or treatment of the number of coronary lesions, complexity, and myocardial ischemia
Among all patients who underwent CAG, the number of severe coronary stenoses (stenosis >50% of the left main trunk and >75% of that other than the left main trunk) and coronary lesion complexity were stratified according to the lesion complexity [6]. To evaluate the myocardial ischemia, they underwent coronary angiography (CAG) combined with exercise stress testing, 201Tl-schintigraphy and/or fractionated flow reserve (FFR) measurements [5].
Average stent length, diameter, and number per patient, restenosis rate, and repeated re-vascularization’s
The average stent length, stent diameter, and stent number per patient were evaluated in the patients that underwent PCI. To evaluate the restenosis rate of the treated coronary arteries, all patients underwent CAG and/or coronary computed-tomography (CCT) 6 to 12-month after the PCI. The patients with the detection of myocardial ischemia by exercise stress testing, 201Tl-schintigraphy, and/or fractionated flow reserve (FFR) measurements [5] in the restenosed coronary arteries received a repeat re-vascularization and/or OMTs.
Statistical analysis
The numerical results are expressed in the text as the mean ± standard deviation. The differences between the SMI, SSAP, and ACS groups were compared using a one-way analysis of variance and Fisher’s exact test. All analyses were performed with SAS version 9.2 software (SAS Institute, Cary, NC). A p of < 0.05 was considered to indicate statistical significance.
Patient characteristics and laboratory analysis (Table 1)
There was no statistical difference in the prevalence of males (75% vs. 74% vs. 77%: p=0.817), mean age (71 ± 1 vs. 72 ± 1 vs. 69 ± 1 years: p=0.057), BMI (23.7 ± 0.4 vs. 23.7 ± 0.3 vs. 24.1 ± 0.4 kg/m2: p=0.698), co-existence of major coronary risk factors including hypertension (71% vs. 73% vs. 75%: p=0.735), dyslipidaemia (62% vs. 64% vs. 79%: p=0.204), smoking (50% vs. 51% vs. 58%: p=0.483), and a family history of CVD (41% vs. 37% vs. 38%: p=0.685), and Hb (14.2 ± 0.2 vs. 14.0 ± 0.3 vs. 13.7 ± 0.2 g/dl: p=0.765), and triglyceride (135 ± 6 vs. 139 ± 6 vs. 170 ± 20 mg/dl: p=0.083) levels between the 3 groups. The duration of the T2DM (16 ± 1 vs. 18 ± 1 vs. 15 ± 1 years: p=0.015) was significantly shorter and prevalence of patients who had a first diagnosis of T2DM on admission (0% vs. 7% vs. 18%: p<0.001) significantly higher in the ACS group than SMI and SSAP groups. The systolic (133 ± 2 vs. 136 ± 1 vs. 142 ± 2 mmHg: p=0.007) and diastolic (70 ± 1 vs. 71 ± 1 vs. 75 ± 2 mmHg: p=0.010) blood pressures, HbA1c level (7.0 ± 0.1 vs. 6.9 ± 0.1 vs. 7.2 ± 0.1%: p=0.036), LDL-cholesterol level (106 ± 3 vs. 107 ± 2 vs. 122 ± 3 mg/dl: p<0.001), LDL to HDL-cholesterol ratio (2.03 ± 0.06 vs. 2.09 ± 0.06 vs. 2.61 ± 0.10 mg/dl: p<0.001), and serum creatinine level (0.89 ± 0.03 vs. 0.94 ± 0.02 vs. 1.12 ± 0.09 mg/dl: p=0.014) were significantly higher, and HDL-cholesterol (55 ± 1 vs. 54 ± 1 vs. 51 ± 1 mg/dl: p=0.032) significantly lower in the ACS group than SMI and SSAP groups.
