Abstract
Objectives: The purpose of this study was to describe treatment strategies for patients with advanced gynecologic malignancies and small bowel obstructions in order to better understand how best to approach treatment and optimize outcomes.
Methods: Women with gynecologic malignancies hospitalized for SBO from 2006-2019 at a single institution were identified. Clinical, treatment, and outcome data were collected. Risk factors were reported using descriptive statistics and compared between treatments. Survival analyses were performed using the Kaplan-Meier method.
Results: We analyzed 288 admissions from 253 unique patients. The majority had ovarian cancer (60.4%). Of the 288 admissions, 163 (56.6%) underwent conservative management, 60 (20.8%) acute surgical intervention, and 65 (22.6%) palliative procedure. In pairwise comparison, patients undergoing surgical intervention had fewer comorbidities (1.98 versus 2.66, p=0.017) and were more likely to have a high-grade versus partial obstruction (p<0.001). Overall survival (OS) was worse for patients who received palliative management. There was no difference in OS among patients who underwent conservative versus surgical management.
Conclusions: Number of comorbidities and type of obstruction were significantly different between patients who failed conservative management and those who required surgical or palliative intervention.
Key words
gynecologic cancer, small bowel obstruction, ovarian cancer
Introduction
Small bowel obstructions (SBO) are frequent complications that occur in patients with advanced gynecologic malignancies. Patients with advanced ovarian cancer are at particularly high risk with a lifetime incidence of 5-51% in published studies [1-4]. Commonly, SBO occurs in patients with poor functional status with advanced disease and limited life expectancy, and these diagnoses often can be considered pre-terminal events.
The initial treatment of SBO is typically conservative management with bowel rest, intravenous fluids, anti-emetics, pain control and nasogastric tube placement for bowel decompression. However, despite similar initial clinical presentations, some women ultimately require acute surgical interventions or palliative procedures to relieve symptoms. Surgical intervention in this setting includes palliative gastrostomy tube placement as well as more invasive procedures including intestinal resection and ostomy creation. These more invasive surgical interventions are associated with high morbidity and perioperative mortality [5]. However, surgical intervention has also been associated with several improved clinical outcomes, including shorter duration of hospitalization, improved pain management, and longer median survival [6-7]. The decision to proceed with surgical intervention can be challenging in this population, as often patients have poor prognoses and are poor surgical candidates due to concurrent medical comorbidities [5].
Several retrospective studies have evaluated risk factors for morbidity and mortality from the surgical management of SBO. Identified risk factors include older age, poor nutritional status, hypoalbuminemia, presence of ascites and carcinomatosis [8]. However, no studies have been performed to identify risk factors that can differentiate which patients who present with a new diagnosis of SBO will fail conservative management and require surgical intervention. The primary aim of this study is to describe clinical features of women with gynecologic malignancies undergoing conservative, surgical and palliative treatments for SBO in order to better understand how best to approach treatment and individualize management for these patients.
Materials and methods
Institutional Review Board approval was obtained at the University of Pittsburgh. International Classification of Disease-10 codes were utilized to retrospectively identify women with gynecologic malignancies hospitalized for SBO from 2006-2019 at a single academic center. Clinical, treatment, and outcome data were collected.
Risk factors for SBO were reported using descriptive statistics. Conservative management was defined as any treatment that did not involve surgical intervention and could include bowel rest with nothing by mouth status, intravenous fluids, administration of antiemetic and pain medications, nasogastric tube placement, and total parental nutrition. Surgical management was defined as requiring any operative procedure to relieve the obstruction with the exception of gastrostomy tube placement. Patients receiving palliative management were those who underwent gastrostomy tube placement for palliation during their admission. Conservative management was compared with surgical and palliative management using chi-square/Fisher’s exact test for categorical risk factors and Mann-Whitney U test for continuous risk factors.
A sensitivity analysis was conducted using Generalized Estimating Equations (GEE) models to account for multiple admissions from the same patients during the study period. Both approaches lead to the same conclusions. We chose to report the results treating all admissions independently. Survival analyses were performed using the Kaplan-Meier method and log-rank test. All analyses were conducted using SPSS version 24 (IBM Corp., Armonk, NY). A p value <0.05 was considered significant.
