Key words
chemotherapy-induced cardiomyopathy, cardioprotection, echocardiograph
Cancer and cardiovascular (CVD) diseases are two separate disease entities but share many different clinicopathologic characteristics [1]. Intensive chemotherapy improves longevity in cancer patients but increases the risk of developing chemotherapy-induced cardiomyopathy (CCM), also known as chemotherapy-induced cardiotoxicity (CIC). In most cases, CCM manifests as left ventricular (LV) systolic dysfunction or clinically overt heart failure (HF). With a large and growing population of cancer survivors, and reports that CVD is a leading cause of death among them [2,3], prevention and treatment of CCM is critical for improved prognosis and survival. Current clinical management approaches have been undermined by the reliance on small singl-center studies and medical experience. However, two position papers on practice guidelines for cardiotoxicity management by the European Society for Medical Oncology (ESMO) and the European Society of Cardiology (ESC) mark an important initial step for the development of strong evidence to guide clinical management of CCM. This article thus reviews CCM with an emphasis on clinical description, risk factors, pathophysiology, prognosis, clinical presentation, diagnosis and clinical management.
Abbreviations
ACE: Angiotensin-Converting Enzyme; ANT: Anthracyclines; BNP: B-type Natriuretic Peptides; CCM: Chemotherapy-Induced Cardiomyopathy; CEF: Cyclophosphamide, Epirubicin, Fluorouracil; CHF: Congestive Heart Failure; CHOP: Cyclophosphamide, Vincristine, Doxorubicin, Prednisone; CIC: Chemotherapy-Induced Cardiotoxicity; CRT: Cardiac Resynchronization Therapy; CT: Computed Tomography; CVD: Cardiovascular Diseases; DNR: Daunorubicin; DOX: Doxorubicin; ESC: European Society of Cardiology; ESMO: European Society for Medical Oncology; FDC: Fluorouracil, Doxorubicin, Cyclophosphamide; HER-2+:Human Epidermal Growth Factor Receptor 2 Positive; HF: Heart Failure; HTx: Heart Transplantation; LV: Left Ventricular; LVD: Left Ventricular Dysfunction; LVEF: Left Ventricular Ejection Fraction; LVSD: Left Ventricular Dysfunction; MRI: Magnetic Resonance Imaging; MUGA: Multiple-Gated Acquisition; NYHA: New York Heart Association; RNVG: Radionuclide Ventriculography; STE: Speckle Tracking Echocardiography; TDI: Tissue Doppler Imaging
Description
Historical Context: Chemotherapy-induced cardiomyopathy is a well-documented adverse event in cancer patients. The initial documentation goes back to between 1963 and 1967 when Di Marco and research associates published a series of reports [4-7] evaluating therapeutic activities of daunomycin in transplanted murine tumor models. Although daunomycin inhibited the growth of tumor cells and prolonged survival, it had a highly toxic effect on neoplastic cells and cardiac function. Later in 1967, Tan and associates [8] were the first to evaluate therapeutic effect of daunomycin in humans and reported its association with CCM with a marked predominance in children with leukemia. The study brought into clinical focus CCM and its role in the development of HF in cancer survivors. Since then, subsequent studies have demonstrated the most common clinical manifestations of CCM is left ventricular dysfunction (LVD) and clinically overt HF [9,10].
Clinical Definition: Despite increased recognition of CCM as a distinct disease entity, both clinical trials and practice guidelines lack consensus on generally accepted international definition. Large randomized trials have proposed various definitions (Table 1).
Table 1. Definitions of CCM in Large Randomized Trials. (CHF: Congestive Heart Failure; LVEF: Left Ventricular Ejection Fraction; NYHA: New York Heart
Association).
1st Author [Ref #] |
Chemotherapeutic Agent |
Suggested Definitions |
Schwartz [11] |
Doxorubicin |
10% LVEF decline;
LVEF < 50% in patients with > 50% at baseline;
LVEF < 30% in patients with < 50% at baseline |
Slamon [12] |
Trastuzumab |
NYHA classification |
O’Brien [13] |
Anthracylines |
20% LVEF decline in patients with > 50%;
10% LVEF decline in patients with < 50%;
Clinical CHF |
Tan-Chiu [14] |
Trastuzumab |
10% LVEF decline to < 55% |
Romond [15] |
Doxorubicin, cyclophosphamide, Trastuzumab |
> 16% LVEF decline |
Ryberg [16] |
Anthracylines |
LVEF < 45%;
15% LVEF decline from baseline |
The basis of the suggested definitions of CCM are the development of LVD (defined as depressed LVEF] and/or manifestations of symptomatic HF. The definitions are supported by reports that the incidence of HF and LVD range between 5% and 65% depending on the diagnostic criteria adopted [17,18]. Broadly, these definitions suggest CCM is a functional or structural heart injury related to anti-cancer treatment. In addition to these definitions, the ESC [19] classifies HF as depressed LVEF (< 40%), mid-range LVEF (40-49%) and preserved LEVF (>50%). On the other hand, oncologists base their definition on the Cardiac Review and Evaluation Committee on trastuzumab-associated cardiotoxicity and the ESMO Clinical Practice Guidelines. They define CCM as a decrease of LVEF by 5% or more to less than 55% in the presence of symptoms of HF or an asymptomatic decrease in LVEF by 10% or more to less than 55% and symptoms of CHF [20]. Both the ESC and ESMO emphasize that a change in LVEF is the basis for clinical definitions of CCM.
Epidemiology
Most studies and registries on LV systolic dysfunction or HF in cancer patients have not specifically analyzed CCM, which has led to an incomplete understanding of its true prevalence. The few clinical trials evaluating the etiology of HF in detail report a prevalence of 1% in all the reported cases of cardiomyopathies [21,22]. In a similar finding, Cardinale et al. [23] report the occurrence of clinical HF in cancer survivors treated with anthracyclines range between 1% and 5%, and usually accompanied by a significant decrease in LV function between 5% and 20%. However, the prevalence is significantly higher in oncology studies, which report more than 50% of cancer survivors exhibit some degree of cardiac dysfunction between 10 and 20 years after chemotherapy and 5% of them go on to develop clinically overt HF [24]. In support, Silber et al. [25] report more than 60,000 cancer patients receive treatment for CCM annually in the Unites States (U.S), suggesting the overall CCM incidence maybe underestimated in literature.
Chemotherapeutic agents: Several chemotherapeutic agents have been associated with the development of CCM and subsequently HF. Of these agents, the most frequently mentioned are (a) cytotoxic agents (anthracyclines [ANT]) such as doxorubicin (DOX) and epirubicin; (b) monoclonal antibodies such as trastuzumab; and (c) alkylating agents such as cyclophosphamide and ifosfamide [26].
Cytotoxic Agents: Cytotoxic agents is a class of anti-cancer drugs that work by preventing the growth or replication (mitosis) of cancerous cells or inducing apoptosis (cell-death). They can be used alone or in combination with radiotherapy [9]. ANT is the most common cytotoxic agent, ranked among the most efficacious anti-cancer drugs ever-developed [27]. For more than three decades, ANT continue to play a prominent role in the treatment and clinical management of a wide variety of hematologic and solid malignant tumors [28]. In the early 1960s, the first ANT were isolated from pigment-producing Streptomyces peucetius and named DOX and daunorubicin (DNR) [29]. While DOX is an essential component in the treatment of breast cancer, childhood tumors, soft tissues sarcomas and aggressive lymphomas, DNR is an essential component in the treatment of acute lymphoblastic or myeloblastic leukemia. Both DOX and DNR demonstrate dose-response relationship in several chemotherapy regimens but suffer from the development of resistance in tumor cells or toxicity necessitating dose reduction [29].
Monoclonal Antibodies: Trastuzumab is an anti-tumor monoclonal antibody used in the treatment of Human Epidermal Growth Factor Receptor 2 Positive (HER2+) breast cancer. The drug works by blocking the HER2+ growth factor receptor, which is over-expressed in about 15% to 20% of breast tumors [30,31]. Trastuzumab was approved for clinical use in 1998 after Phase III trials demonstrated significant improvement in the overall survival and a reduction in the risk of relapse [32]. However, trastuzumab monotherapy was associated with a significantly depressed cardiac function in many patients. Slamon et al. [12] reviewed trastuzumab-ANT/cyclophosphamide combination therapy and reported incidences of 27% and 16% of cardiac dysfunction and NYHA Class III or IV symptoms respectively.
Alkylating Agents: Alkylating agents such as cyclophosphamide, ifosfamide and mitomycin represent another group of chemotherapy drugs used in the treatment of cancer. They work by adding alkyl group to DNA protein molecules causing the breakage of DNA strands, which interferes with the ability of cancerous cells to multiply, and consequently induces apoptosis [9]. Cyclophosphamide has dose-response effect on cardiac myocytes. With doses of greater than (≥) 150mg/kg, cyclophosphamide is associated with HF events in 7% to 28% of cancer patients while Ifosfamide is associated with 17% events in cancer patients receiving doses ≥ 12.5g/m2 [17,33]. Mitomycin enhances DOX-induced cardiomyopathy when administered concurrent with or sequential to DOX therapy [34-36]. Mitomycin-induced cardiomyopathy is dose-dependent occurring at doses ≥ 30 mg in cancer patients treated previously or concomitantly with DOX [37].
Other Agents: Several other chemotherapeutic agents have also been associated with the development of HF in cancer patients (Table 2).
Table 2. Other Chemotherapeutic Agents and their Association with HF
Other Chemotherapeutic Agents |
Association with Heart Failure |
Microtubule Agents (Paclitaxel & Docetaxal) |
Used to treat multiple malignancies with low incidence of HF at 1.68% [38] |
Proteosome Inhibitors (Bortezomib) |
For treating multiple Myeloma & mantle cell lymphoma with low HF incidence at 2% to 5% [39]. |
Small Molecule Tyrosine Kinase Inhibitors (Lapatinib/Suntinib) |
Small-scale study on Lapatinib reports low rates of symptomatic cardiac failure (1.7%) but events increases to 2.2% and 1.7% with prior treatment of ANT or Trastuzumab respectively [40]. Reports on Suntinib suggest 10% incidence of asymptomatic drop in LVEF > 10% with full recovery when treatment is completed [41]. |
Monoclonal Antibody-based Tyrosine Kinase Inhibitors (Bevacizumab) |
The reported incidence of HF is about 1.7% and 3.0% [34]. |
Types of CCM: Classically, CCM has been classified into two: Type I and Type II mainly based on reversibility of myocardial injury and morphological myocardial abnormalities. The basis of this classification has been the effect of chemotherapeutic agents: ANT and trastuzumab [28]. However, this classification is not very useful in clinical practice since patients usually receive a cocktail of chemotherapy drugs making it challenging to determine the specific mechanism of each drug. Table 3 lists the main features of Type I and Type II CCM.
Table 3. Features and Types of CCM. Adapted from Shakir et al., 2009, p. 9 [28].