Baseline therapies for T2DM and CVD (Table 2)
There was no statistical difference in the prevalence of the internal use of insulin (25% vs. 17% vs. 16%: p=0.251), sulfonylurea (20% vs. 14% vs. 16%: p=0.369), metformin (36% vs. 30% vs. 24%: p=0.395), α-glucosidase inhibitors (α-GI) (16% vs. 11% vs. 16%: p=0.431), thiazolidinedione (4% vs. 3% vs. 4%: p=0.711), rapid insulin secretagogue (5% vs. 9% vs. 7%: p=0.337), dipeptidyl peptidase (DPP)-4 inhibitors (49% vs. 46% vs. 35%: p=0.672), glucagon-like peptide (GLP)-1 receptor agonists (8% vs. 6% vs. 4%: p=0.593), sodium-glucose cotransporter (SGLT)-2 inhibitors (5% vs. 4% vs. 2%: p=0.852), anti-platelets (28% vs. 26% vs. 27%: p=0.907), renin-angiotensin system (RAS) inhibitors (52% vs. 53% vs. 43%: p=0.806), and beta-blockers (6% vs. 9% vs. 8%: p=0.907), between the 3 groups. On the other hand, the prevalence of a diet alone (9% vs. 7% vs. 26%: p=0.013) and being untreated (1% vs. 7% vs. 26%: p<0.01) for T2DM was significantly higher, and the internal use of statins (47% vs. 46% vs. 30%: p=0.038) significantly lower in the ACS group than the SMI and SSAP groups. There were 12 (7%) and 22 (18%) patients who had a first diagnosis of T2DM on admission in the SSAP and ACS groups, respectively. They were included in the untreated T2DM patients in the SSAP and ACS groups, respectively.
Evaluation of the number of coronary vessel lesions and complexity, and evaluation and treatment of myocardial ischemia (Table 3 and Figure 1)
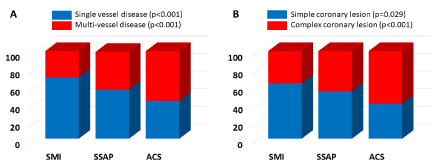
Figure 1. The prevalence of single vessel disease (blue) and multi-vessel disease (red) (A) and simple (blue) and complex (red) coronary lesions (B) in patients with type 2 diabetes mellitus with silent myocardial ischemia (SMI), symptomatic stable angina pectoris (SSAP), and acute coronary syndrome (ACS). The single vessel disease (blue) (A) and coronary simple lesions (blue) (B) decreased in the order of the SMI group, SSAP group, and ACS group. On the contrary, the multi-vessel disease (red) (A) and coronary complex lesions (red) (B) increased in the order of the SMI group, SSAP group, and ACS group
There were 4, 9, and 14 of severe LMT lesions (stenosis >50%), and 146, 273, and 227 severe coronary lesions (stenosis >75%) of that other than the left main trunk in the SMI, SSAP, and ACS groups, respectively. There was no statistical difference in the prevalence of left main trunk disease (LMTD) (4% vs. 5% vs. 11%: p=0.067), and the performance during the exercise stress testing (79% vs. 79% vs. 72%: p=0.895), 201Tl-schintigraphy (21% vs. 19% vs. 15%: p=0.424), FFR (33% vs. 30% vs. 23%: p=0.195), and an angiography-guided PCI (3% vs. 2% vs. 5%: 0.130) between the 3 groups. The prevalence of coronary single-VD (70% vs. 56% vs. 42%: p<0.001) and simple lesions (type A/B1) (63% vs. 54% vs. 40%: p=0.029) was significantly higher, and the prevalence of coronary multi-VDs (30% vs. 43% vs. 57%: p<0.001) and complex lesions (type B2/C) (37% vs. 46% vs. 60%: p<0.001) significantly lower in the SMI group than SSAP and ACS groups.
Although the prevalence of OMTs alone (6% vs. 3% vs. 0%: p=0.014) was significantly higher in the SMI group than in the SSAP and ACS groups, there was no statistical difference in the prevalence of a CABG alone (9% vs. 12% vs. 9%: p=0.103), PCI plus CABG (0% vs. 0% vs. 2%: p-0.281) and PCI alone (85% vs. 84% vs. 89%: p=0.463) between the 3 groups.
Average stent number, diameter, and length per patient, restenosis rates, and repeated re-vascularization (Table 3)
One hundred thirty-six, 136, and 236 stents were implanted in 102, 172, and 122 patients in the SMI, SSAP, and SMI groups, respectively. Although the average stent diameter did not statistically differ significantly between the 3 groups (3.05 ± 0.04 vs. 3.03 ± 0.03 vs. 3.03 ± 0.03 mm: p=0.573), the average stent number per patient (1.5 ± 0.1 vs. 1.8 ± 0.1 vs. 2.2 ± 0.2: p<0.001) was significantly lower, and average stent length (38 ± 3 vs. 46 ± 3 vs. 59 ± 4 mm: p<0.001) significantly shorter in the SMI group than SSAP and ACS groups in accordance with a greater number of complex lesions and multi-VDs. As a matter of course, after treating the myocardial ischemia, all patients received OMTs for CVD including anti-platelets, statins, RAS inhibitors, beta-blockers, nitrates, and/or diuretics. The restenosis rates (9% vs. 10% vs. 20%: p=0.016) and ischemic-driven repeated revascularizations (8% vs. 8% vs. 16%: p=0.023) were significantly lower in the SMI and SSAP groups than ACS group.