Results
Two hundred eighty-eight admissions from 253 unique patients were identified, of which 90.5% (n=229) had one admission and 10.4% (n=24) had multiple admissions (Table 1). Most patients were white (n=234, 92.5%), and the mean BMI was 27.1 kg/m2 (range 14.0-57.6 kg/m2). The majority of patients had ovarian cancer (n=184, 72.7%).
For approximately half of the admissions (n=163, 56.6%), the SBO resolved with conservative management. Of the admissions for SBO that did not resolve with conservative measures, 60/125 (48%) underwent acute surgical intervention, and 65/125 (52%) underwent palliative gastrostomy tube placement.
In univariate pairwise comparison, patients undergoing acute surgical intervention had a lower mean number of comorbidities (1.98 versus 2.66, p=0.017) and were more likely to have a high grade versus partial bowel obstruction (p<0.001) when compared to those who resolved with conservative management (Table 2). Patients undergoing palliative gastrostomy tube placement had received a higher mean number of prior chemotherapy lines (2.69 versus 2.0, p=0.01), were more likely to have platinum resistant disease (p=0.003), chronic opioid use (p=0.003), high grade versus partial bowel obstruction (p=0.04), active cancer on presentation (p=0.015), and were more likely to have a nasogastric tube placed on admission (p=0.011) when compared to those who resolved with conservative management.
Table 1. Demographics and clinical characteristics of patient cohort
|
n = 288 admissions |
Total Cohort |
Conservative Management |
Surgical Management |
Palliative Management |
Number, n (%) |
253 (100) |
163 (56.6) |
60 (20.8) |
65 (22.6) |
Age, mean ± SD |
62.1 ± 11.5 |
62.4 ± 11.7 |
59.6 ± 9.2 |
60.4 ± 13.1 |
BMI (kg/mg2), mean ± SD |
27.1 ± 7.4 |
28.2 ± 8.2 |
26.5 ± 7.7 |
27.1 ± 7.8 |
Race/Ethnicity, n (%)
White
Black
Other |
234 (92.5)
13 (5.1)
6 (2.4) |
145 (88.9)
12 (7.3)
6 (3.7) |
59 (98.3)
1 (1.7)
0 |
60 (92.3)
4 (6.2)
1 (1.5) |
Type of Cancer, n (%)
Ovarian*
Uterine
Cervical
Other** |
184 (72.7)
43 (17.0)
14 (5.5)
12 (4.7) |
117 (71.8)
28 (17.2)
10 (6.1)
8 (4.9) |
40 (66.7)
15 (25.0)
4 (6.7)
1 (1.67) |
52 (80.0)
7 (10.8)
2 (3.1)
4 (6.2) |
Stage, n (%)
I
II
III
IV
Unknown/Recurrent |
23 (9.1)
20 (7.9)
126 (49.8)
35 (13.8)
49 (19.4) |
18 (13.6)
15 (11.4)
69 (52.3)
30 (22.7) |
7 (12.9)
2 (3.7)
34 (63.0)
11 (20.4) |
1 (2.1)
3 (6.4)
36 (76.6)
7 (14.9) |
* Includes ovarian, fallopian tube and primary peritoneal cancer.