Feature |
Type I |
Type II |
Cellular |
Cellular death beginning with first drug administration |
Cellular (mitochondrial) dysfunction |
Biopsy |
Changes in biopsy |
No changes in biopsy |
Dose-relation |
Cumulative dose-relation |
No cumulative dose-relation |
Damage |
Permanent damage (Myocyte death) |
Predominantly reversible |
Risk Factors |
Combination therapy – prior or concurrent radiotherapy; age; previous cardiac disease; hypertension |
Paclitaxel, prior or concurrent anthracylines; age; previous cardiac disease; obesity (BMI > 25kg/sm) |
Type I: Anthracycline-Induced: Type I CCM is usually associated with ANT class of anti-cancer drugs. It is a more serious cardiac condition usually leading to permanent damage to the myocardium characterized with myocyte apoptosis. Its cardiotoxicity is cumulative dose-related effect, where each administration conveys additive myocardial damage [26]. Prior or concurrent radiotherapy, age, hypertension or concomitant cardiac disease may aggravate Type I associated cardiotoxicity [28].
Type II: Trastuzumab-Induced: Type II CCM is associated with trastuzumab induced cardiac dysfunction. It is less serious and potentially reversible [26]. Its effect is not dose-dependent and conveys no changes in biopsy. However, CCM classification based on myocardial damage does have important limitations. Trastuzumab (Type II drug) can also trigger irreversible myocardial damage in patients with severe pre-existing cardiac disease or aggravate ANT-induced (Type I) cardiomyopathy [28].
Risk factors: Repeatedly cited risk factors associated with chronic progressive cardiotoxicity in cancer patients following exposure to chemotherapy include (a) cumulative dose [23,42,43]; (b) rate of administration [42,44-49]; (c) female gender [50,51]; (d) younger and older age [23,42,49,50]; (e) pre-existing cardiac condition and hypertension [42]; and (f) mediastinal irradiation [52,53,54]. Whereas younger and older age are risk factors, for pediatric patients receiving anthracyclines in their childhood, the time interval since first administration is also a potential a risk factor cited in several clinical trials [42,43,50,55].
Cumulative Dose: Several chemotherapeutic agents could cause CCM but current research has a strong focus on ANT-induced cardiotoxicity, particularly the association between DOX and cardiotoxicity or HF. Von Hoff et al. [42] was one of the initial studies to report an association between cumulative dose and CHF secondary to doxorubicin-induced cardiomyopathy. Doxorubicin dose < 400 mg/m2 was associated with 0.14% CHF events between 0 and 231 days after the completion of ANT therapy. The CHF events increased significantly to 7% and 18% at higher doses of 550 mg/m2 and 700 mg/m2 respectively. The rapid increase in cardiotoxicity at doses > 550 mg/m2 informed current maximum dose to minimize DOX-induced cardiotoxicity and HF [33]. Despite dose limitation, there is great individual variability in DOX doses in both adults and children. Von Hoff et al. [42] series reported in 3,943 cancer patients receiving > 1,000 mg/m2, none of them exhibited clinically diagnosed CHF. However, accumulative dose of ANT that does not induce cardiotoxicity has not been established [55]. Whereas ANT-induced cardiotoxicity is related to peak plasma drug-concentration, its anti-neoplastic activity is proportional to the total systemic drug exposure or tissue concentration over time [44]. DOX is less toxic when administered in a prolonged continuous intravenous infusion over more than 48 to 96 hours. Dosage in excess of 50 mg/m2 per day increases the risk of cardiotoxicity by 2.81 times [49]. Less toxicity associated with exposure to ANT over time may explain the current administration of ANT regimens given as weekly injections instead of a single bolus injection every three weeks [45-48].
Age of Exposure: Several clinical trials and reviews have reported pediatric patients appear to be at a greater risk of developing anthracycline-induced cardiotoxicity [42,49,50]. Exposure to ANT therapy at the age of < 4 years appears to be a significant risk factor associated with increased afterload because of reduced ventricular wall thickness [42]. In children, anthracyclines alters the transcription of myocellular proteins [56], which could explain the reduction in ventricular wall in children exposed to ANT [42,55]. In adults, the risk of ANT-induced CHF increases relative to patient age [42], with females appearing more vulnerable [50,51]. The study also reports an increasing risk of ANT-induced CHF in patients with previous cardiac disease and hypertension.
Mediastinal Irradiation: ANT chemotherapy may be used alone or in combination with radiotherapy in cancer patients with hematological and solid neoplasms. However, mediastinal irradiation has been associated with elevate risk of ANT-induced cardiotoxicity [52-54]. In a survey of 1,273 cancer patients, Praga et al. [52] report significant higher (p=0.01) severity of histopathological changes in patients with prior exposure to mediastinal irradiation. Multivariate analysis based on histological evidence shows previous mediastinal irradiation and higher rate of drug administration are the most important independent risk factors for the development of CCM in cancer survivors [28].
Pathophysiology
Anthracycline-Induced CCM: The pathophysiologic mechanism of ANT-induced CCM is not completely understood. Although thought to be multifactorial, the most widely accepted pathophysiologic mechanism is the formation of ANT-iron complexes and the stimulation of free-radical formation [57-60]. Evidence supporting this finding are reports of iron-chelating compounds inhibiting cardiotoxic effect of ANT chemotherapy [61]. In particular, the mammalian myocyte has been shown to be more susceptible to free radical damage because of relatively less superoxide dismutase and catalase activities, and that, DOX suppresses glutathione peroxidase, which is the principal myocyte defense against free radical damage [28]. Superhydroxide free radicals accumulate and lead to severe lipid peroxidation resulting into destruction of mitochondrial membranes, endoplasmic reticulum and nucleic acid [62,63].
Besides iron-dependent free radical formation, Zhang et al. [64] study on molecular basis of DOX-induced cardiotoxicity based on murine models reported a defective mitochondrial biogenesis and the formation of reactive oxygen species (ROS) maybe a possible pathologic mechanism for the development of CCM. The study observed the deletion of the enzyme Top2b (encoding topoisomerase-II beta) in cardiac myocytes conveyed a protective effect against DOX-induced breaks and transcription changes in DNA double strands that causes defective mitochondrial biogenesis, ROS and HF. The findings suggest topoisomerase-II beta may mediate DOX-induced cardiotoxicity in mammals. Other probable pathologic mechanisms for CCM include reduced production of adenosine triphosphates, inhibited synthesis of nucleic acid and protein synthesis, impaired mitochondrial synthesis, induced myocyte apoptosis and increased immune functions [16,65,66].
Trastuzumab-Induced CCM: The pathophysiology of Trastuzumab-Induced CCM is mostly associated with the blockade of HER2+ receptor signals in cardiac myocytes, which is important for repairing cardiac myocyte [67]. Trastuzumab administered in combination with anthracylines exacerbates cardiotoxicity. Anthracyclines causes initial oxidative damage to cardiac myocytes. The heavily damaged cells then undergo apoptosis or necrosis while the remaining ones undergo repair processes but remain temporarily vulnerable, a condition referred to as “vulnerable window hypothesis” [68]. In the presence of trastuzumab, the inhibition of HER2+ interferes with the usual repair mechanism, exacerbating myocyte apoptosis and necrosis [9]. Three recent clinical trials examining sequential therapy of anthracylines and trastuzumab support this hypothesis [12,69,70]. The initiation of a three-week interval between treatment was associated with a 3.8% reduction of NYHA class III and IV, and HF, [69] which reduced to 0.6% with a 90-day interval with 7% systolic dysfunction [70] compared to 27% reported by salmon et al. [12]. In a majority of cases, Trastuzumab induced HF or LVD is a sub-acute phenomenon with more cases reported during treatment. However, the condition is reversible after drug withdrawal as well as is not dose-responsive [34].
Prognosis: In comparison with other more frequently encountered forms of cardiomyopathies, ANT-induced cardiomyopathy has an unfavorable prognosis with a two-year mortality rate of up to 60% [21,42,72] as well as refractory to conventional therapy. However, a majority of evidence on the natural history of CCM and its therapy are anecdotal or based on findings from older studies, in which standard therapy was the administration of digoxin and diuretics [42,72]. In addition, there is a paucity of clinical trials examining response to therapy of CCM-associated HF as well as the efficacy of angiotensin-converting enzyme inhibitors (ACE-I) and β-blockers in the treatment of CCM-associated HF. Further, limited data on treated and untreated CCM patients has undermined the understanding of prognostication of CCM. As a result, evidence-based recommendation for clinical management of cancer survivors with symptomatic and asymptomatic HF are lacking, which has contributed to the absence of universally accepted clinical guidelines of CCM [24]. However, available evidence suggests the onset of CCM has a negative effect on cardiac outcomes on cancer patients limiting treatment options [73,74]. About 30% to 60% of CCM patients with poor prognosis may require adjunctive chemotherapy for the prevention of disease relapse after initial chemotherapy within 5 years [75,76]. The presence of CCM could also restrict the choice of oncologic treatment within five years [17,77-79].
Clinical presentation: Cardiac events associated with CCM may manifest as mild changes in blood pressure, thrombosis, ECG abnormalities, arrhythmias, myocarditis, myocardial infarction (MI), LV failure and CHF. As such, using LVEF changes alone in CCM patients is substantially limited by reports that about half of HF patients may have normal LVEF with their overall cardiac outcomes being similar to patients with low LVEF [28]. Clinical presentation of CCM may be categorized into acute, sub-acute or chronic progressive sub-types. The disease may also present as late sequalae, several years after the end of treatment [28].
Acute/Sub-Acute: Acute or sub-acute cardiotoxicity occurs within a week after chemotherapy. It may occur even after a single dose of chemotherapeutic agent but conveys no serious clinical consequences. In most cases, transient electrophysiologic abnormalities manifest as electrocardiogram (ECG) changes in about 20% to 30% of CCM patients. These ECG abnormalities include non-specific ST and T wave changes, T- wave flattening, suppressed QRS voltage or prolonged QRS interval [33]. About 0.5% to 3% of patients with CCM may present with arrhythmias such as ventricular, supraventricular and junctional tachycardia with reported overall incidence of 0.7% [80]. However, lethal arrhythmias such as atrial fibrillation and atrial flutter are rare occurrence in CCM patients [80]. Sub-acute cardiotoxicity usually leads to LV failure, pericarditis or, in rare cases, fatal pericarditis-myocarditis syndrome [81]. Arrhythmias or ECG changes have no relation with the chronic progressive cardiotoxicity [17].
Chronic Progressive: Chronic progressive cardiotoxicity is the more frequently encountered and clinically significant sub-type of CCM. The chronic subtype is associated with ANT-induced cardiomyopathy, which may present as an early onset during the first year of treatment or may progress gradually and manifest years or even decades after the completion of chemotherapy [42]. Some studies categorize chronic progressive CCM into (a) early onset and (b) late onset [17,82] based on Von Hoff’s et al. [42] retrospective study on 4,018 patients records where HF occurred between 0 and 231 days after the completion of ANT therapy. However, Steinherz et al. [23] observe that chronic progressive occurs along a continuum of time with the incidence and severity of LV systolic dysfunction increasing with the length of follow-up. Other factors such as cumulative dose and the presence of other risk factors such as exposure to mediastinal irradiation and time of follow-up may explain the variable study findings of systolic complications in cancer patients [17]. The incidence of systolic dysfunction as high as 65% has been reported with low cumulative doses of 228 mg/m of DOX in leukemia patients ten years after completion of chemotherapy [83]. On the other hand, the incidence of HF during the first year of treatment or within a year after the completion of treatment has been as low as 3% [17,83,84]. Early onset chronic progressive sub-type is the strongest predictor of late onset chronic progressive sub-type [17,50,55,83].