Laboratory analysis on discharge and hospitalization (Table 4)
On discharge, the brain natriuretic peptide (BNP) level (64 ± 12 vs. 71 ± 19 vs. 219 ± 36 pg/ml: p<0.001) and medical expense (130 ± 13 vs. 149 ± 11 vs. 256 ± 19 hundred dollars: p<0.001) were significantly higher, left ventricular ejection fraction (LVEF) (64 ± 1 vs. 63 ± 1 vs. 57 ± 1%: p=0.001) significantly lower, and inpatient hospitalization days (8 ± 1 vs. 8 ± 1 vs. 23 ± 5 days: p<0.001) significantly longer, in the ACS group than in the SMI and SSAP groups.
Major adverse cardiovascular events (MACE) (Table 4)
The evaluation during the follow-up period of 24-months after the treatment of myocardial ischemia demonstrated that the prevalence of all MACE (4% vs. 6% vs. 12%: p<0.001) including hospitalizations for HF (1% vs. 1% vs. 7%: p=0.011), cerebrovascular apoplexy/transient ischemic attacks (1% vs. 1% vs. 2%: p=0.471), acute MIs (0% vs. 1% vs. 1%: p=0.860), major/minor bleeding (1% vs. 2% vs. 2%: p=0.899), and death (1% vs. 1% vs. 2%: p=0.792) was significantly higher in the ACS group than in the SMI and SSAP groups, but there was no significant difference in deaths between the 3 groups.
Table 4. Laboratory Analysis on Discharge, Hospitalization, and Major Adverse Cardiovascular Events. SMI=silent myocardial ischemia, SSAP=symptomatic stable angina pectoris, ACS=acute coronary syndrome, TIA=transient ischemic attack
|
SMI group
(n=102)
|
SSAP group
(n=172)
|
ACS group
(n=122)
|
p
value
|
Laboratory Analysis on discharge
|
|
|
|
|
Brain natriuretic peptide (pg/ml)
|
64 ± 12
|
71 ± 19
|
219 ± 36
|
<0.001
|
Left ventricular ejection fraction (%)
|
64 ± 1
|
63 ± 1
|
57 ± 1
|
0.011
|
Hospitalization
|
|
|
|
|
Inpatient hospitalization day (days)
|
8 ± 1
|
8 ± 1
|
23 ± 5
|
<0.001
|
Medical expenses (hundred dollars)
|
130 ± 13
|
149 ± 11
|
256 ± 19
|
<0.001
|
Major Adverse Cardiovascular Events
|
|
|
|
|
All
|
4 (4%)
|
10 (6%)
|
15 (12%)
|
<0.001
|
Hospitalization for heart failure
|
1 (1%)
|
2 (1%)
|
8 (7%)
|
0.011
|
Cerebrovascular apoplexy/TIA
|
1 (1%)
|
1 (1%)
|
2 (2%)
|
0.471
|
Acute myocardial infarction
|
0 (0%)
|
2 (1%)
|
1 (1%)
|
0.860
|
Major/minor bleeding
|
1: 0/1 (1%)
|
3: 0/3 (2%)
|
2: 1/1 (2%)
|
0.899
|
Death
|
1 (1%)
|
2 (1%)
|
2 (2%)
|
0.292
|
Management of T2DM and risk factors of CVD (Tables 1 and 2)
This study revealed the characteristics of the clinical background in patients with T2DM as follows: 1) the control of both the systolic and diastolic blood pressure, blood sugar, and LDL-cholesterol, and renal function were poor in the ACS group compared to that in the SMI and SSAP groups (Table 1), however, the co-existing coronary risk factors including hypertension, dyslipidemia, current or ex- smoking, and a family history of CVD were equal, 2) a poor control of the blood sugar may be associated with a treatment of T2DM of the diet alone or being untreated T2DM in the ACS group (Tables 2 and 3) poor control of LDL-cholesterol may be associated with a low prevalence of internal use of statins in the ACS group (Tables 2 and 4) poor renal function may be associated with a poor control of hypertension, and the blood sugar in the ACS group (Table 1). Among the patients with T2DM, even though their HbA1c level was controlled under 6.9% or less, and they had a normal renal function, an excessive risk of death associated with