** Other = Mullerian not otherwise specified, vulvar, multiple primaries.
Abbreviations: n=number, SD=standard deviation, BMI=body mass index
Table 2. Univariate pairwise comparison of factors associated with SBO management strategy
|
Conservative vs. Surgical
p value |
Conservative vs. Palliative
p value |
Age |
0.093 |
0.264 |
BMI |
0.153 |
0.415 |
Tobacco Use Status |
0.297 |
0.614 |
Race/Ethnicity |
0.119 |
1.00 |
Years Since Initial Cancer Diagnosis |
0.425 |
0.747 |
Type of Cancer |
0.789 |
0.127 |
Prior Radiation Therapy |
0.159 |
0.435 |
History of Prior SBO |
0.077 |
0.711 |
Number of Prior Abdominal Surgeries |
0.392 |
0.221 |
Platinum Resistance |
0.359 |
0.003 |
Chronic Opioid Use |
0.908 |
0.003 |
Active Cancer at SBO Diagnosis |
0.482 |
0.001 |
Receiving Cancer-Directed Therapy at Time of SBO Diagnosis |
0.993 |
0.111 |
Type of Bowel Obstruction
High grade
Partial |
<0.001 |
0.040 |
Number of Prior Chemotherapy Lines (mean ± SD) |
0.209 |
0.010 |
Number of Medical Comorbidities (mean ± SD) |
0.017 |
0.202 |
Abbreviations: SD=standard deviation, BMI=body mass index, SBO=small bowel obstruction
Numerous demographic and clinical factors were not significantly different on univariate analysis between the patient groups, and these included age, smoking status, years since cancer diagnosis, type of cancer, history of prior bowel obstruction, history of prior radiation therapy, history of prior abdominal surgery, and receiving cancer-directed therapy at the time of SBO diagnosis. Moreover, abdominal pain or emesis on admission, peritoneal carcinomatosis or ascites on admission imaging, number of obstruction points noted on imaging, and serum laboratory values were also not significantly different between groups.
Multivariable analysis for risk factors was limited due to small sample sizes, missing data and closely correlated clinical variables.
A minority of patients went on to receive additional cancer-directed therapy after discharge (n=80, 31.6%). There was no significant difference in overall survival among patients who underwent conservative management versus surgical management (42 versus 36 months, p=0.449, Figure 1). Patients who received palliative management had significantly shorter overall survival when compared to those who underwent conservative management (3 versus 42 months, p<0.001, Figure 2).

Figure 1. Overall survival in patients undergoing surgical versus conservative management
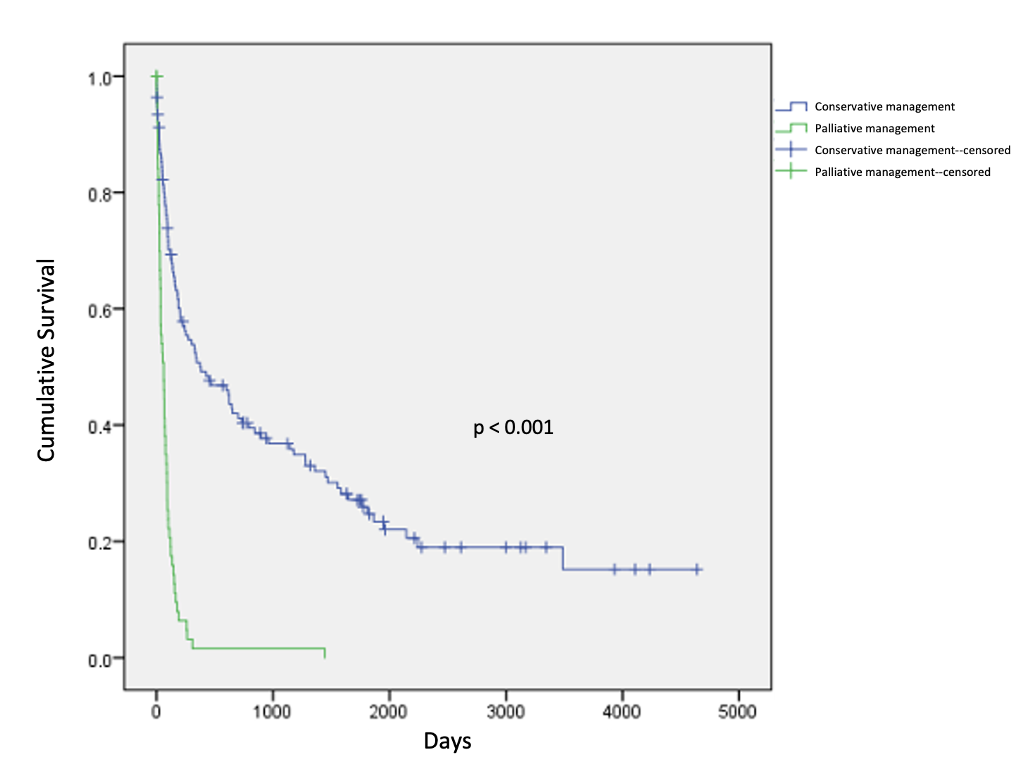
Figure 2. Overall survival in patients undergoing palliative versus conservative management
Discussion
SBO is a frequent complication in patients with advanced stage gynecologic malignancies. Given the high morbidity but potential for significant clinical benefit of surgical intervention in the management of SBO in patients with gynecologic malignancies, careful selection of surgical candidates is critical.