Diagnosis: Diagnosis aims to identify cancer survivors at a higher risk of developing CCM or to detect already developed CCM [85]. The most frequently used modality for the detection of CCM is periodic measurement of LVEF using echocardiography or multigated acquisition scanning (MUGA). However, the lack of evidence-based guidelines to monitor CCM during and after chemotherapy seriously undermines diagnosis. Although ESC [19] and ESMO [20] have provided practice guidelines, they do not specify the frequency, method or length of time required for the assessment of cardiac function both during and after chemotherapy. In addition, LVEF measurement is relatively insensitive for assessing CCM during the sub-clinical stage due to the lack of any significant changes in LV function until a substantial amount of myocardial damage has occurred, which is usually after the exhaustion of compensatory mechanisms [20].
Electrocardiography: Electrocardiography (ECG) is an inexpensive and readily available tool for monitoring and managing many cardiovascular diseases. Although in CCM ECG may show no changes or non-specific polarization abnormalities that do not predict the development of CCM, for cancer patients presenting with chest discomfort, palpitation or dyspnea, it may suggest a contributing or an underlying cardiac condition such as acute coronary syndrome (ACS), arrhythmia or electrolyte imbalance [86]. Further, in cancer patients with infiltrative diseases, low voltage on ECG often suggests cardiac involvement [85].
Chest Radiography: Chest radiographs are used to diagnose conditions that affect the chest, its contents and nearby structures. It may reveal an enlarged cardiac silhouette caused by pericardial effusion or cardiac chamber enlargement. In cancer patients with HF symptoms, chest radiograph presents clues on volume overload suggested by pulmonary artery enlargement due to elevated pulmonary pressures, pulmonary edema or pleural effusions. Further, coronary artery or aortic calcification may indicate atherosclerotic disease [85].
Echocardiography: Echocardiography due to its availability and reproducibility is considered a gold standard in the assessment and monitoring of cardiac function in cancer patients [87]. Current echocardiography imaging standards suggest the assessment of LV size and systolic function using Simpson’s method of discs. Further, 3D imaging achieves improved measurement of global and regional systolic and diastolic function as well as accurate evaluation of valvular, hemodynamics and pericardial diseases, and their functional effects [85]. Although used extensively, echo-defined changes in systolic function alone are non-specific in early detection of subclinical cardiac injury. Tjeerdsma et al. [88] reports after chemotherapy, 50% of asymptomatic patients with normal LVEF have diastolic dysfunction on echocardiogram. In support, Stoddard et al. [89] and Ganz et al. [90] report the addition of diastolic dysfunction in the diagnosis of CCM improves the accuracy of predicting early cardiotoxicity. In addition, although significant echocardiography changes are usually noted following chemotherapy in 25% of patients with no observable symptoms of cardiotoxicity [98], reliance on only LVEF could underestimate the extent of cardiac dysfunction [91].
Despite the loss of myocyte function, the heart has compensatory reserve to maintain LVEF and LVEF depressed only after exhausting the compensatory reserve. Speckle-tracking strain imaging (or myocardial deformation imaging) is a recent echocardiographic technique used as an independent measure of preload deformation useful for detecting early CCM-related cardiac changes. It has been demonstrated that subclinical diastolic dysfunction and changes in strain imaging precede systolic dysfunction and thus are important in facilitating early detection of CCM in cancer patients following chemotherapy [92,93]. Further, echocardiography is also important in the assessment of co-occurring disease such as amyloid and carcinoid as well as reveal incidental finding such as thrombus [85]. However, echocardiography in cancer patients is subjective and suffers from inter-observer variability, certain imaging protocols contrast and 3D imaging may affect LVEF measurement, and LVEF may depended on loading and unloading conditions on the vasculature [85].
Radionuclide Ventriculography: Radionuclide Ventriculography (RNVG) or multiple-gated acquisition (MUGA) scan is used to assess global LV systolic function and ventricular size and regional wall motion. However, RNVG is not very accurate in the assessment of LV function in cancer patients with arrhythmias as well as does not detect valvular lesions and pericardial diseases [85]. Further, although the correlation of echocardiographic visual and quantitative radionuclide LVEF is good, RNVG is preferred for monitoring cardiac function due to its reproducibility in quantifying LVEF [94]. Schwartz et al. [11] retrospective study of 1,487 patients treated with Doxorubicin over seven-year period reports RNVG is a valuable imaging method for the prediction of CHF. The study defined the risk of Doxorubicin-induced cardiomyopathy based on one of the following three criteria: (a) Decline in LEVF > 10%; (b) Baseline LVEF < 50%; and (c) Total Doxorubicin dose of 450 mg/m. Still, detailed analysis of cardiac structure is better suited to other imaging techniques such as echocardiography and MRI [11].
Cardiac Magnetic Resonance: Cardiac magnetic resonance imaging (MRI) is a newer technique for evaluating ventricular function, valves, anatomy and myocardial perfusion. It has limited use in evaluating coronary artery and strategies to evaluate discology are still experimental [85].
Endomyocardial Biopsy: Endomyocardial biopsy has high sensitivity for assessing cardiac damage. It is an invasive test associated with risks of right heart catheterization and complications of RV biopsy. Endomyocardial biopsy can detect cardiotoxicity very early even before ventriculography or echocardiography can detect LV dysfunction [95]. However, endomyocardial biopsy is limited since it cannot detect all forms of CCM and is impractical for continuous monitoring and histological changes do not correlate to subsequent risk of CCM nor provide data on myocardial function and clinical state of the patient [85].
Cardiac Nuclear Imaging: There is an emerging role for cardiac nuclear imaging in the diagnosis and monitoring of CCM [96]. Flotats and Carrio [97] report indium-111 (in-111) antimyosin binds to the intra-cellular heavy cardiac myocyte after cell damage. It is incorporated inversely to blood circulation to damaged tissue such that it has clinical value in detecting myocarditis and doxorubicin-induced cardiomyopathy and other causes of cardiomyopathy [97]. Iodine-123-meta-iodobenzylguanidine (MIBG) is another useful radioactive material for imaging sympathetic innervation of the myocardium in HF patients [98]. Both in-111 antimyosin and MIBG have been used for early detection of doxorubicin-induced cardiotoxicity at the cellular level at cumulative doses of 240-300 mg/m [99]. At higher cumulative doses (420-600 mg/m), there is increased myosin uptake with decreased MIBG uptake, suggesting the value of antimyosin and MIBG in detecting cell damage and dysfunctional adrenergic neuron activity in chemotherapy patients [99].
Cardiac Biomarkers: In the last decade, cardiac biomarkers such as troponin and B-type natriuretic peptides (BNP) have proven to be sensitive and specific in early and real-time identification, evaluation and monitoring of CCM [20,99]. When examining the prognostic value of troponin in cardiac risk stratification of cancer patients receiving high doses of chemotherapy, Cardinale et al. [73] report higher incidence of cardiac events in patients with persistently high levels of troponin. The study also finds patients with no troponin elevation after chemotherapy show low incidence of cardiac complications. Troponin elevation subsequent to chemotherapy could also help identify patients at higher risk of developing a depressed LVEF [73,74]. Persistently elevated levels of BNP has also been linked to increased incidence of cardiac complications [100].
BNP levels are also useful in differentiating cardiac from pulmonary causes of dyspnea [101]. BNP levels has been used to monitor the effect of cardiotoxicity in cancer patients after chemotherapy [102,103]. Elevated BNP levels suggests impairment of LV diastolic function as well as assist in differentiating symptoms of HF and cancer [104]. Assessment of BNP biomarker should be considered early in cancer survivors presenting with symptoms of heart failure. However, elevated BNP levels in the absence of clinical signs of volume overload other causes of BNP elevation should be considered such as ischemic heart disease [105], pulmonary embolism [106] or sepsis [107]. Further, biomarkers are non-sensitive and non-specific in clearly predicting cancer patients who should avoids chemotherapy or should have an alternative therapy to chemotherapy [108-111].
Meta-Analysis of Echocardiography Diagnosis: Echocardiography is considered the cornerstone imaging modality for the assessment and monitoring of cardiac function in cancer patients [87]. Although LVEF measurement is relatively insensitive for assessing CCM during the sub-clinical stage, changes in global systolic myocardial strains (myocardial deformation) evaluated using speckle-tracking echocardiography (STE) strain imaging has been recently shown to predict LVD [92,93]. This meta-analysis aims to examine the value of echocardiographic assessment of myocardial strain for early detection of myocardial damage in cancer patients compared to LVEF.
Search Criteria: Search for studies on diagnosis of CCM were conducted in three leading online databases: Medline, EMBASE and Cochrane Library using a combination of free text and thesaurus terms for chemotherapy-induced cardiomyopathy and diagnostic methods: chemotherapy-induced cardiomyopathy, anthracycline-induced cardiomyopathy, doxorubicin-induced cardiomyopathy, echocardiography, cardiac MRI, and ventriculography. Additional studies were identified by screening bibliographies. Abstracts were included only if they reported sufficient detail enabling data extraction. There was no restriction on publication language.
Inclusion Criteria: Prospective and retrospective studies assessing at least ten patients using echocardiographic criteria as the primary diagnostic method for all CCM patients. The included studies had to provide empirical data on LV parameters (strain data) during or after therapy. Studies lacking data on the pre- and post-strain analysis were excluded. Table 4 provides a summary of the studies on diagnostic features.
Table 4. Summary of STE Studies on Myocardial Deformation. (2D: Two Dimensional: ANT: Anthracyclines: DOX: Doxorubicin: NHL: Non-Hodgkin Lymphoma STE: Speckle Tracking Echocardiography; TDI: Tissue Doppler Imaging; R-CHOP: (Cyclophosphamide, Vincristine, Epirubicin, Prednisone); GLS: Global Longitudinal Strain).