CVD still existed and was approximately twice as high as the risk among patients without T2DM [7]. Further interestingly, the HbA1c level was significantly higher in the untreated patients than treated patients with medications for T2DM in the 3 groups (7.7 ± 0.3% vs. 7.0 ± 0.1%: p=0.014) in this study. Moreover, surprisingly, with a greater renal dysfunction, the excessive risk of death from cardiovascular death, increased to up to approximately 30-fold as high as the risk among patients without T2DM [7,8]. Furthermore, maintenance of the renal function may be necessary to control the individual cardiovascular risk factors to prevent or slow the CVD in patients with T2DM [9,10]. Because the poor control of coronary risk factors and renal dysfunction in patients with T2DM may accelerate the progression of the atherosclerosis and formation of unstable plaque developing to ACS, the duration of T2DM in the ACS group may be shorter than that in the SMI and SSAP groups (Table 1). Thus, not only a comprehensive and aggressive treatment of T2DM, but also an intensified multifocal intervention for common conditions such as hypertension [11,12] and dyslipidemia [11,12] and maintenance of renal function may be important to prevent the cardiovascular events in patients with T2DM [13].
Table 1. Patient Characteristics. SMI=silent myocardial ischemia, SSAP=symptomatic stable angina pectoris, ACS=acute coronary syndrome, T2DM= type 2 diabetes mellitus
|
SMI group
(n=102)
|
SSAP group
(n=172)
|
ACS group
(n=122)
|
p value
|
Male
|
76 (75%)
|
128 (74%)
|
94 (77%)
|
0.817
|
Age (years)
|
71 ± 1
|
72 ± 1
|
69 ± 1
|
0.057
|
Body mass index (kg/m2)
|
23.7 ± 0.4
|
23.7 ± 0.3
|
24.1 ± 0.4
|
0.698
|
Duration of T2DM (years)
|
16 ± 1
|
18 ± 1
|
15 ± 1
|
0.015
|
First diagnosis of T2DM on admission
|
0 (0%)
|
12 (7%)
|
22 (18%)
|
<0.001
|
Systolic blood pressure (mmHg)
|
133 ± 2
|
136 ± 1
|
142 ± 2
|
0.007
|
Diastolic blood pressure (mmHg)
|
70 ± 1
|
71 ± 1
|
75 ± 2
|
0.010
|
Co-existence
|
|
|
|
|
Hypertension
|
72 (71%)
|
126 (73%)
|
92 (75%)
|
0.735
|
Dyslipidemia
|
63 (62%)
|
110 (64%)
|
96 (79%)
|
0.204
|
Smoking
|
51 (50%)
|
88 (51%)
|
71 (58%)
|
0.483
|
Family history of cardiovascular disease
|
42 (41%)
|
64 (37%)
|
46 (38%)
|
0.685
|
Laboratory Analysis on admission
|
|
|
|
|
Hb (g/dl)
|
14.2 ± 0.2
|
14.0 ± 0.3
|
13.7 ± 0.2
|
0.765
|
HbA1c (%)
|
7.0 ± 0.1
|
6.9 ± 0.1
|
7.2 ± 0.1
|
0.036
|
LDL-cholesterol (mg/dl)
|
106 ± 3
|
107 ± 2
|
122 ± 3
|
<0.001
|
HDL-cholesterol (mg/dl)
|
55 ± 1
|
54 ± 1
|
51 ± 1
|
0.032
|
LDL- to HDL-cholesterol ratio
|
2.03 ± 0.06
|
2.09 ± 0.06
|
2.61 ± 0.10
|
<0.001
|
Triglyceride (mg/dl)
|
135 ± 6
|
139 ± 6
|
170 ± 20
|
0.083
|
Serum creatinine (mg/dl)
|
0.89 ± 0.03
|
0.94 ± 0.02
|
1.12 ± 0.09
|
0.014
|
Table 2. Baseline Therapies for T2DM and CVD on Admission. SMI=silent myocardial ischemia, SSAP=symptomatic stable angina pectoris, ACS=acute coronary syndrome, T2DM=type 2 diabetes mellitus, CAD=cardiovascular disease, DPP=Dipeptidyl peptidase, GLP=glucagon-like peptide, SGLT=sodium- glucose cotransporter, RAS=renin-angiotensin system, ACEI=angiotensin-converting enzyme inhibitor, ARB=angiotensin II receptor blocker
|
SMI group
(n=102)
|
SSAP group
(n=172)
|
ACS group
(n=122)
|
p
value
|
Insulin
|
25 (25%)
|
29 (17%)
|
19 (16%)
|
0.251
|
Sulfonylurea
|
20 (20%)
|
24 (14%)
|
19 (16%)
|
0.369
