N and Pt doped titania (Pt/TiO2-xNx) nanoparticles were prepared by the microemulsion method. The as-prepared products were characterized by using X-ray photoelectron spectroscopy, nitrogen adsorption-desorption, and transmission electronic microscopy. Results reveal that the photocatalysts possess a particle size of 4-8 nm with a specific surface area of 130-160 m2/g and exhibit a narrow pore size distribution and high total pore volume. The effect of Pt loading content on the photocatalytic activity was investigated. The degradation of methylene blue over as-prepared titania in aqueous solution under visible light irradiation was remarkably enhanced by codoping N and Pt elements.
photocatalysis, titania, visible light, XPS, methylene blue
In photocatalysis, TiO2 is the most attractive semiconductor among transition metal oxides because of its excellent performance in photocatalytic reactions [1-4]. TiO2 has also been widely used as a photocatalyst in waste air and wastewater treatment due to its relatively high photocatalytic activity, chemical stability, nontoxicity, low cost, and environmentally friendly property [1,3,5-7]. However, TiO2 with a band gap of ca. 3.2 eV can be photoexcited under irradiation of UV light (λ<395 nm), which is less than 5 % of sunlight. Therefore, considerable effort has been made to increase the absorption of TiO2 in the visible region to improve its visible light response through various modifications such as doping of various metal, metal oxides or nonmetal. Recently, N-doped TiO2 has been studied by many researchers [8-12] and demonstrated its photoactivity for decomposition of many organic compounds under visible light irradiation.
If the light energy is equal to or greater than the bandgap of the semiconductor, the electron in the valance band can be excited to the conduction band. This energy change will result in the formation of positive holes in the valance and free electrons in the conduction band. However, the positive hole and electron are easily recombined in a very short time, which will therefore lead to a very low activity for the photocatalyst. The loaded platinum on the surface of TiO2 can play an important role in preventing the rapid recombination between positive hole and electron. It is because the platinum can capture the transferred electrons onto the surface of the TiO2 [1,13].
The importance of nanophase technology has resulted in tremendous research efforts towards the development of new techniques for the synthesis of nanosized particles. One such technique with the ability to precisely control the size and shape of the particles formed was to use water-in-oil microemulsions as a reaction medium. The aqueous cores/water pools of these microemulsions can be used as nanosize reactors for the precipitation of a wide variety of nanoparticles. Compared to other methods, the microemulsion method has several advantages in producing high-purity particles with uniform small size, narrow size distribution, and low aggregation properties [14-16].
Accordingly, the N and Pt doped titania (Pt/TiO2-xNx) photocatalysts in this work were prepared by using the microemulsion method, their characterizations by X-ray photoelectron spectroscopy (XPS), nitrogen adsorption-desorption analysis, and transmission electronic microscopy (TEM) were relatively described. The photocatalytic activities of the as-prepared photocatalysts were evaluated by degrading methylene blue under visible light irradiation (as a model reaction). The effect of Pt loading content on the photoactivity was also studied.
Materials
n-hexane (≥ 99 % purity, Acros), citric acid monohydrate (≥ 99.5 % purity, Riedel-de Haёn), titanium(IV) isopropoxide (TIP, 98 % purity, Fluka), ammonia solution (15 M, Showa), hydroxypropyl cellulose (HPC, m. w. = 100,000, Aldrich), potassium hexachloroplatinate (99 % purity, Showa), sorbitan monopalmitate (Span 40, HLB = 6.7, Sigma ), and isopropyl alcohol (IPA, ≥ 99.5 % purity, ECHO) were used for this study. All chemicals were used without further purification. Span 40 and IPA were used as a surfactant and cosurfactant respectively. HPC behaved as a steric dispersant. Citric acid serving as a modifying agent was applied to moderate the hydrolysis and condensation processes of titanium precursor.
Preparation of Pt/TiO2-xNx photocatalysts
Nanosized Pt/TiO2-xNx powders were synthesized by the microemulsion method, as shown in Figure 1. Both the oil phase (continuous phase) of the each microemulsion were composed of n-hexane, Span 40, and IPA. The two solutions of microemulsion, one contained 5.0×10-5 M hydroxypropyl cellulose, citric acid / titanium isopropoxide with molar ratio 3, and a necessary amount of potassium hexachloroplatinate for desired Pt loading of 0-0.7 wt% aqueous droplets, the other contained aqueous ammonia droplets with the same volume ratio of water/oil (w/o) at 1/100, were mixed together and kept continuously stirring at 50 °C for 1 h to form a milky slurry of titania precursor. After the consecutive procedures of evaporation, drying, calcination at 500 °C, and grind, the desired photocatalysts were obtained.