1st Author [Ref. #] |
Year |
No. of Patients |
Mean Age (yrs.) |
Female (%) |
Type of Cancer |
Chemotherapeutic Agent |
Baseline GLS |
Follow-up GLS |
Baseline LVEF |
Follow-up LVEF |
Fallah-Rad et al. [112] |
2011 |
42 |
47.0 |
100 |
Breast Cancer |
Epirubicin + DOX ± Trastuzumab |
-19.8 |
-16.4 |
64.0 |
58.0 |
Stoodley et al. [113] |
2011 |
52 |
49.0 |
100 |
Breast Cancer |
Epirubicin, DOX |
-17.8 |
-16.3 |
58.6 |
56.0 |
Sawaya et al. [114] |
2011 |
43 |
49.0 |
100 |
Breast Cancer |
Epirubicin + DOX ± Trastuzumab |
-20.5 |
-19.3 |
65.0 |
63.0 |
Sawaya et al. [115]. |
2012 |
81 |
50.0 |
100 |
Breast Cancer |
ANT, Taxanes + Trastuzumab |
-21.00 |
-19.00 |
64.0 |
62.0 |
Mavinkurve-Groothuis et al. [116] |
2012 |
60 |
6.0 |
38 |
Lymphoblastic Leukemia |
ANT |
-18.2 |
-16.7 |
|
|
Poterucha et al. [117] |
2012 |
19 |
15.3 |
37 |
Various Pediatric Cancer |
ANT |
-19.9 |
-18.1 |
62.0 |
61.0 |
Al-Biltagi et al. [118] |
2012 |
25 |
9.0 |
48 |
Various Pediatric Cancers |
DOX |
-18.7 |
-15.1 |
|
|
Kang et al. [119] |
2013 |
67 |
52.6 |
46 |
Large B-cell NHL |
Epirubicin |
-18.30 |
-16.2 |
63.6 |
63.8 |
Negishi et al. [120] |
2013 |
159 |
49.0 |
80 |
Breast Cancer |
ANT±Trastuzumab |
-20.7 |
-18.3 |
62.5 |
56.7 |
Stoodley et al. [121] |
2013 |
78 |
52.0 |
99 |
Breast Cancer |
Epirubicin, DOX |
-18.6 |
-17.0 |
|
|
Baratta et al. [122] |
2013 |
36 |
47.0 |
58 |
Breast Cancer |
DOX ± Trastuzumab |
-20.3 |
-18.9 |
65.0 |
63.5 |
Mornos et al. [123] |
2013 |
74 |
51.0 |
58 |
Breast, Lymphoma |
ANT |
-21.2 |
-19.0 |
|
|
Kang et al. [124] |
2014 |
75 |
53.9 |
45 |
NHL |
R-CHOP |
-18.48 |
-15.56 |
65.0 |
64.8 |
Total/Average |
|
811 |
40.83 |
69.92 |
|
|
-19.49 |
-17.37 |
65 |
64.8 |
Results
Thirteen (13) peer-reviewed publications that met the inclusion criteria were included in this meta-analysis [112-124]. Data extracted from the studies included publication year, number of patients, mean age, percentage of women, type of cancer, chemotherapeutic agent, baseline and follow-up GLS and LVEF. Most of the studies assessed patients with breast cancer [112-115,120-123] while the remaining studies assessed hematological malignancies such as non-Hodgkin lymphoma [119,124], and lymphoblastic leukemia [116]. The included patients were treated with ANT regimens (mainly DOX and epirubicin) either alone or in combination with trastuzumab. All the studies assessed myocardial damage using speckle-tracking echocardiography (STE) for between 3 and 12 months. The combined patient population was 811, which included both adults and pediatrics (mean age = 40.83 years; female = 69.9%).
Despite heterogeneity in patient age, types of cancer and follow-up periods, all the 13 studies reported myocardial deformation (changes in myocardial strain) occurred much earlier than changes in LVEF or LV fractional shortening. Using STE, the studies report at baseline (during or immediately after treatment) and at 1 to 3 months follow-up, there was no significant change in mean LVEF (65% t0 64.8%). However, there was a marked reduction of global longitudinal strain (GLS) from -19.49% at baseline to -17.37% at follow-up, translating into a reduction of 2.17%, range 9% to 19%. In addition, early changes in GLS > 11% predicted subsequent cardiotoxicity in patients with asymptomatic and symptomatic LV dysfunction [113,120] and GLS < 19% has a sensitivity of 74%, specificity of 73%, positive predictive value of 53% and negative predictive value of 87% for developing cardiotoxicity [115].
Discussion
Echocardiographic-defined changes in LVEF remains the basis of many current definitions and clinical diagnosis of CCM (or cardiotoxicity) in cancer patients [87]. However, it is non-specific in the detection of subclinical CCM or subtle alterations in LV function in asymptomatic patient [85,88,89]. With reports that changes in myocardial strain may precede systolic dysfunction and help predict patients at a higher risk of developing cardiotoxicity, research interest in the use of STE to assess myocardial strain in cancer patients has grown [92,93]. Thus, the aim of this meta-analysis was to evaluate the value of echocardiographic-defined changes in myocardial strain in predicting patients at risk of developing CCM.
Several key messages emerge from this meta-analysis. The reduction of echocardiographic measures of LV myocardial deformation signal subclinical myocardial changes due to cancer therapy. These reductions precede changes in LVEF, which occur much later. The early reduction of myocardial deformation parameters predict subsequent development of CCM defined as a decrease of LVEF ≥ 5% to < 55% [19,20]. The most consistent myocardial deformation parameter was global longitudinal strain (GLS). The threshold of GLS changes range between 10% and 15% or values lower than 19% as measured by STE [113]. However, significant changes in GLS are more common during ANT treatment or immediately after.
Other myocardial deformation parameters – global radial strain (GRS) and global circumferential strain (GCS) were also reviewed. A reduction of 6% to 17% in GRS [113,115,116,122,123] and 11% to 16% in GCS [114,115,116] was associated with a higher risk of developing subsequent LV systolic dysfunction. However, both GRS and GCS have two important limitations. First, they are less consistent. Second, they have very low reproducibility. These two limitations makes identification of changes between pre- and post-treatment challenging [115].
The early detection of subtle myocardial abnormalities in cancer patients treated with ANT and/or trastuzumab has important clinical implications. Specifically, Type I cardiomyopathy associated with ANT-treatment usually detected several years after therapy [33]. Thus, there was a growing interest in detecting CCM during the subclinical phase in cancer survivors using myocardial deformation to prognosticate clinically relevant outcomes. This is important since once LVEF is significantly depressed, it may be too late to prevent further LV remodeling or it would undermine the efficacy of treatment [115]. Early detection of at risk patient may help guide subsequent treatment to avoid adverse cardiac side effects and its progression to HF [87]. Although myocardial deformation measured by STE could predict the development of subsequent cardiomyopathy, there is need to examine strain changes in larger multi-center studies as well as in other types of cancers that use potentially cardiotoxic regimens. There is also need to understand the long-term effect of strain changes occurring during therapy and the effect of the current interventions on the natural course of CCM.
Whereas several imaging modalities including magnetic resonance imaging and MUGA scans fare available or assessing early myocardial changes in cancer patients, echocardiography remains the mainstay imaging modality. It is versatile, low cost, available, able to assess more than ventricular function and avoids repeat radiation [87]. The widespread use of echocardiography supports the current research interest in using STE to assess myocardial deformation in cancer patients. In echocardiography, Tissue Doppler Imaging (TDI) and STE-based strains have been used to assess myocardial deformation in cancer patients on chemotherapy but STE has been shown to provide the most clinically relevant data. Compared to STE, TDI has important challenges limiting its use (a) it requires data acquisition for each myocardial segment and careful attention given to frame rate and aligning walls with Doppler beam and (b) measurement of strain is noisy requiring significant expertise [115]. On the other hand, STE is able to easily measure GLS from all the 18 myocardial segments as well as allows measurement of GCS and GRS in multiple segments and rotation parameters. Finally, STE has superior reproducibility to TDI-based strain analysis.
Clinical management: The ideal goal for the management of cancer patients would be chemotherapeutic agents that protect them from cardiotoxicity without altering cytotoxicity of abnormal cells. The ESMO clinical practice guidelines propose algorithms for clinical management of cardiomyopathy due to ANT and trastuzumab chemotherapy as illustrated in Figures 1 and 2.
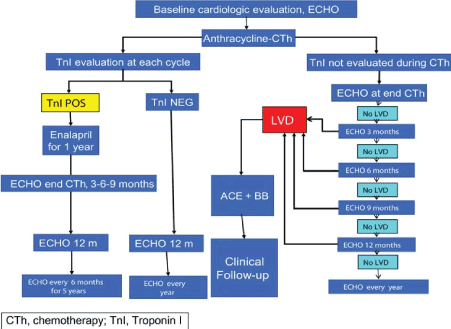
Figure 1. Algorithm for Management of ANT-Induced Cardiomyopathy (Adapted from Curigliano, 2012, p. 159 [20]).
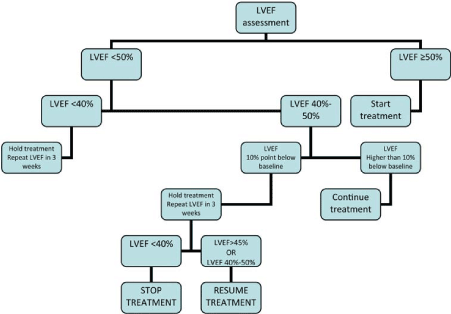
Figure 2. Algorithm for Management of Trastuzumab-Induced Cardiomyopathy. Adapted from Curigliano, 2012, p. 159 [20].
The ESMO clinical guidelines propose an algorithm for the management of ANT-induced cardiomyopathy based primarily on serial evaluation of myocardial damage by echocardiography-defined LV dysfunction and cardiac biomarker Troponin 1. LV dysfunction should be assessed by echocardiography at baseline (presentation), and during and after chemotherapy. If LVD is detected, traditional HF treatment using ACE-inhibitors and beta-blockers should be initiated, and the patient followed-up. Troponin should also be assessed at every treatment cycle. If Troponin is positive, the guidelines recommend ACE-inhibitors (Enalapril) for one year followed with echocardiograph assessment at the end of chemotherapy, at the 12th month after completion of therapy and thereafter every six months for five years.
For management of trastuzumab-induced cardiomyopathy, the ESMO clinical guidelines propose repeat evaluation of LVEF. Treatment should be initiated only if baseline LVEF ≥ 50%. In subsequent LVEF evaluation, if a change in LVEF > 10 points, treatment should be continued. However, if the change in LVEF < 10 points below baseline, treatment should be halted until subsequent evaluation indicate LVEF ≥ 45% or 40%-50%. If LVEF < 40%, treatment should be halted and repeat LVEF assessment held every three weeks. Repeat evaluation guides treatment options as per the ESMO [20] and ESC [19] clinical guidelines. Treatment includes dose continuation or dose modulation especially for cardiomyopathy due to ANT chemotherapy, which is dose-responsive. Other treatment options include the use of cardioprotective adjuvants and traditional HF treatment as discussed below.
Dose Modulation: The most effective approach for managing Type I CCM due to ANT therapy is dose modulation because of its cumulative dose-related cardiotoxic effect [26]. A growing evidence demonstrates a lower weekly dosage or continuous infusion rather than boluses achieves adequate tumor suppression while limiting myocardial damage [126,127]. However, dose modulation may also interfere with the efficacy of chemotherapy since its maximal effect depends on concentration and the amount of time the drug is present in the body [125].
Cardioprotective Adjuvants: Dexrazoxane is the most studied and efficacious cardioprotective adjuvant tested to date for the management of CCM. Human and murine studies demonstrates that the drug directly competes with topoisomerase II, interacts with iron to diminish oxidative stress in cardiomyocytes and may stimulate the expression of mitochondrial antioxidant enzymes [128,129,130]. Lipshultz et al. [131] clinical trial on the cardioprotective role of dexrazoxane reports children with acute lymphoblastic leukemia treated with doxorubicin and dexrazoxane duo therapy reduced myocardial injury indicated by decreased serum troponin T. Although the optimal dose of dexrazoxane has not been empirically established, the American Society of Clinical Oncology 2008 clinical guidelines recommend a ratio of 10:1 dexrazoxane-to-anthracycline administered 15 to 30 minutes before administering doxorubicin [132]. However, dexrazoxane use has been limited to cancer patients receiving a cumulative dose of 300 mg/m2 because of potential impact on antitumor efficacy [132].