|
Metformin
|
37 (36%)
|
51 (30%)
|
29 (24%)
|
0.395
|
α-glucosidase inhibitor
|
16 (16%)
|
19 (11%)
|
20 (16%)
|
0.431
|
Thiazolidinedione
|
4 (4%)
|
5 (3%)
|
5 (4%)
|
0.711
|
Rapid insulin secretagogue
|
5 (5%)
|
15 (9%)
|
9 (7%)
|
0.337
|
DPP-4 inhibitor
|
50 (49%)
|
79 (46%)
|
43 (35%)
|
0.672
|
GLP-1 receptor agonist
|
8 (8%)
|
10 (6%)
|
5 (4%)
|
0.593
|
SGLT-2 inhibitor
|
5 (5%)
|
7 (4%)
|
3 (2%)
|
0.852
|
Diet alone
|
9 (9%)
|
12 (7%)
|
32 (26%)
|
0.013
|
Untreated
|
1 (1%)
|
12 (7%)
|
32 (26%)
|
<0.001
|
|
|
|
|
|
Statins
|
48 (47%)
|
79 (46%)
|
36 (30%)
|
0.038
|
Anti-platelets
|
29 (28%)
|
45 (26%)
|
33 (27%)
|
0.907
|
RAS inhibitors: ACEI/ARB
|
53: 2/51 (52%)
|
92: 3/39 (53%)
|
52: 2/50 (43%)
|
0.806
|
Beta-blockers
|
6 (6%)
|
15 (9%)
|
10 (8%)
|
0.90
|
Association between the management of t2dm and coronary risk factors, and coronary lesions (Table 3 and Figure 1)
This study also revealed that the coronary complex lesions and multi-vessel disease increased in the order of the SMI group, SSAP group, and ACS group, and the coronary simple lesions and single vessel disease contrarily decreased in the order of the SMI group, SSAP group, ACS group in patients with T2DM. As mentioned above, the poor control of hypertension, dyslipidaemia, and T2DM and renal dysfunction may have been closely related to the progression of coronary atherosclerosis contributing to the coronary complex lesions and multi-VDs.
Table 3. Evaluation and Treatment of Coronary Lesions and Myocardial Ischemia. MI=silent myocardial ischemia, SSAP=symptomatic stable angina pectoris, ACS=acute coronary syndrome, VD=vessel disease, OMT=optimal medical therapy, CABG= coronary artery bypass graft, PCI= percutaneous coronary intervention
|
SMI group
(n=102)
|
SSAP group
(n=172)
|
ACS group
(n=122)
|
p
value
|
Coronary Lesions
|
|
|
|
|
Single-VD
|
71 (70%)
|
97 (56%)
|
51 (42%)
|
<0.001
|
Multi-VD: 2VD/3VD
|
31: 20/11
(30%)
|
74: 53/21
(43%)
|
70: 46/24
(57%)
|
<0.001
|
Left main trunk disease: alone/plus VD
|
4: 1/3
(4%)
|
9: 1/8
(5%)
|
14: 1/13
(11%)
|
0.067
|
Coronary Lesion Complexity
|
|
|
|
|
Simple lesion: A/B1
|
92: 54/38 (63%)
|
147: 64/83
(54%)
|
90: 15/75
(40%)
|
0.029
|
Complex lesion: B2/C
|
54: 32/22 (37%)
|
126: 82/44
(46%)
|
137: 87/50
(60%)
|
<0.001
|
Evaluation of Myocardial Ischemia
|
|
|
|
|
Exercise stress testing
|
81 (79%)
|
136 (79%)
|
88 (72%)
|
0.895
|
201Tl-schintigraphy
|
21 (21%)
|
32 (19%)
|
18 (15%)
|
0.423
|
Fractionated flow reserve
|
34 (33%)
|
52 (30%)
|
28 (23%)
|
0.195
|
Angiography-guided
|
3 (3%)
|
3 (2%)
|
6 (5%)
|
0.130
|
Treatment of Myocardial Ischemia
|
|
|
|
|
OMT alone
|
6 (6%)
|
6 (3%)
|
0 (0%)
|
0.014
|
CABG alone
|
9 (9%)
|
21 (12%)
|
11 (9%)
|
0.103
|
PCI plus CABG
|
0 (0%)
|
0 (0%)
|
2 (2%)
|
0.281
|
PCI alone
|
87 (85%)
|
145 (84%)
|
109 (89%)
|
0.463
|
Stent implantation
|
|
|
|
|
Total stent number
|
136
|
136
|
236
|
-
|
Average stent number per patient
|
1.5 ± 0.1
|
1.8 ± 0.1
|
2.2 ± 0.2
|
<0.001
|
Average stent diameter (mm)
|
3.05 ± 0.04
|
3.03 ± 0.03
|
3.03 ± 0.03
|
0.573
|
Average stent length (mm) per patient
|
38 ± 3
|
46 ± 3
|
59 ± 4
|
<0.001
|
Patients with restenosis
|
8 (9%)
|
17 (10%)
|
25 (20%)
|
0.016
|
Patients underwent ischemic-driven
repeated revascularization (PCI/CABG)
|
7:
7/0 (8%)
|
14:
14/0 (8%)
|
20:
19/1 (16%)
|
0.023
|