This study demonstrates that many patients with gynecologic malignancies admitted for SBO will ultimately require a procedural intervention, including surgical intervention or palliative gastrostomy tube placement, for successful management of the obstruction. In this cohort, patients with a lower mean number of comorbidities and patients with evidence of high-grade obstruction on imaging were more likely to undergo acute surgical intervention as compared to those patients who achieved resolution of SBO with conservative management. When compared to patients who were successfully managed with conservative strategies, patients ultimately requiring palliative gastrostomy tube placement were more likely to have features of advanced malignancy including platinum resistance, chronic opioid use, active cancer at the time of SBO diagnosis, evidence of high grade obstruction on imaging, and higher mean number of prior chemotherapy lines.
Etiologies of SBO in patients with advanced gynecologic malignancies include widespread carcinomatosis, extrinsic compression or intraluminal occlusion from disease, and adhesive disease. The clinical course and prognosis for patients diagnosed with SBO can vary depending on the etiology of the obstruction. Thus, careful appraisal of the details of a patient’s presentation as well as her disease status, comorbidities and life expectancy is critical when making decisions regarding surgical intervention for alleviation of SBO in this patient population.
There are numerous potential benefits to acute surgical intervention for patients with gynecologic malignancies and a diagnosis of SBO. A systematic review of patients with advanced abdominal malignancies and SBO demonstrated that surgical intervention is successful in relieving obstructive symptoms, enabling resumption of a diet, and allowing discharge to home [5]. Several retrospective studies demonstrate that surgical intervention in appropriately selected patients with advanced and recurrent ovarian cancer is associated with longer overall survival when compared to patients conservatively managed [6, 9-10]. Surgical intervention has also been associated shorter hospitalization duration, improved pain management, and decreased re-obstruction rates compared to conservative management [6]. However, while real benefits to acute surgical intervention exist, there are also significant risks to these procedures. Surgical intervention in patients with advanced abdominal cancer, including gynecologic malignancies, are associated with high morbidity (7-44%), high perioperative mortality (6-32%) and frequent readmissions (38-74%) [5].
Thus, appropriate patient selection for surgical intervention is critical. However, there are few published studies that evaluate clinical variables as factors predictive of surgical outcome. Moreover, these studies group acute surgical intervention and palliative gastrostomy tube placement together and also include patients with large bowel obstruction which are inherently different in terms of general management. Additionally, these studies tend to focus on clinical factors predictive of prolonged survival in order to aid in appropriate patient selection for surgery rather than identifying risk factors that specifically predict failure of conservative management. Factors correlated to survival in these studies include age, nutritional status, stage, presence of ascites, as well as the amount of prior chemotherapy and/or radiation therapy previously received [1-2,5,6,11].
This study focuses on identifying unique clinical factors of women with gynecologic malignancies diagnosed with SBO according to their ultimate management strategy (conservative, surgical or palliative) in order to better define risk factors for failure of conservative management to relieve SBO. We found that patients with fewer medical comorbidities and with evidence of high-grade obstruction on imaging were more likely to undergo acute surgical intervention than have resolution with conservative management. We also found that patients with features of advanced malignancy were more likely to require palliative gastrostomy tube placement. These differences can be used as a part of anticipatory counseling during a trial of conservative management and incorporated into overall decision making given survival outcomes reported in this study.
Strengths of this study include its heterogeneous population of women with gynecologic malignancies, as most available studies focus solely on patients with ovarian cancer. Additionally, this study focuses exclusively on SBO and does not include patients with large bowel obstructions which are typically managed as surgical emergencies. Our study is limited by its retrospective design and single institution nature, and lack of outcome data. Additionally, multivariable analysis was limited due to small sample sizes and missing data.