Another common cardioprotective adjuvant is flavonoid monoHER. In both in vivo and ex vivo murine models, pretreatment with monoHER protects against doxorubicin-induced cardiotoxicity [133,134]. Both in vivo and ex vivo experiments report that monoHER does not interfere with antitumor effect of anthracycline [135]. High doses of monoHER > 1,500 mg/m are suggested for potentiating the antitumor effect and low doses for cardioprotection [136]. However, there is need for further clinical trials to establish dose response characteristics of monoHER cardioprotective effect for patients administered with chemotherapy.
Heart Failure Management: Classical pharmacotherapy management of HF using β-adrenergic antagonists and angiotensin-converting enzyme (ACE) inhibitors have also been used in the management of CCM. Carvedilol (a β-blocker) has cardioprotective effect against ANT-induced cardiomyopathy, which maintains LV diameters and better preserves diastolic function [137]. Early administration of β-blocker in combination with ACE-inhibitors improves cardiac contractility in DOX-induced cardiomyopathy [138]. In CCM patients secondary to Epirubicin treatment, administration of ACE-inhibitors such as Enalapril or Ramipril restore systolic function after stopping administration of diuretic [139]. In mouse models, Sildenafil (a phosphodiesterase 5 inhibitor) has been shown to attenuate cardiomyocytes apoptosis, preserve mitochondrial function, and prevent ST-interval prolongation and LV dysfunction [140]. A combined therapy of Sildenafil and Doxorubicin attenuates cell-killing effect of Doxorubicin on cancer cells [141]. In addition to the traditional HF medical therapy, the value of mechanical circulatory support and heart transplantation have also been examined in CCM patients. Cardiac resynchronization therapy (CRT) also has a beneficial effect on patients with DOX-induced cardiomyopathy, refractory to medical therapy and satisfy the criteria for device implantation. CRT improves LVEF, and reduces end-diastolic dimensions and HF symptoms and improves LV function. However, long-term outcomes of CRT remain unknown [142]. Ward et al. [143] studied heart transplantation (HTx) on CCM patients and observed HTx is a viable treatment option for pediatric patients with intractable cardiac failure due to ANT-therapy. Survival outcomes were comparable to those of International Society for Heart and Lung Transplantation (ISHLT) Registry data for all pediatric recipients and tumor recurrence was rare.
Meta-Analysis of Cardioprotective Therapy for CCM: Cardiomyopathy due to chemotherapy has had several reviews [17,34,1342,145]. However, these reviews have a very wide scope, examine very many different chemotherapeutic agents or are opinion pieces prone to bias in selection and results presentation. Further, many systematic reviews on CCM have been published but specifically focus on a particular type of cancer (such as Hodgkin's lymphoma) [146], or type of patients (pediatrics) [147,148] or adults [149,150]. This meta-analysis specifically goes further to analyze randomized controlled trials (RCTs) that examine cardioprotective adjuvants. The aim is to determine their overall value in the prevention of CCM in both adult and pediatric cancer patients.
Search and Inclusion Criteria: The search for studies on cardioprotective adjuvants was conducted in two online databases EMBASE and PUBMED using a combination of free text and thesaurus terms for individual chemotherapy drugs combined with terms for different tumor types, cardiotoxicity and RCTs. Additional studies were identified from reviewing of bibliographies of the selected studies and reviews. Studies with only abstracts were included for further review if they provided sufficient numerical data. Studies were included if they (a) reported on chemotherapeutic agents given alone (control group) or in combination with cardioprotective adjuvants (study/intervention group); (b) assessed at least one of the following outcomes: clinical cardiotoxicity (presence of CHF) or subclinical cardiotoxicity (changes in LV function [a reduction in LVEF] as defined by echocardiographic findings). For studies that used a duplicate cohort of cancer patients, the latest study was included. There was no restriction on the publication language. The following data was extracted from the included studies: author, year of publication, population of both the study and control groups, intervention used (chemotherapy agent with and without cardioprotective adjuvant), cardiotoxic events, CHF, mean change in LVEF and cardiac deaths. Table 5 provides a summary of details of the included studies and their outcomes on cardiotoxicity.
Table 5. Summary of Cardioprotection Agents and Outcomes for Managing Cardiotoxicity (CHF: Congestive Heart Failure; CHOP: Cyclophosphamide, Vincristine, Doxorubicin, Prednisone; Dox: Doxorubicin; FDC: Fluorouracil, Doxorubicin, Cyclophosphamide; CEF: Cyclophosphamide, Epirubicin, Fluorouracil).
1st Author [Ref. #] |
Year |
Study Group (N) |
Control Group (N) |
Intervention
(Cardioprotective Agent) & Control Group |
Cardiotoxic Events |
CHF |
Mean Δ LVEF |
Cardiac Death (%) |
Myers et al. [137] |
1983 |
24 |
30 |
Dox + N-acetylcysteine |
|
3 |
|
|
Dox |
|
3 |
|
|
Milei et al. [138] |
1987 |
13 |
13 |
Adriamycin + Prenylamine |
0 |
0 |
|
0 |
Adriamycin + Placebo |
3 |
2 |
|
3 |
Speyer et al. [139] |
1992 |
76 |
74 |
FDC + Dexrazoxane |
6 |
|
|
1 |
FDC |
10 |
|
|
1 |
Venturini et al. [140] |
1996 |
82 |
78 |
CEF/Epirubicin + Dexrazoxane |
6 |
2 |
|
|
CEF/Epirubicin |
9 |
4 |
|
|
Gallegos-Castorena et al. [141] |
2007 |
15 |
13 |
Dox + Amifostine |
0 |
|
|
|
Dox |
2 |
|
|
|
Lopez et al. [142] |
1998 |
129 |
NR |
Epirubicin + Dexrazoxane |
4 |
0 |
1.0 |
|
Epirubicin |
6 |
4 |
-8.0 |
|
Swain et al. [143] |
1999 |
534 |
NR |
FDC + Dexrazoxane |
9 |
2 |
|
0 |
FDC |
17 |
4 |
|
2 |
Kalay et al. [151] |
2006 |
25 |
25 |
Anthracycline + Carvedilol |
|
|
0.8 |
1 |
Anthracycline |
|
|
-16.6 |
4 |
Marty et al. [152] |
2006 |
85 |
79 |
Dox/Epirubicin + Dexrazoxane |
1 |
1 |
|
|
Dox/Epirubicin |
11 |
8 |
|
|
Waldner et al. [153] |
2006 |
20 |
20 |
CHOP + L-Cartinine |
|
|
-1.6 |
|
CHOP |
|
|
-1.6 |
|
Acar et al. [154] |
2011 |
20 |
20 |
Anthracycline + Statin (Atorvastatin) |
|
|
1.3 |
|
Anthracycline |
|
|
-7.9 |
|
Bosch et al [10] |
2013 |
45 |
45 |
Anthracycline + Enalapril + Carvedilol |
|
|
-0.17 |
|
Anthracycline |
|
|
-3.28 |
|
Silber et al. [25] |
2004 |
69 |
66 |
Anthracycline + Enalapril |
1 |
|
|
|
Anthracycline |
6 |
|
|
|
Cardinale et al. [155] |
2006 |
56 |
58 |
Anthracycline + ACE-Inhibitor |
0 |
|
-0.3 |
|
Anthracycline |
25 |
|
-10.9 |
|
Seicean, et al. [156] |
2012 |
67 |
134 |
Anthracycline + Statin |
|
4 |
|
|
Anthracycline |
|
23 |
|
|
Seicean et al [157] |
2013 |
106 |
212 |
Anthracycline /Trastuzumab + β-Blocker |
|
5 |
|
|
Anthracycline /Trastuzumab |
|
27 |
|
|
Georgakopoulos et al. [158] |
2010 |
85 |
40 |
Dox + Metoprolol + Enalapril |
3 |
|
|
|
Dox |
3 |
|
|
|
Study Characteristics
Seventeen (17) studies [10,25,137-143,151-158] meeting the inclusion criteria were included in this meta-analysis. The studies assessed the efficacy of cardioprotective adjuvants on preventing the development of CCM on cancer patients treated with chemotherapy. The studies assessed several clinical outcomes: cardiotoxicity events [25,138-143,152,155,158]; congestive heart failure [137,138,140,143,144,152,156,157]; LVEF [10,142,151,153-155] and cardiac deaths [138,139,143,151]. Dexrazoxane was the most studied cardioprotective adjuvant in seven studies [137,139-143,152]. Other cardioprotective adjuvants assessed were N-acetylcysteine [137], Prenylamine [138], Carvedilol [10,151], L-Cartinine [153], and statin (Atorvastatin) [154]. Two studies assess ACE-inhibitor [25,155,158] and beta-blocker [157]. The combined patient population in the 11 studies was 1,332 randomly assigned into either study (intervention) group or control group.
Results
Cardiotoxicity events were the common outcomes assessed in ten (10) studies [25,138-143,152,155,158]. Cancer patients administered with chemotherapeutic agent in combination with a cardioprotective adjuvant (study group) had fewer total cardiotoxicity events (39) compared to the control group (receiving only chemotherapy) that had more events (92). Eight studies [137,138,140,143,144,152,156,157] reported incidence of congestive heart failure (CHF), which was much higher in control group (75 cases), compared to study group (17 cases). Although the overall LV parameter (LVEF) had no significant difference between baseline and follow-up, changes in the control group were significant. Four studies that assessed cardiac death report lower number of cases in study group (two cases) compared to control or placebo group (10 cases).
Despite heterogeneity on patient age, follow-up periods, chemotherapeutic agents and cardioprotective adjuvants used in the individual studies, overall analysis of the study reveals a lower risk of cancer patients developing CCM with a dual therapy of chemotherapeutic agents and cardioprotective adjuvants compared to monotherapy of chemotherapeutic agents. (Odds Ratio: 0.319, 95%, Confidence Interval: 0.234-0.434). Figure 3 shows the summary of results on individual studies and overall comparing the effect of chemotherapy alone and chemotherapy with cardioprotective adjuvants on the development of CCM.
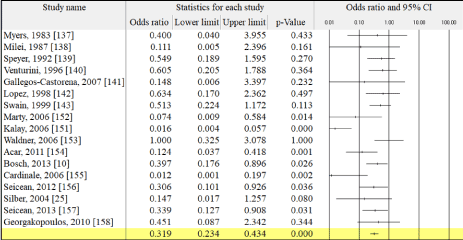
Figure 3. Chemotherapy and Cardioprotective Adjuvants Compared to Chemotherapy Alone
Discussion
Although chemotherapy remains one of the mainstay of treatment for a variety of hematologic and solid malignant tumors, it conveys an additional risk of developing cardiomyopathy. For instance, Anthracyclines increase the risk of developing clinical CCM by 5.43, subclinical CCM by 6.25 and cardiac death by 4.94 [159]. Modulation or temporary discontinuation of chemotherapy dosage limits its cardiotoxic effect but limit its efficacy since it is optimal effect depends on concentration and the period of exposure to the drug [125]. To address this conundrum, research interest on cardioprotective adjuvants has grown. The intention is to allow optimal chemotherapy dosage (increases) for maximal tumor suppression while at the same time limiting its myocardial damage. Thus, the aim of this meta-analysis is to examine the value of cardioprotective adjuvants on predicting and consequently preventing the development of CCM on cancer patients treated with chemotherapeutic agents.