Cost-effectiveness of SMI, SAP, and ACS in Patients with T2DM (Table 3)
It has been reported as a big problem that the US health care spending has continued to increase and now accounts for more than 17% of the US economy, and diabetes and CAD accounted for the highest and second-highest amount of health care spending in 2013 with an estimated spending of 101.4 billion and 88.1 billion dollars, respectively [14]. This study also revealed that the medical expense increased in the order of the SMI group, SSAP group, ACS group in accordance with an increase in the average stent number, average stent length, restenosis rate, ischemic-driven repeated revascularization, inpatient hospitalization day, and MACE.
The greater number of complex coronary lesions [15,16] and multi-VDs, elevated BNP [17], and deceased LVEF [18] are high risk factor for cardiovascular events and death [15,16] in patients with T2DM. Thus, the increased MACE may be associated with those factors in the ACS group. In addition, because the coronary culprit lesions were small coronary arteries in diameter in which the perfusion area was comparably small and there was no overt myocardial ischemia within their daily exercise tolerance of 6 patients in the SMI group, they received only OMTs without any coronary revascularization. It may contribute to a decreased medical expense. There was no statistical difference in the prevalence of PCIs, PCI plus CABG, and CABGs between the 3 groups. Almost all patients in the ACS group with an ACS underwent an emergency PCI for the culprit lesion(s) for an ACS even though they had LMTD and/or multi-VDs. Further, almost all other residual coronary lesion(s) were treated with a staged PCI and there were only 2 cases of a CABG, contributing to the increased medical expense and lesser cost-effectiveness.
Screening tests for CVD
In 2013, the Evidence-based Practice Guideline for the Treatment for Diabetes in Japan 2013 recommends screening tests for CVD, and it is desirable that those examinations should be performed in patients with T2DM once a year [3]. Thus, we have been started the screening tests for CVD for the outpatients with T2DM in our hospitals since April in 2014. Moreover, we recently demonstrated that SMI could be detected in 19% of patients with asymptomatic T2DM without a previous history of CVD [4] by those screening tests for CVD. Furthermore, the factors, including a longer history of T2DM and co-existence of a family history of CVD, were demonstrated as independent risk factors of SMI by a multivariate analysis (Odds ratio 1.060 and 4.000, respectively) [4]. Further, the number of patients with SMI is gradually increasing in accordance with the disease duration of T2DM [4]. In this study, about half of the patients with SMI were detected by screening tests for CVD in our hospitals. Moreover, the coronary lesions in the SMI group revealed more simple and single vessel disease. It is well known that the prognosis of patients with T2DM with SMI is worse than that in those without SMI [19]. Thus, the detection and treatment of more simple and single vessel coronary lesions before more complex and multi-vessel lesions may be one of the reasonable optional therapies for patients with T2DM with SMI. However, in order to determine whether those therapies can improve the prognosis of those patients, further studies may be needed.