Conclusions
Many patients with gynecologic malignancies admitted for SBO will ultimately require a procedural intervention, including surgical intervention and palliative gastrostomy tube placement, for successful management of the obstruction. A better understanding of risk factors for surgical intervention and palliative procedures can aid in counseling, medical optimization, and treatment decisions for patients at high risk for failure of SBO resolution with conservative management. If specific risk factors for an increased probability of requiring surgical management for SBO resolution can be identified early in a patient’s clinical course, improved preoperative planning, optimization of medical comorbidities and nutritional status, and more informed goals of care discussions can be performed prior to surgical intervention. Additionally, more timely surgical intervention can be enacted for patients at high risk of failure of conservative management. Further investigation is necessary to further delineate these risk factors.
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Synopsis
Small bowel obstruction is a frequent complication in patients with advanced stage gynecologic malignancies, and many patients admitted for SBO will ultimately require a procedural intervention. Patients with a lower mean number of comorbidities and patients with evidence of high-grade obstruction on imaging were more likely to undergo acute surgical intervention compared to patients whose symptoms improved with conservative management.
Conflict of interest
The authors have no conflicts of interest or financial disclosures.
Funding
This project was supported in part by the National Institutes of Health through Grant Number UL1-TR-001857.
References
- Sartori E, Chiudinelli F, Pasinetti B, Maggino T (2009) Bowel obstruction and survival in patients with advanced ovarian cancer: analysis of prognostic variables. Int J Gynecol Cancer 19: 54-57. [Crossref]
- Feuer DJ, Broadley KE, Shepherd JH, Barton DP (2000) Surgery for the resolution of symptoms in malignant bowel obstruction in advanced gynaecological and gastrointestinal cancer. Cochrane Database Syst Rev CD002764. [Crossref]
- Mooney SJ, Winner M, Hershman DL, Wright JD, Feingold DL, et al. (2013) Bowel obstruction in elderly ovarian cancer patients: a population-based study. Gynecol Oncol 129: 107-112. [Crossref]
- Lee YC, Jivraj N, O’Brien C, Chawla T, Shlomovitz E, et al. (2018) Malignant bowel obstruction in advanced gynecologic cancers: An updated review from a multidisciplinary perspective. Obstet Gynecol Int 2018: 1867238. [Crossref]
- Paul Olson TJ, Pinkerton C, Brasel KJ, Schwarze ML, Schwarze ML, et al. (2014) Palliative surgery for malignant bowel obstruction from carcinomatosis: a systematic review. JAMA Surg 149: 383-392. [Crossref]
- Daniele A, Ferrero A, Fuso L, Mineccia M, Porcellana V, et al. (2015) Palliative care in patients with ovarian cancer and bowel obstruction. Support Care Cancer 23: 3157-3163. [Crossref]
- Pothuri B, Vaidya A, Aghajanian C, Venkatraman E, Barakat RR, et al. (2003) Palliative surgery for bowel obstruction in recurrent ovarian cancer: An updated series. Gynecol Oncol 89: 306-313. [Crossref]
- Jin M, Shen F, Li M, Chen Y (2020) Palliative treatment for bowel obstruction in ovarian cancer: a meta-analysis. Arch Gynecol Obstet 302: 241-248. [Crossref]
- Bais JMJ, Schilthuis MS, Slors JFM, Lammes FB (1995) Intestinal obstruction in patients with advanced ovarian cancer. Int J Gynecol Cancer 5: 346-350. [Crossref]
- Mangili G, Aletti G, Frigerio L, Franchi M, Panacci N, et al. (2005) Palliative care for intestinal obstruction in recurrent ovarian cancer: a multivariate analysis. Int J Gynecol Cancer 15: 830-835. [Crossref]
- Krebs HB, Goplerud DR (1983) Surgical management of bowel obstruction in advanced ovarian carcinoma. Obstet Gynecol 61: 327-330. [Crossref]