Several key messages have emerged from this meta-analysis. Chemotherapy with concomitant cardioprotective adjuvants conveys a protective effect against subsequent development of CCM. Cardioprotective adjuvants help to maintain LV function and are associated with reduced incidences of congestive heart failure and cardiac-related deaths. A recent systematic review and meta-analysis of single-center prospective studies on cardiotoxicity of ANTs in the treatment of cancer [158] also associate ANT-regimens with an elevated risk of subsequent development of CCM compared to ANT-regimens given concomitant with cardioprotective adjuvants (OR: 0.21. 95%, CL; 0.13-0.33) [158]. When ANT regimens include concomitant trastuzumab administration, the total cardiotoxic effect increases significantly. According to ESMO clinical guidelines, while cardioprotective adjuvants are helpful in preventing CCM, serial evaluation of LV function is recommended to inform the decision to choose the most appropriate cardioprotective adjuvant to prevent the development of CCM [19,20]. Besides cardioprotective adjuvants, traditional HF pharmacotherapy has also been shown to convey a protective effect against the development of CCM. A combined therapy of ACE-inhibitors/ARBs with or without beta-blockers attenuates the cardiotoxic effect of trastuzumab by improving LV function within 3 to 12 months after completion of therapy [160].
In summary, chemotherapy with concomitant cardioprotective adjuvants protect against the development of CCM. They aid in the preservation of the LV function and a reduction in the incidence of HF. However, prior evaluation of LV function is required to choose the most appropriate cardioprotective adjuvants with minimal side effect. In addition to cardioprotective adjuvants, the traditional HF therapy also plays a protective effect on the development of CCM and CHF in cancer patients undergoing chemotherapy.
Conclusion
Chemotherapy is a well-established clinical management approach for several malignancies but its clinical efficacy is limited by an elevated risk of inducing cardiomyopathy and possibly progression to heart failure (HF). Chemotherapy-induced cardiomyopathy (CCM) is cardiac condition marked by a significant decrease in LVEF > 10% to values less than 55% in cancer patients treated with chemotherapy. The main classes of chemotherapeutic agents potentially causing CCM are cytotoxic such as Anthracyclines (ANT), monoclonal antibodies such as trastuzumab, and alkylating agents such as cyclophosphamide and mitomycin. Reversibility of myocardial damage and pathological structural changes form the basis of CCM classification into Type I (irreversible damage, ANT-associated) and Type II (reversible damage, trastuzumab-associated). The main risk factors for the development of CCM are cumulative dose and concomitant treatment such as medication and radiotherapy. Predominant pathophysiologic mechanisms for Type I are the formation of ANT-iron complexes and stimulation of free-radical formation while for Type II is the blockade of HER2+ receptor signals in cardiac myocytes. Prognosis of CCM is poor, conveying a negative effect on cardiac outcomes necessitating adjunctive therapy or restricting the choice of oncologic treatment. Clinical presentation of CCM may be classified into acute and sub-acute phases that occur within a week of chemotherapy or chronic progressive whose onset is at the first year, progresses gradually and manifests years after the completion of chemotherapy.
Diagnosis of CCM is made by assessing LV dysfunction mainly based on LEVF values using imaging modalities such as echocardiography, multigated acquisition scanning (MUGA) or cardiac magnetic resonance (CMR). Echocardiography is considered the cornerstone modality for assessing LV dysfunction in cancer patients because it is readily available and reproducible. Although LVEF is insensitive to myocardial changes in subclinical CCM, speckle tracking echocardiography (STE) based strain analysis is able to predict patients at an increased risk of developing CCM. Recently, cardiac biomarkers such as troponin and B-type natriuretic peptides (BNP) have also proved sensitive and specific in the identification of subclinical CCM. Finally, clinical management of CCM depends on serial evaluation of LVEF changes and cardiac biomarker Troponin useful for guiding treatment options. Significantly depressed LVEF (<40%) requires halting treatment while positive Troponin requires traditional HF treatment using ACE-inhibitors and beta-blockers. Other frequently used treatment options are dose modulation and cardioprotective adjuvants, which aim to prevent cardiotoxic effects of chemotherapy while allowing optimal suppression of cancer tumors.
References
- Koene RJ (2016) Shared risk factors in cardiovascular disease and cancer. Circulation 133: 1104-1114.
- Armstrong GT (2009) Late mortality among 5-year survivors of childhood cancer: a summary from the Childhood Cancer Survivor Study. Journal of Clinical Oncology 27: 2328-2338.
- Tukenova M, Guibout C, Oberlin O (2010) Role of cancer treatment in long-term overall and cardiovascular mortality after childhood cancer. Journal of clinical oncology 28: 1308-1315.
- Di Marco A, Gaetani M, Orezzi P, Soldati M (1963) Antitumor activity of a new antibiotic: Daunomycin. In Proceedings of the Third International Congress of Chemotherapy 2: 1023-1031.
- DiMarco A, Gaetani M, Dorigotti L, Soldati M, Bellini O (1964) Daunomycin: A new antibiotic with antitumor activity. Cancer Chemotherapy Reports 38: 31-38
- Di Marco A, Gaetani M, Orezzi P, Scarpinato BM (1964) ‘Daunomycin’, a new antibiotic of the rhodomycin group. Nature 201: 706-707.
- Di Marco A (1967) Daunomycin and related antibiotics. In Antibiotics (pp. 190-210), Springer Berlin Heidelberg.
- Tan C, Tasaka H, Yu KP, Murphy ML, Karnofsky DA, et al. (1967) Daunomycin, an antitumor antibiotic, in the treatment of neoplastic disease. Clinical evaluation with special reference to childhood leukemia. Cancer 20: 333-353.
- Piper SE, McDonagh TA (2015) Chemotherapy-related cardiomyopathy. European Cardiology Review 10: 19-24.
- Bosch X, Rovira M, Sitges M (2013) Enalapril and carvedilol for preventing chemotherapy-induced left ventricular systolic dysfunction in patients with malignant hemopathies: the OVERCOME trial (preventiOn of left Ventricular dysfunction with Enalapril and caRvedilol in patients submitted to intensive ChemOtherapy for the treatment of Malignant hEmopathies). Journal of the American College of Cardiology 61: 2355-2362.
- Schwartz RG, McKenzie WB, Alexander J, Sager P (1987) Congestive heart failure and left ventricular dysfunction complicating doxorubicin therapy. The American Journal of Medicine 82: 1109-1118.
- Slamon DJ (2001) Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. New England Journal of Medicine 344: 783-792.
- O’brien MER, Wigler N, Inbar M, Rosso R, Grischke E, et al. (2004) Reduced cardiotoxicity and comparable efficacy in a phase III trial of pegylated liposomal doxorubicin HCl (CAELYX™/Doxil®) versus conventional doxorubicin for first-line treatment of metastatic breast cancer. Annals of Oncology 15: 440-449.
- Tan-Chiu E, Yothers G, Romond E (2005) Assessment of cardiac dysfunction in a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel, with or without trastuzumab as adjuvant therapy in node-positive, human epidermal growth factor receptor 2–overexpressing breast cancer: NSABP B-31. Journal of Clinical Oncology 23: 7811-7819.
- Romond EH, Perez EA, Bryant J, Suman VJ (2005) Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. New England Journal of Medicine 353: 1673-1684.
- Ryberg M, Nielsen D, Cortese G, Nielsen G (2008) New insight into epirubicin cardiac toxicity: competing risks analysis of 1097 breast cancer patients. Journal of the National Cancer Institute 100: 1058-1067.
- Pai VB, Nahata MC (2000) Cardiotoxicity of chemotherapeutic agents. Drug Safety 22: 263-302.
- Cardinale D (2008) Prevention and treatment of cardiomyopathy and heart failure in patients receiving cancer chemotherapy. Current Treatment Options in Cardiovascular Medicine 10: 486-495.
- Eschenhagen T (2011) Cardiovascular side effects of cancer therapies: a position statement from the Heart Failure Association of the European Society of Cardiology. European Journal of Heart Failure 13: 1-10.
- Curigliano G Guidelines Working Group (2012) Cardiovascular toxicity induced by chemotherapy, targeted agents and radiotherapy: ESMO Clinical Practice Guidelines. Annals of Oncology, 23(suppl_7), vii155-vii166.\
- Felker GM, Thompson RE, Hare JM (2000) Underlying causes and long-term survival in patients with initially unexplained cardiomyopathy. New England Journal of Medicine 342: 1077-1084.
- Adams KF, Dunlap SH, Sueta CA (1996) Relation between gender, etiology and survival in patients with symptomatic heart failure. Journal of the American College of Cardiology 28: 1781-1788.
- Cardinale D, Colombo A, Lamantia G (2010) Anthracycline-induced cardiomyopathy: Clinical relevance and response to pharmacologic therapy. Journal of the American College of Cardiology 55: 213-220.
- Steinherz LJ, Steinherz PG, Tan CT (1991) Cardiac toxicity 4 to 20 years after completing anthracycline therapy. JAMA 266: 1672-1677.
- Silber JH, Cnaan A, Clark BJ, Paridon SM (2004) Enalapril to prevent cardiac function decline in long-term survivors of pediatric cancer exposed to Anthracyclines. Journal of Clinical Oncology 22: 820-828.
- Saidi A, Alharethi R (2011) Management of chemotherapy induced cardiomyopathy. Current Cardiology Reviews 7: 245-249.
- Weiss RB (1992) The Anthracyclines: Will we ever find a better doxorubicin? In Seminars in Oncology 19: 670-686.
- Shakir DK, Rasul KI (2009) Chemotherapy induced cardiomyopathy: pathogenesis, monitoring and management. Journal of Clinical Medicine Research 1: 8-12.
- Minotti G, Menna P, Salvatorelli E, Cairo G (2004) Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacological Reviews 56: 185-229.
- Pegram M, Slamon D (2000) Biological rationale for HER2/neu (c-erbB2) as a target for monoclonal antibody therapy. In Seminars in oncology 5: 13-19).
- Baselga J, Albanell J (2001) Mechanism of action of anti-HER2 monoclonal antibodies. Annals of Oncology 12: S35-S41.
- Research CFDEA (1998) Therapeutic Biologic Applications (BLA) - Trastuzumab Product Approval Information – Licensing Action 9/25/98, 1998.
- Yeh ET, Bickford CL (2009) Cardiovascular complications of cancer therapy: incidence, pathogenesis, diagnosis, and management. Journal of the American College of Cardiology 53: 2231-2247.
- Gharib MI, Burnett AK (2002) Chemotherapy‐induced cardiotoxicity: Current practice and prospects of prophylaxis. European Journal of Heart Failure 4: 235-242.
- Buzdar AU (1978) Adriamycin and mitomycin C: possible synergistic cardiotoxicity. Cancer treatment reports 62: 1005-1008.