Past and new drugs for T2DM
Because there was no evidence that these past drugs could adequately prevent cardiovascular events in patients with T2DM [20-23], there may be limitations that can prevent cardiovascular events only by treating the T2DM with past drugs. However, it has been recently reported that a recent advancement in new drugs for T2DM such as SGLT-2 inhibitors [8] and GLP-1 receptor agonists [24,25] could significantly prevent cardiovascular death, nonfatal MIs, or nonfatal strokes, and improve the prognosis of the patients with T2DM [26,27]. Since these new drugs have not been on the market for very long after their release in Japan, there was little use of them in this study. When they become commercially available all over the world, they may improve and help the treatment of T2DM as breakthrough therapies in the near future.
Limitations of the study
Although our study was a multi-centre trial, it is limited by its retrospective design and relatively small number of patients. Because the disease duration of T2DM obtained from the patient histories recorded and the blood pressures of almost all patients in the ACS group obtained in the emergency room, they may not be necessarily accurate values. Moreover, the timing of the detection of myocardial ischemia was comparably long (>15 years) for T2DM in the 3 groups and may have influenced the results. Thus, whether our results can safely be extrapolated to a larger number of patients should be determined in further prospective studies.
This study revealed that the patients with ACS or SSAP have a lot of or a few points that they should correct, such as a poor control of common conditions including T2DM, hypertension, and dyslipidaemia, no internal use of statins, being untreated for T2DM, and the existence of renal dysfunction, as compared with SMI with T2DM without previous histories of CVD. Those points may contribute to the development of atherosclerosis, such as complex coronary lesions and multi-vessel disease and/or formation of unstable plaque developing into the onset of ACS in those patients. Thus, an intensified multifocal intervention for common conditions and the maintenance of the renal function may be as important as the control of T2DM to prevent or slow CVD associated with more coronary multi-VDs and complex lesions probably progressing to ACS in patients with T2DM. Moreover, this study also demonstrated that the coronary lesions in patients with SMI revealed more simple and single vessel disease. In view of these findings, the detection and treatment of more simple and single vessel coronary lesions before more complex and multi-vessel lesions may be one of the reasonable optional therapies for patients with T2DM with SMI even though they have no previous histories of CVD, because that the prognosis of patients with T2DM with SMI is worse than that in those without SMI19. However, in order to determine whether those therapies can improve the quality of life and/or prognosis of those patients, further studies may be needed. Finally, not only the cardiologists, but also physicians, especially diabetologists, should be aware of these conditions when examining patients with T2DM even though they are without a previous history of CVD.
We thank Mr. John Martin for his linguistic assistance with this paper.
None
None
- International Diabetes Federation (2013) IDF Diabetes Atlas 6th Edition. International Diabetes Federation, Brussels, p7-p8.
- Ali MK, Bullard KM, Gregg EW (2013) Achievement of goals in U.S. diabetes care, 1999-2010. N Engl J Med 369: 287-288. [Crossref]
- Kawano Y, Takemoto M, Mito T, Morisaki H, Tanaka A, et al. (2016) Silent myocardial ischemia in asymptomatic patients with type 2 diabetes mellitus without previous histories of cardiovascular disease. Int J Cardiol 216: 151-155.
- De Bruyne B, Fearon WF, Pijls NH, Barbato E, Tonino P, et al. (2014) Fractional flow reserve-guided PCI for stable coronary artery disease. N Engl J Med 371: 1208-1217.
- Ryan TJ, Faxon DP, Gunnar RM, Kennedy JW, King SB, et al. (1988) Guidelines for percutaneous transluminal coronary angioplasty. A report of the American college of cardiology/american heart association task force on assessment of diagnostic and therapeutic cardiovascular procedures (Subcommittee on percutaneous transluminal coronary angioplasty). J Am Coll Cardiol 78: 486-502. [Crossref]
- Tancredi M, Rosengren A, Svensson AM, Kosiborod M, Pivodic A, et al. (2015) Excess mortality among persons with type 2 diabetes. N Engl J Med 373: 1720-1732. [Crossref]
- Wanner C, Inzucchi SE, Lachin JM, Fitchett D, von Eynatten M, et al. (2016) Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med 375: 323-334. [Crossref]
- Gaede P, Lund-Andersen H, Parving HH, Pedersen O (2008) Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med 358: 580-591. [Crossref]
- Griffin SJ, Borch-Johnsen K, Davies MJ, Khunti K, Rutten GE, et al. (2011) Effect of early intensive multifactorial therapy on 5-year cardiovascular outcomes in individuals with type 2 diabetes detected by screening (ADDITION-Europe): a cluster-randomised trial. Lancet 378:156-167.