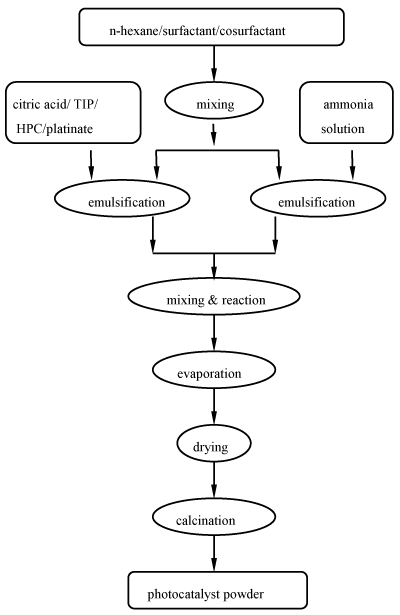
Figure 1. Preparation of Pt/TiO2-xNx photocatalyst powders using the microemulsion method.
Characterization of photocatalysts
The surface area of the Pt/TiO2-xNx powders was determined by a BET analyzer (Micromeritics, ASAP 2101), and the surface structure and particle morphology were examined by a transmission electronic microscopy (TEM) (Philips, CM-200). The surface composition and bonding of the Pt/TiO2-xNx powders were detected by X-ray photoelectron spectroscopy (XPS) (Ulvac-Phi, Model ES 650). The binding energy was referenced to the C1s line at 284.6 eV for calibration.
Photocatalytic activity experiments
The photocatalytic activity experiments on Pt/TiO2-xNx powders for the photodegradation of a dye in water were carried out in a Pyrex glass reactor under visible light irradiation. Analytical grade methylene blue was used as the model dye chemical. Its aqueous suspension was prepared by adding 0.2 g of photocatalyst powder to 300 mL of 10 ppm aqueous methylene blue solution. The visible light irradiation was provided by a high-pressure mercury lamp and an appropriate cutoff filter was placed in the front of the reactor to remove the part of UV radiation. At given irradiation time intervals, samples of 5 mL volume were taken from the suspension and immediately centrifuged at 5000 rpm for 10 min, then passed through a 0.45 μm Millipore filter to remove the particles. The concentration of methylene blue was measured at the maximum absorption wavelength of 664 nm by a UV-Vis spectrophotometer.
Characterization of Pt/TiO2-xNx catalyst
The morphology of the as-synthesized Pt/TiO2-xNx powders was examined by TEM as shown in Figure 2. The Pt/TiO2-xNx particles were clearly observed with a typical size of 4-8 nm.

Figure 2. TEM image of 0.2 wt% Pt/TiO2-xNx prepared by microemulsion method.
The Brunauer-Emmett-Teller (BET) gas adsorption method has become the most widely used standard procedure for the determination of the surface area of finely-divided and porous materials. Figure 3 shows the nitrogen adsorption-desorption isotherms of the TiO2-xNx and 0.6 wt% Pt/TiO2-xNx prepared by microemulsion method. The isotherms exhibit typical Type IV pattern with hysteresis loop, characteristic of mesoporous material according to the classification of IUPAC [17]. A sharp increase in adsorption volume of N2 was observed and located in the P/Po range of 0.45-1.00. This sharp increase can be attributed to the capillary condensation, indicating the good homogeneity of the sample. As can be seen from Figure 4, the pore size distributions obtained by BJH approach are noticeably narrow, confirming good quality of the samples. It is found that the mean pore diameter of the TiO2-xNx photocatalysts is obviously decreased with the increased amount of Pt loading. The textural properties of the photocatalysts prepared by microemulsion method are given in Table 1. The higher the Pt content was loaded, the larger the decrease in the BET surface area of the samples was observed. The mean pore diameter and the total pore volume of the photocatalysts decreased in similar trend to the surface area. It may be concluded that such the small amount of Pt loaded disturbed the mesopore volume by depositing at the surface of the TiO2-xNx aggregates, while maintaining the general pore size characteristic. By comparison, the BET surface area of the synthesized samples are quite higher than that of Degussa P-25 (a commercial TiO2 photocatalyst), which is widely used in photocatalysis. It is concluded that Pt/TiO2-xNx prepared by microemulsion method in this work has the advantage of giving high specific surface area. It should be good for the photocatalytic reaction.