- Villani F (1985) Possible enhancement of the cardiotoxicity of doxorubicin when combined with mitomycin C. Medical Oncology 2: 93-97.
- Verweij J (1988) A prospective study on the dose dependency of cardiotoxicity induced by mitomycin C. Medical oncology and tumor pharmacotherapy 5: 159-163.
- Marty M (2005) Randomized phase II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2–positive metastatic breast cancer administered as first-line treatment: the M77001 study group. Journal of Clinical Oncology 23: 4265-4274.
- Richardson PG (2005) Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. New England Journal of Medicine 352: 2487-2498.
- Perez EA (2008) Cardiac safety of lapatinib: Pooled analysis of 3689 patients enrolled in clinical trials. In Mayo Clinic Proceedings 83: 679-686.
- Schmidinger M (2008) Cardiac toxicity of sunitinib and sorafenib in patients with metastatic renal cell carcinoma. Journal of Clinical Oncology 26: 5204-5212.
- Von Hoff DD (1979) Risk factors for doxorubicin-induced congestive heart failure. Annals of Internal Medicine 91: 710-717.
- Lipshultz SE (1991) Late cardiac effects of doxorubicin therapy for acute lymphoblastic leukemia in childhood. New England Journal of Medicine 324: 808-815.
- Legha SS (1982) Reduction of doxorubicin cardiotoxicity by prolonged continuous intravenous infusion. Annals of Internal Medicine 96: 133-139.
- Categories R (1983) Reduced cardiotoxicity of doxorubicin delivered on a weekly schedule. Annals of Internal Medicine 99: 745-749.
- Weiss AJ (1976) Studies on adriamycin using a weekly regimen demonstrating its clinical effectiveness and lack of cardiac toxicity. Cancer Treatment Reports 60: 813-822.
- Weiss AJ (1977) Experience with the use of adriamycin in combination with other anticancer agents using a weekly schedule, with particular reference to lack of cardiac toxicity. Cancer 40: 2046-2052.
- Chlebowski RT (1980) Adriamycin given as a weekly schedule without a loading course: clinically effective with reduced incidence of cardiotoxicity. Cancer Treatment Reports 64: 47-51.
- Krischer JP (1997) Clinical cardiotoxicity following anthracycline treatment for childhood cancer: the Pediatric Oncology Group experience. Journal of Clinical Oncology 15: 1544-1552.
- Lipshultz SE (1995) Female sex and higher drug dose as risk factors for late cardiotoxic effects of doxorubicin therapy for childhood cancer. New England Journal of Medicine 332: 1738-1744.
- Silber JH (1993) Increased risk of cardiac dysfunction after anthracyclines in girls. Pediatric Blood & Cancer 21: 477-479.
- Bristow MR (1978) Doxorubicin cardiomyopathy: evaluation by phonocardiography, endomyocardial biopsy, and cardiac catheterization. Annals of Internal Medicine 88: 168-175.
- Praga C (1979) Adriamycin cardiotoxicity: a survey of 1273 patients. Cancer Treatment Reports 63: 827-834.
- Pihkala J (1996) Myocardial function in children and adolescents after therapy with anthracyclines and chest irradiation. European Journal of Cancer 32: 97-103.
- Grenier MA (1998) Epidemiology of anthracycline cardiotoxicity in children and adults. Seminars in Oncology 25: 72-85.
- Boucek Jr (1999) Persistent effects of doxorubicin on cardiac gene expression. Journal of Molecular and Cellular Cardiology 31: 1435-1446.
- Gewirtz D (1999) A critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics adriamycin and daunorubicin. Biochemical Pharmacology 57: 727-741.
- Horenstein MS (2000) Molecular basis of anthracycline-induced cardiotoxicity and its prevention. Molecular Genetics and Metabolism 71: 436-444.
- Hershko C (1993) The role of iron and iron chelators in anthracycline cardiotoxicity. Leukemia and Lymphoma 11: 207-214.
- Link G (1996) Role of iron in the potentiation of anthracycline cardiotoxicity: identification of heart cell mitochondria as a major site of iron-anthracycline interaction. Journal of Laboratory and Clinical Medicine 127: 272-278.
- Hershko C (1996) Prevention of anthracycline cardiotoxicity by iron chelation. Acta Haematologica, 95: 87-92.
- Doroshow JH (1983) Effect of anthracycline antibiotics on oxygen radical formation in rat heart. Cancer Research 43: 460-472.
- Ehrke MJ (1986) Correlation between adriamycin-induced augmentation of interleukin 2 production and of cell-mediated cytotoxicity in mice. Cancer research 46: 54-60.
- Zhang S (2012) Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nature Medicine 18: 1639-1642.
- Wouters KA (2005) Protecting against anthracycline‐induced myocardial damage: a review of the most promising strategies. British Journal of Hematology 131: 561-578.
- Kalyanaraman BJSSES (2002) Doxorubicin-induced apoptosis: implications in cardiotoxicity. In Oxygen/Nitrogen Radicals: Cell Injury and Disease, (pp. 119-124). Springer US.
- Crone SA (2002) ErbB2 is essential in the prevention of dilated cardiomyopathy. Nature Medicine 8: 459-465.
- Morris PG (2011) Troponin I and C-reactive protein are commonly detected in patients with breast cancer treated with dose-dense chemotherapy incorporating trastuzumab and lapatinib. Clinical Cancer Research 17: 3490-3499.
- Tan-Chiu E (2005) Assessment of cardiac dysfunction in a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel, with or without trastuzumab as adjuvant therapy in node-positive, human epidermal growth factor receptor 2–overexpressing breast cancer: NSABP B-31. Journal of Clinical Oncology 23: 7811-7819.
- Piccart-Gebhart MJ (2005) Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. New England Journal of Medicine 353: 1659-1672.
- Saini J (1987) Reversibility of severe left ventricular dysfunction due to doxorubicin cardiotoxicity: report of three cases. Annals of Internal Medicine 106: 814-816.
- Haq MM (1985) Doxorubicin‐induced congestive heart failure in adults. Cancer 56: 1361-1365.
- Cardinale D (2004) Prognostic value of troponin I in cardiac risk stratification of cancer patients undergoing high-dose chemotherapy. Circulation 109: 2749-2754.
- Cardinale D (2000) Left ventricular dysfunction predicted by early troponin I release after high-dose chemotherapy. Journal of the American College of Cardiology 36: 517-522.
- Rodenhuis S (2003) High-dose chemotherapy with hematopoietic stem-cell rescue for high-risk breast cancer. New England Journal of Medicine 349: 7-16.
- Schmitz N (2006) Four versus six courses of a dose‐escalated cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) regimen plus etoposide (megaCHOEP) and autologous stem cell transplantation. Cancer 106: 136-145.
- Bearman SI (1990) Radionuclide ejection fractions in the evaluation of patients being considered for bone marrow transplantation: risk for cardiac toxicity. Bone Marrow Transplantation 5: 173-177.
- Kakavas PW (1995) Angiotensin converting enzyme inhibitors in bone marrow transplant recipients with depressed left ventricular function. Bone Marrow Transplantation 15: 859-861.
- Perez EA (2004) Clinical cardiac tolerability of trastuzumab. Journal of Clinical Oncology 22: 322-329.
- Frishman WH (1997) Cardiovascular toxicity with cancer chemotherapy. Current Problems in Cancer 21: 301-360.
- Ferrans VJ (1978) Overview of cardiac pathology in relation to anthracycline cardiotoxicity. Cancer Treatment Reports 62: 955-961.
- Shan K (1996) Anthracycline-induced cardiotoxicity. Annals of Internal Medicine 125: 47-58.
- Lipshultz SE (1991) Late cardiac effects of doxorubicin therapy for acute lymphoblastic leukemia in childhood. New England Journal of Medicine 324: 808-815.
- Bu'Lock FA (1999) Left ventricular diastolic filling patterns associated with progressive anthracycline-induced myocardial damage: a prospective study. Pediatric Cardiology 20: 252-263.
- Yusuf SW (2011) Chemotherapy-induced cardiomyopathy. Expert Review of Cardiovascular Therapy 9: 231-243.
- Morandi P (2001) Serum cardiac troponin I levels and ECG/Echo monitoring in breast cancer patients undergoing high-dose (7 g/m 2) cyclophosphamide. Bone Marrow Transplantation 28: 272-277.
- Fernandez AE (2017) Chemotherapy-induced dysfunction. European Society of Cardiologist 14: 17 March 2017.
- Tjeerdsma G (1999) Early detection of anthracycline induced cardiotoxicity in asymptomatic patients with normal left ventricular systolic function: autonomic versus echocardiographic variables. Heart 81: 419-423.
- Stoddard MF (1992) Prolongation of isovolumetric relaxation time as assessed by Doppler echocardiography predicts doxorubicin-induced systolic dysfunction in humans. Journal of the American College of Cardiology 20: 62-69.
- Ganz WI (1993) Detection of early anthracycline cardiotoxicity by monitoring the peak filling rate. American Journal of Clinical Oncology 16: 109-112.
- Ewer MS (1984) A comparison of cardiac biopsy grades and ejection fraction estimations in patients receiving Adriamycin. Journal of Clinical Oncology 2: 112-117.
- Jassal DS (2009) Utility of tissue Doppler and strain rate imaging in the early detection of trastuzumab and anthracycline mediated cardiomyopathy. Journal of the American Society of Echocardiography 22: 418-424.
- Hoit BD (2009) Detection of myocardial dysfunction during cancer chemotherapy with tissue Doppler imaging: a canary in the coalmine? Journal of the American Society of Echocardiography 22: 425-426
- Van Royen N (1996) Comparison and reproducibility of visual echocardiographic and quantitative radionyclide left ventricular ejection fractions. American Journal of Cardiology 77: 843-850.
- Ewer MS (2005) Reversibility of trastuzumab-related cardiotoxicity: new insights based on clinical course and response to medical treatment. Journal of Clinical Oncology 23: 7820-7826.
- Panjrath GS (2006) Monitoring chemotherapy-induced cardiotoxicity: role of cardiac nuclear imaging. Journal of nuclear Cardiology 13: 415-426.
- Flotats A (2003) Non-invasive in vivo imaging of myocardial apoptosis and necrosis. European Journal of Nuclear Medicine and Molecular Imaging 30: 615-630.
- Boogers MJ (2011) The role of nuclear imaging in the failing heart: myocardial blood flow, sympathetic innervation, and future applications. Heart Failure Reviews 16: 411-423.
- Urbanova D (2006) Cardiac troponins--biochemical markers of cardiac toxicity after cytostatic therapy. Neoplasma 53: 183-190.
- Price JF (2006) B-type natriuretic peptide predicts adverse cardiovascular events in pediatric outpatients with chronic left ventricular systolic dysfunction. Circulation 114: 1063-1069.
- Morrison LK (2002) Utility of a rapid B-natriuretic peptide assay in differentiating congestive heart failure from lung disease in patients presenting with dyspnea. Journal of the American College of Cardiology 39: 202-209.
- Suzuki T (1998) Elevated B-type natriuretic peptide levels after anthracycline administration. American Heart Journal 136: 362-363.
- Okumura H (2000) Brain natriuretic peptide is a predictor of anthracycline-induced cardiotoxicity. Acta haematologica104: 158-163.