- Bangalore S, Fakheri R, Toklu B, Messerli FH (2016) Diabetes mellitus as a compelling indication for use of renin angiotensin system blockers: systematic review and meta-analysis of randomized trials. BMJ 352: 438. [Crossref]
- Brunstrom M, Carlberg B (2016) Effect of antihypertensive treatment at different blood pressure levels in patients with diabetes mellitus: systematic review and meta-analyses. BMJ 352: 717. [Crossref]
- Rawshani A, Rawshani A, Franzen S, Sattar N, Eliasson B, et al. (2018) Risk factors, mortality, and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 379: 633-644. [Crossref]
- Dieleman JL, Baral R, Birger M, Bui AL, Bulchis A, et al. (2016) US spending on personal health care and public health, 1996-2013. JAMA 316: 2627-2646. [Crossref]
- Goldberg S, Savage MP, Fischman DL (1996) The interventional cardiologist and the diabetic patient. Have we pushed the envelope too far or not far enough? Circulation 94: 1804-1806. [Crossref]
- Kip KE, Faxon DP, Detre KM, Yeh W, Kelsey SF, et al. (1996) Coronary angioplasty in diabetic patients (1996) The National heart, lung, and blood institute percutaneous transluminal coronary angioplasty registry. Circulation 94: 1818-1825. [Crossref]
- Jarolim P, White WB, Cannon CP (2018) Serial measurement of natriuretic peptides and cardiovascular outcomes in patients with type 2 diabetes in the EXAMINE trial. Diabetes Care 41: 1510-1515. [Crossref]
- Chandramouli C, Teng TK, Tay WT, Yap J, MacDonald MR, et al. (2018) Impact of diabetes and sex in heart failure with reduced ejection fraction patients from the ASIAN-HF registry. Eur J Heart Fail 10.1002/ejhf.1358.
- The Japanese Diabetes Society (2013) Diabetec macroangiopathy: Screening for macroangiopathy. practice guideline for the treatment for diabetes in Japan 2013. The Japanese diabetes society japan p15-p30.
- Kwong RY, Sattar H, Wu H, Vorobiof G, Gandla V, et al. (2008) Incidence and prognostic implication of unrecognized myocardial scar characterized by cardiac magnetic resonance in diabetic patients without clinical evidence of myocardial infarction. Circulation 118: 1011-1020. [Crossref]
- Liu J, Li L, Deng K, Xu C, Busse JW, et al. (2017) Incretin based treatments and mortality in patients with type 2 diabetes: systematic review and meta-analysis. BMJ 357: j2499. [Crossref]
- American diabetes association (2016) Standards of medical care in diabetes-2016 Abridged for primary care providers. Clin Diabetes 34: 3-21. [Crossref]
- American diabetes association (2016) 8. Cardiovascular disease and risk management. Diabetes care 39 Suppl 1: S60-S71. [Crossref]
- American Diabetes Association (2016) 8. Cardiovascular disease and risk management. Diabetes care 39 Suppl 1: S72-S80.
- Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, et al. (2016) Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 375: 311-322. [Crossref]
- Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jodar E, et al. (2016) Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 375: 1834-1844. [Crossref]
- Cavender MA, Norhammar A, Birkeland KI, Jorgensen ME, Wilding JP, et al. (2018) SGLT-2 Inhibitors and Cardiovascular risk: An analysis of CVD-REAL. J Am Coll Cardiol 71: 2497-2506. [Crossref]
- Kosiborod M, Lam CSP, Kohsaka S, Kim DJ, Karasik A, et al. (2018) Cardiovascular events associated with sglt-2 inhibitors versus other glucose-lowering drugs: The CVD-REAL 2 Study. J Am Coll Cardiol 71: 2628-2639. [Crossref]