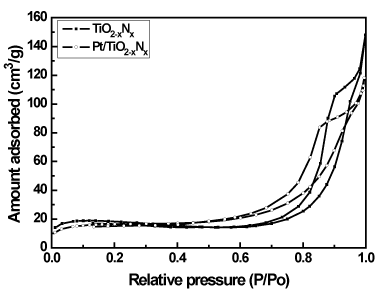
Figure 3. N2 adsorption-desorption isotherms of TiO2-xNx and 0.6 wt% Pt/TiO2-xNx.
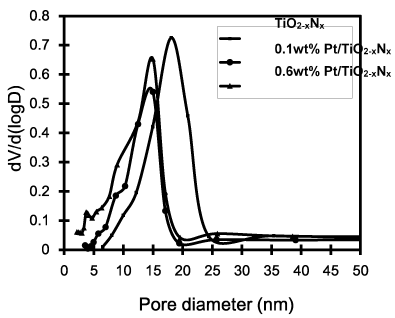
Figure 4. Pore size distributions of Pt/TiO2-xNx at various Pt loadings.
Table 1. Textural properties of photocatalysts from N2 adsorption-desorption analysis.
Photocatalyst |
BET surface area Mean pore diameter Total pore volume |
(m2 g-1) |
(nm) |
(cm3 g-1) |
TiO2-xNx |
158.2 |
9.5 |
0.332 |
0.1 wt%Pt/TiO2-xNx |
157.6 |
9.3 |
0.303 |
0.6 wt%Pt/TiO2-xNx |
135.1 |
7.8 |
0.259 |
P-25 |
48.0 |
12.6 |
0.120 |
The surface composition and bonding of the as-synthesized Pt/TiO2-xNx powders were analyzed by X-ray photoelectron spectroscopy (XPS). Figure 5 shows the XPS signals over the whole scanned energy range (0-1100 eV). As seen, the presence of Ti 2p, O 1s, N 1s and Pt 4f signals in the spectra of the sample demonstrated the as-synthesized powders consist of Ti, O, N, and Pt elements. Figures 6-9 show the high-resolution Ti 2p, O 1s, N 1s and Pt 4f spectra of the Pt/TiO2-xNx powders.
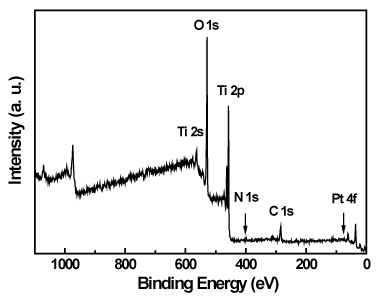
Figure 5. XPS spectrum of the Pt/TiO2-xNx powders.

Figure 6. Ti 2p XPS spectrum of the Pt/TiO2-xNx powders.
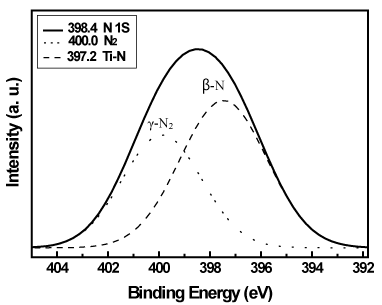
Figure 7. N 1s XPS spectrum of the Pt/TiO2-xNx powders.
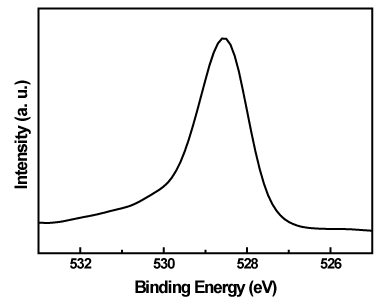
Figure 8. O 1s XPS spectrum of the Pt/TiO2-xNx powders.