- Nousiainen T (2002) Natriuretic peptides during the development of doxorubicin‐induced left ventricular diastolic dysfunction. Journal of Internal Medicine 251: 228-234.
- Jaffe AS (2006) Biomarkers in acute cardiac disease. Journal of the American College of Cardiology 48: 1-11.
- Tulevski II (2007) Combined utility of brain natriuretic peptide and cardiac troponine T may improve rapid triage and risk stratification in normotensive patients with pulmonary embolism. International Journal of Cardiology 116: 161-166.
- Favory R (2006). Bench-to-bedside review: significance and interpretation of elevated troponin in septic patients. Critical Care 10: 220-224.
- Polena S (2005) Troponin I as a marker of doxorubicin induced cardiotoxicity. In Proceedings of the Western Pharmacology Society 48: 142-144.
- Gaze DC (2005) Cardiac troponins as biomarkers of drug-and toxin-induced cardiac toxicity and cardioprotection. Expert Opinion on Drug Metabolism and Toxicology 1: 715-725.
- Nousiainen T (1999) Natriuretic peptides as markers of cardiotoxicity during doxorubicin treatment for non‐Hodgkin's lymphoma. European Journal of Hematology 62: 135-141.
- Carrió I (1995) Indium-111-antimyosin and iodine-123-MIBG studies in early assessment of doxorubicin cardiotoxicity. Journal of Nuclear Medicine: Official Publication, Society of Nuclear Medicine 36: 2044-2049.
- Fallah-Rad N (2011) The utility of cardiac biomarkers, tissue velocity and strain imaging, and cardiac magnetic resonance imaging in predicting early left ventricular dysfunction in patients with human epidermal growth factor receptor II–positive breast cancer treated with adjuvant trastuzumab therapy. Journal of the American College of Cardiology 57: 2263-2270.
- Stoodley PW (2011) Two-dimensional myocardial strain imaging detects changes in left ventricular systolic function immediately after anthracycline chemotherapy. European Journal of Echocardiography 12: 945-952.
- Sawaya H (2011) Early detection and prediction of cardiotoxicity in chemotherapy-treated patients. The American Journal of Cardiology 107: 1375-1380.
- Sawaya H (2012) Assessment of Echocardiography and Biomarkers for the Extended Prediction of Cardiotoxicity in Patients Treated With Anthracyclines, Taxanes, and Trastuzumab Clinical Perspective. Circulation: Cardiovascular Imaging 5: 596-603.
- Mavinkurve-Groothuis AM (2012) Myocardial 2D strain echocardiography and cardiac biomarkers in children during and shortly after anthracycline therapy for acute lymphoblastic leukaemia (ALL): a prospective study. European Heart Journal–Cardiovascular Imaging 14: 562-569.
- Poterucha JT (2012) Changes in left ventricular longitudinal strain with anthracycline chemotherapy in adolescents precede subsequent decreased left ventricular ejection fraction. Journal of the American Society of Echocardiography 25: 733-740.
- Al-Biltagi M (2012) Strain echocardiography in early detection of doxorubicin-induced left ventricular dysfunction in children with acute lymphoblastic leukemia. ISRN Pediatrics, 2012.
- Kang Y (2013) Early detection of anthracycline-induced cardiotoxicity using two-dimensional speckle tracking echocardiography. Cardiology Journal 20: 592-599.
- Negishi K (2013) Use of speckle strain to assess left ventricular responses to cardiotoxic chemotherapy and cardioprotection. European Heart Journal–Cardiovascular Imaging 15: 324-331.
- Stoodley PW (2013) Left ventricular systolic function in HER2/neu negative breast cancer patients treated with anthracycline chemotherapy: a comparative analysis of left ventricular ejection fraction and myocardial strain imaging over 12 months. European Journal of Cancer 49: 3396-3403.
- Baratta S (2013) Serum markers, conventional Doppler echocardiography and two-dimensional systolic strain in the diagnosis of chemotherapy-induced myocardial toxicity. Argentine Journal of Cardiology 81: 133-138.
- Mornos C (2013) Early detection of anthracycline-mediated cardiotoxicity: the value of considering both global longitudinal left ventricular strain and twist. Canadian Journal of Physiology and Pharmacology 91: 601-607.
- Kang Y (2014) Two‐dimensional speckle tracking echocardiography combined with high‐sensitive cardiac troponin T in early detection and prediction of cardiotoxicity during epirubicine‐based chemotherapy. European Journal of Heart Failure 16: 300-308.
- Octavia Y (2012) Doxorubicin-induced cardiomyopathy: From molecular mechanisms to therapeutic strategies. Journal of Molecular and Cellular Cardiology 52: 1213-1225.
- Erttmann R (1988) Pharmacokinetics of doxorubicin in man: dose and schedule dependence. Journal of Cancer Research and Clinical Oncology 114: 509-513.
- Creech RH (1980) An effective low‐dose adriamycin regimen as secondary chemotherapy for metastatic breast cancer patients. Cancer 46: 433-437.
- Mitry MA (2016) Doxorubicin induced heart failure: Phenotype and molecular mechanisms. IJC Heart and Vasculature 10: 17-24.
- Schroeder PE (2005) Metabolism of the one-ring open metabolites of the cardioprotective drug dexrazoxane to its active metal-chelating form in the rat. Drug Metabolism and Disposition 33: 1367-1372.
- Hasinoff BB (1990) The hydrolysis activation of the doxorubicin cardioprotective agent ICRF-187 [+)-1, 2-bis (3, 5-dioxopiperazinyl-1-yl) propane). Drug Metabolism and Disposition 18: 344-349.
- Lipshultz SE (2004). The effect of dexrazoxane on myocardial injury in doxorubicin-treated children with acute lymphoblastic leukemia. New England Journal of Medicine 351: 145-153.
- Hensley ML (2009) American Society of Clinical Oncology 2008 clinical practice guideline update: use of chemotherapy and radiation therapy protectants. Journal of Clinical Oncology 27: 127-145.
- Van Acker FA (2001) New synthetic flavonoids as potent protectors against doxorubicin-induced cardiotoxicity. Free Radical Biology and Medicine 31: 31-37.
- Acker DA (1995) Monohydroxyethylrutoside as protector against chronic doxorubicin‐induced cardiotoxicity. British Journal of Pharmacology 115: 1260-1264.
- Van Acker SA (1997) Monohydroxyethylrutoside, a dose-dependent cardioprotective agent, does not affect the antitumor activity of doxorubicin. Clinical Cancer Research 3: 1747-1754.
- Bruynzeel AM (2007) The effect of monohydroxyethylrutoside on doxorubicin-induced cardiotoxicity in patients treated for metastatic cancer in a phase II study. British Journal of Cancer 97: 1084-1089.
- Myers C (1983) A randomized controlled trial assessing the prevention of doxorubicin cardiomyopathy by N-acetylcysteine. In Seminars in Oncology 10: 53-55.
- Milei J (1987) Prevention of adriamycin-induced cardiotoxicity by prenylamine: a pilot double blind study. Cancer Drug delivery 4: 129-136.
- Speyer JL (1992) ICRF-187 permits longer treatment with doxorubicin in women with breast cancer. Journal of Clinical Oncology 10: 117-127.
- Venturini M (1996) Multicenter randomized controlled clinical trial to evaluate cardioprotection of dexrazoxane versus no cardioprotection in women receiving epirubicin chemotherapy for advanced breast cancer. Journal of Clinical Oncology 14: 3112-3120
- Gallegos-Castorena S (2007) Toxicity prevention with amifostine in pediatric osteosarcoma patients treated with cisplatin and doxorubicin. Pediatric Hematology and Oncology 24: 403-408.
- Lopez M (1998) Randomized prospective clinical trial of high-dose epirubicin and dexrazoxane in patients with advanced breast cancer and soft tissue sarcomas. Journal of Clinical Oncology 16: 86-92.
- Swain SM (1997) Cardioprotection with dexrazoxane for doxorubicin-containing therapy in advanced breast cancer. Journal of Clinical Oncology 15: 1318-1332.
- Murphy CA (2007) Drug-induced cardiovascular disorders. Drug Safety 30: 783-804.
- Frei BL (2008) A review of the cardiovascular effects of oncology agents. Journal of Pharmacy Practice, 21: 146-158.
- Brandt L (2001) A systematic overview of chemotherapy effects in Hodgkin's disease. Acta Oncologica 40: 185-197.
- Bryant J (2007) Clinical and cost-effectiveness of cardioprotection against the toxic effects of anthracyclines given to children with cancer: a systematic review. British Journal of Cancer 96: 226-230.
- Kremer LCM (2002) Frequency and risk factors of subclinical cardiotoxicity after anthracycline therapy in children: a systematic review. Annals of Oncology 13: 819-829.
- Van Dalen EC (2009) Different dosage schedules for reducing cardiotoxicity in cancer patients receiving anthracycline chemotherapy. Cochrane Database Syst Rev, 4.
- Van Dalen EC (2008) Cardioprotective interventions for cancer patients receiving anthracyclines. Cochrane Database Syst Rev, 2, CD003917.
- Kalay N (2006) Protective effects of carvedilol against anthracycline-induced cardiomyopathy. Journal of the American College of Cardiology 48: 2258-2262.
- Marty M (2006) Multicenter randomized phase III study of the cardioprotective effect of dexrazoxane (Cardioxane®) in advanced/metastatic breast cancer patients treated with anthracycline-based chemotherapy. Annals of Oncology 17: 614-622.
- Waldner R (2006). Effects of doxorubicin-containing chemotherapy and a combination with L-carnitine on oxidative metabolism in patients with non-Hodgkin lymphoma. Journal of Cancer Research and Clinical Oncology 132: 121-128.
- Acar Z (2011) Efficiency of atorvastatin in the protection of anthracycline-induced cardiomyopathy. Journal of the American College of Cardiology 58: 988-989.
- Cardinale D (2006) Prevention of high-dose chemotherapy–induced cardiotoxicity in high-risk patients by angiotensin-converting enzyme inhibition. Circulation 114: 2474-2481.
- Seicean S (2012) Effect of statin therapy on the risk for incident heart failure in patients with breast cancer receiving anthracycline chemotherapy: An observational clinical cohort study. Journal of the American College of Cardiology 60: 2384-2390.
- Seicean S (2013). Cardioprotective effect of Beta-Adrenoceptor blockade in breast cancer patients undergoing chemotherapy: a follow-up study of heart failure. Circulation: Heart Failure CIRCHEARTFAILURE-112.
- Georgakopoulos P (2010) Cardioprotective effect of metoprolol and enalapril in doxorubicin‐treated lymphoma patients: a prospective, parallel‐group, randomized, controlled study with 36‐month follow‐up. American Journal of Hematology 85: 894-896.
- Smith LA (2010) Cardiotoxicity of anthracycline agents for the treatment of cancer: systematic review and meta-analysis of randomised controlled trials. BMC Cancer 10: 331-337.
- Oliva S (2012) Administration of angiotensin-converting enzyme inhibitors and β-blockers during adjuvant trastuzumab chemotherapy for nonmetastatic breast cancer: marker of risk or cardioprotection in the real world? The oncologist 17: 917-924.