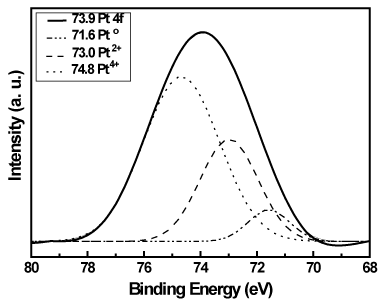
Figure 9. Pt 4f XPS spectrum of the Pt/TiO2-xNx powders.
The Ti 2p XPS peaks of the pure TiO2 appeared at 458.2 eV and 464.0 eV for Ti 2p3/2 and Ti 2p1/2, respectively [18]. For the as-synthesized powders, the Ti 2p3/2 and Ti 2p1/2 peaks shifted to 457.2 eV and 463.1 eV (not shown), respectively. The lower binding energy of the Ti 2p should be attributed to the incorporation of substitutional N into TiO2 lattice [19-20]. In Figure 6, the Ti 2p3/2 peak can be fitted into two Gaussian peaks at 457.4 and 456.9 eV, respectively. The former is attributed to the Ti4+ on the surface; the latter is ascribed to the Ti3+ on the surface. It is clear that the presence of Ti3+ arose from the depositing of Pt on the surface of TiO2 [21].

Figure 6. Ti 2p XPS spectrum of the Pt/TiO2-xNx powders.
The N 1s XPS spectrum of the as-synthesized powders showed a broad peak that could be deconvoluted into two peaks, as shown in Figure 7. The binding energies centered at 400.0 and 397.2 eV were assigned to molecularly chemisorbed nitrogen ϒ-N2 and substitutional nitrogen β-N [12,22-23], respectively. The β-N species were nitrogen interacting with Ti through Ti-N bond formation and contributed to the band-gap narrowing which lead to a significant red shift of the absorption edge to the visible-light region [20]. Using the Gaussian fitting and quantitative analysis, the relative percentage of the two N species are 58.6 % and 41.4 % for β-N and ϒ-N2, respectively. It is revealed the substitutional nitrogen is more than the chemisorbed nitrogen on the surface of the as-prepared powders.

Figure 7. N 1s XPS spectrum of the Pt/TiO2-xNx powders.
According to the reports [18,24-27], the typical O 1s electron binding energy for TiO2 molecure is 529.3 eV. Figure 8 shows the O 1s spectrum of Pt/TiO2-xNx centered at 528.5 eV. In this case, the binding energy was shifted to a lower value, which could be due to the substitutional N-doping into TiO2 lattice and affected the binding energy of the O 1s peak.

Figure 8. O 1s XPS spectrum of the Pt/TiO2-xNx powders.
In Figure 9, the Pt 4f peak of Pt/TiO2-xNx consisted of three individual peaks, corresponding to metallic Pt, PtO, and PtO2, respectively. The peak at 71.6 eV can be attributed to metallic Pt, the peaks at 73.0 eV and 74.8 eV can be assigned to Pt oxides PtO and PtO2, respectively. The different species of Pt on the surface of TiO2-xNx have different photoactivity and optical absorption properties. Therefore, the photosensitization of Pt/TiO2-xNx samples to visible light was partially caused by the presence of Pt, PtO, and PtO2 on the surface of TiO2-xNx. Using the Gaussian fitting and quantitative analysis, the relative percentage of the three Pt species are 6.7 %, 30.0 %, and 63.3 % for Pt, PtO, and PtO2, respectively. The XPS results revealed that PtO2 is the predominant species on the TiO2-xNx surface.

Figure 9. Pt 4f XPS spectrum of the Pt/TiO2-xNx powders.
Photocatalytic activity
Methylene blue (MB), an organic dye, was selected as target compound because MB is ubiquitously used and the removal of the dye from wastewaters has been an acute problem [28]. The photocatalytic activity of the Pt/TiO2-xNx was quantitatively examined by photocatalytic degradation of methylene blue in water for 24 h irradiation using visible light. As can be seen from Figures 10 and 11, the concentration of methylene blue decreased with increasing the irradiation time. The activity of Pt/TiO2-xNx with Pt loading in the range of 0.1–0.6 wt% is much higher than that of TiO2-xNx (0 wt% Pt) and the commercial TiO2 photocatalyst P-25 in the methylene blue degradation. An optimum content of Pt impurity was 0.2 wt%, and any lower or higher content of Pt impurity was detrimental to the advancement of photoactivity. The presence of Pt on TiO2-xNx favors the migration of photoproduced electron to platinum thus improving the electron–hole separation. On the basis of the relevant band positions of Pt and TiO2-xNx, Pt clusters at a lower concentration act as a separation center. The photogenerated electrons are transferred from TiO2-xNx conduction band to the Pt conduction band and the holes accumulate in the TiO2-xNx valence band. Hence, photogenerated electrons and holes were efficiently separated. However Pt clusters at a higher concentration act as a recombination center and the recombination rate between electrons and holes increases exponentially with the increase of Pt concentration because the average distance between trap sites decreases by increasing the number of Pt clusters confined within a particle [21]. Furthermore, too much Pt clusters on TiO2-xNx would shield the photosensitive TiO2-xNx surface, scatter the visible light to decrease the absorption by TiO2-xNx, and subsequently decrease the surface concentration of the electrons and holes available for further reactions.
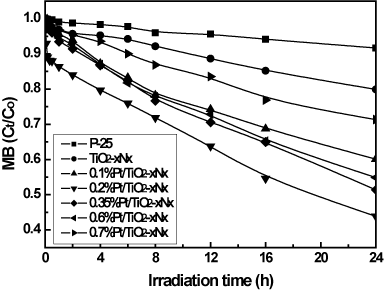
Figure 10. Time profiles of photocatalytic degradation of MB by Pt/TiO2-xNx with different Pt content in comparison with P-25.
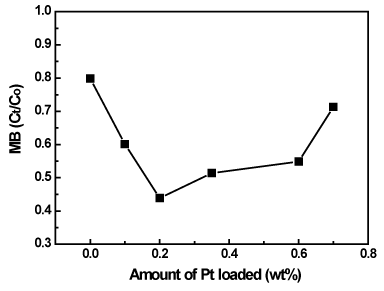
Figure 11. Influence of Pt content on the photocatalytic activity of Pt/TiO2-xNx photocatalysts in photocatalytic degradation of MB.
Nanosized Pt/TiO2-xNx photocatalysts with a typical size of 4-8 nm were prepared by the microemulsion method. XPS analysis revealed the existence of Pt0, Pt2+, and Pt4+ species on the surface of the Pt/TiO2-xNx and nitrogen interacting with Ti through Ti-N bond formation. Both the Pt-doping and substitutional N contribute to the response to visible light. The pore size distributions are noticeably narrow, indicating the good homogeneity of the as-prepared powders. The specific surface area and total pore volume of the as-prepared powders are much higher than that of commercial TiO2 photocatalyst P-25 (Degussa), which is beneficial to increase the opportunities of contacts between photocatalysts and dyes in water, resulting in high efficiency of destruction for dye compounds. The photocatalytic degradation of MB dye in aqueous solutions under visible light irradiation was promoted with doping an appropriate amount of Pt on the TiO2-xNx surface. The optimal content of Pt was found to be 0.2 wt%.
The authors gratefully acknowledge the financial support of the Ministry of Science and Technology of the Republic of China (Taiwan).
- Linsebigler AL, Lu GQ, Yates JT Jr. (1995) Photocatalysis on TiO2 surfaces principles, mechanisms, and selected results. Chem Rev 95: 735-758.
- Mills A, Hunte SL (1997) An overview of semiconductor photocatalysis. J Photochem Photobiol A: Chem 108: 1-35.
- Hoffmann MR, Martin ST, Choi WY, Bahnemann DW (1995) Environmental applications of semiconductor photocatalysis. Chem Rev 95: 69-96.
- Sreethawong T, Yoshikawa S (2006) Enhanced photocatalytic hydrogen evolution over Pt supported on mesoporous TiO2 prepared by single-step sol–gel process with surfactant template. Inter J Hydrogen Energy 31: 786-796.
- Li CH, Hsieh YH, Chiu WT, Liu CC, Kao CL (2007) Study on preparation and photocatalytic performance of Ag/TiO2 and Pt/TiO2 phototocatalysts. Sep Purif Technol 58: 148-151.
- Torimoto T, Ito S, Kuwabata S, Yoneyama H (1996) Effects of adsorbents used as supports for titanium dioxide loading on photocatalytic degradation of propyzamide. Environ Sci Technol 30: 1275-1281.
- Li X, Xiong R, Wei G (2009) Preparation and photocatalytic activity of nanoglued Sn-doped TiO2. J Hazard Mater 164: 587-591. [Crossref]
- Rhee CH, Bae SW, Lee JS (2005) Template-free hydrothermal synthesis of high surface area nitrogen-doped titania photocatalyst active under visible light. Chem Lett 34: 660-661.
- Irie H, Watanabe S, Yohino N, Hashimoto K (2003) Visible-light induced hydrophilicity on nitrogen-substituted titanium dioxide films. Chem Commun 11: 1298-1299.
- Jang JS, Kim HG, Ji SM, Bae SW, Jung JH, et al. (2006) Formation of crystalline TiO2-xNx and its photocatalytic activity. J Solid State Chem 179: 1067-1075.
- Asahi R, Morikawa T, Ohwaki T, Aoki K, Taga Y (2001) Visible-light photocatalysis in nitrogen-doped titanium oxides. Science 293: 269-271. [Crossref]
- Sakatani Y, Koike H (2001) Japan Patent P2001-72419A.
- Fox MA, Dulay MT (1993) Heterogeneous photocatalysis. Chem Rev 93: 341-357.
- Tai CY, Lee MH, Wu YC (2001) Control of zirconia particle size by using two-emulsion precipitation technique. Chem Eng Sci 56: 2389-2398.
- Lee JS, Lee JS, Choi SC (2005) Synthesis of nano-sized ceria powders by two-emulsion method using sodium hydroxide. Mater Lett 59: 395-398.
- Wang J, Sun J, Bian X (2004) Preparation of oriented TiO2 nanobelts by microemulsion technique. Mater Sci Eng A 379: 7-10.
- Sing FKSW, Everett DH, Haul RAW, Moscou L, Pierotti RA, et al. (1985) Reporting physisorption data for gas/solid systems with specific reference to the determination of surface area and porosity. Pure Appl Chem 57: 603-619.
- Zhang X, Zhang F, Chanb KY (2006) The synthesis of Pt-modified titanium dioxide thin films by microemulsion templating, their characterization and visible-light photocatalytic properties. Mater Chem Phys 97: 384-389.
- Zerkout S, Achour S, Mosser A, Tabet N (2003) On the existence of superstructure in TiNx thin films. Thin Solid Films 441: 135-139.
- Miao L, Tanemura S, Watanabe H, Mori Y, Kaneko K, et al. (2004) The improvement of optical reactivity for TiO2 thin films by N2–H2 plasma surface-treatment. J Cryst Growth 260: 118-124.
- Li FB, Li XZ (2002) The enhancement of photodegradation efficiency using Pt-TiO2 catalyst. Chemosphere 48: 1103-1111. [Crossref]
- Saha NC, Tompkins HG (1992) Titanium nitride oxidation chemistry: an X-ray photoelectron spectroscopy study. J Appl Phys 72: 3072-3079.
- Shinn ND, Tsang KL (1991) Strain-induced surface reactivity: low temperature Cr/W(110) nitridation. J Vac Sci Technol 9: 1558-1562.
- Li FB, Li XZ (2002) Photocatalytic properties of gold/gold ion-modified titanium dioxide for waste treatment. Appl Catal A 228: 15-27.
- Wong MS, Chou HP, Yang TS (2006) Reactively sputtered N-doped titanium oxide films as visible-light photocatalyst. Thin Solid Films 494: 244-249.
- Jang HK, Whangbo SW, Kim HB, Im KY, Lee YS, et al. (2000) Titanium oxide films on Si100 deposited by electron-beam evaporation at 250°C. J Vac Sci Technol 18: 917-921.
- Sayers CN, Armstrong NR (1978) X-ray photoelectron spectroscopy of TiO2 and other titanate electrodes and various standard titanium oxide materials: surface compositional changes of the TiO2 electrode during photo-electrolysis. Surf Sci 77: 301-320.
- Gnaser H, Savina MR, Calaway W, Tripa CE, Veryovkin IV, et al. (2005) Photocatalytic degradation of methylene blue on nanocrystalline TiO2: surface mass spectrometry of reaction intermediates. Int J Mass Spectrom 245: 61-67.











