Abstract
Endogenous clocks determine almost all circadian rhythms in physiology. Circadian rhythms are patterns of physiology and behavior that follow a 24-hour cycle. They are autonomous and consist of self-sustained oscillations in biological processes driven or stimulated by environmental processes, the most important being light. Examples are, to name a few, sleep-wake cycle, learning, memory, daily variations in blood pressure, temperature, mood, heart rate, exercise capacity, ventilation, and coagulation, and it is now realized that the time at which our immune system is triggered (by infection, vaccination, surgery), appears to be critical to the way we respond to these insults. Also, circadian clocks give physiological systems the ability to anticipate daily changes. The complex network consists of a master pacemaker in the central nervous system and peripheral molecular clocks neurologically or hormonally connected and trained by the central node. These peripheral clocks govern a vast panel of cellular and molecular processes at virtually every level of regulation, maintaining the stability of the internal environment and anticipating disturbances. The objective of this work is to review the molecular biology of the system, to understand how it can be impacted, negatively or positively, and to review its dysfunction in respiratory diseases. It is also discussed how the understanding of circadian rhythms in respiratory medicine can have significance in the treatment optimization. At the end, some future recommendations for research on this topic are made.
Keywords
circadian rhythm, endogenous clocks, circadian pacemaker, respiratory diseases, chronotherapy
Introduction
Jürgen Aschoff traced back the interest in biological rhythms to the Greek poet Archilochus of Paros (ca. 680–640 BC) who wrote “recognize which rhythms govern man” [1]. The notion that the respiratory system and its diseases exhibit circadian rhythms was first noted in the 5th century by Roman physician Caelius Aurelianus, who described worsening nocturnal asthma symptoms compared with the daytime [2]. More than 2500 years later biological rhythms are known to ‘govern’ many aspects in human behavior, physiology, metabolism, disease symptoms and response to treatment in a rhythmic fashion with the circadian clock as timekeeper. A breakthrough in understanding how circadian clocks “tick” followed the discovery of the Period gene (Per) in the fruit fly Drosophila melanogaster and the Clock gene in the mouse. Surprisingly, Per encodes a protein that represses its own transcription, resulting in daily Per rhythms [3,4]. Subsequently, the Per activator was discovered in mammals and named Clock, revealing that the gears of the clock are composed of activators that induce the expression of their own repressors, forming a negative feedback loop that is highly conserved from flies to humans [5]. Over the past 17 years, our understanding of the body clock and biological rhythms has increased immeasurably. In 2017, the Nobel Prize in Physiology or Medicine was awarded jointly to Jeffrey C. Hall, Michael Rosbash, and Michael W. Young “for their discoveries of molecular mechanisms controlling the circadian rhythm” [6-8].
For the purposes of this review, entrainment will refer to the central clock aligning to the external time cues, while synchronization will refer to the alignment of the central and peripheral clocks relative to each other. Those external time cues (light intensity, temperature, food availability and predator pressure amongst many others have led to the evolution of biological clocks in most species) are external synchronizer or Zeitgeber (from German Zeit “time” and Geber “giver”). Clock proteins will be written in capital letters and genes in italics.
Neurophysiology of the circadian rhythm
The term circadian originates from the Latin circa diem, about a day. Circadian rhythms persist even in constant conditions with a period that is almost 24 hours. Some endogenous circadian rhythms do not have a 24-hour periodicity, such as the metabolic rhythm that shifts or changes the rhythm with fasting-feeding or temperature or nutritional changes that disrupt the clock, conditioning adipogenesis with ultradian cycles of one hour [9]. Light entrains the clock to the 24-hour rotation of Earth. The molecular circuitry of circadian clocks is encoded by an autoregulatory 24-hour transcription loop in the brain, where the clocks align sleep–wake and feeding cycles with the rotation of Earth on its axis. Clocks are also present in nearly all tissues of the body, composing a network of timekeepers that anticipate varying environmental conditions each day [10] (Figure 1). The evolution of these clocks coincided with the great oxygen expansion 3 billion years ago, fundamentally tying circadian processes with oxygenic respiration [11].

Figure 1. Alignment between the SCN and the lung
Light is the main external cue (Zeitgeber) that activates the circadian rhythm. SCN: suprachiasmatic nucleus.
Pacemaker neurons housing circadian clocks are the master node in a hierarchical network of internal clocks, driving sleep–wake rhythms and orchestrating clocks in peripheral tissues. Central pacemaker neurons are found in the central nervous system, in the suprachiasmatic nucleus (SCN) in the hypothalamus. The (SCN) comprising approximately 20,000 neurons and glia, contains the pacemaker neurons that are both necessary and sufficient to drive these rhythms [12]. Circadian pacemaker neurons display high-amplitude day–night variation in the spontaneous firing rate and resting membrane potential [13]. Neurons have excitatory and inhibitory activity that depend on currents of sodium and potassium. Both activities offer rhythmic activity, and the oscillations of this rhythmicity depend on the expression of ion channels or their regulators, including metabolic signals within the cell (e.g., nicotinamide adenine dinucleotide [NAD+]) [14,15]. Molecular clocks are also present in many cells of peripheral tissues and have oscillations coupled with the central oscillator (SCN). Communication is established through neuroendocrine systems that function as homeostatic sensors that respond to environmental changes (for example, insulin secretion in response to glucose or glucocorticoids in response to stress). Although peripheral clocks are normally entrained by the master SCN pacemaker, feeding can independently synchronize peripheral clocks in the liver and kidneys, leading to misalignment in clock cycles when food is consumed at the wrong time of day. Peripheral clocks reinforce rhythmic regulation at the local tissue level and can be entrained by timed meals, even in animals lacking the master brain clock [16].
Melanopsin is a photopigment of the receptors of the cones, rods, and retinal ganglion cells (RGC) of the retina, which transmits light information by activating the master clock in the SCN of the hypothalamus [17]. This information entrains (aligns) the circadian rhythm to the most important environmental key, the light [18,19] (Figure 2). This light-dark (Zeitgeber) signal releases information downstream through the neuroendocrine network for the rhythmicity of feeding behavior [20]. During darkness, as the shadows lengthen, the internal clock recognizes that bedtime is approaching the pineal gland produces melatonin, the hormone that promotes and controls deep sleep, which acts on two receptors: MT1 and MT2 coupled to G protein. This secretion is directly regulated by the central clock and this circadian regulation of the hormone and its signals influences sleep-wake times. The greatest sensitivity of the photopigments is to the short wavelength of blue and green and less for the color red. Exposure to blue light at night can cause problems falling asleep as it activates wakefulness and inhibits sleep-inducing neurons, subsequently reducing melatonin production. In addition, light stimulates the sympathetic nervous system, increasing consciousness and alertness [21].
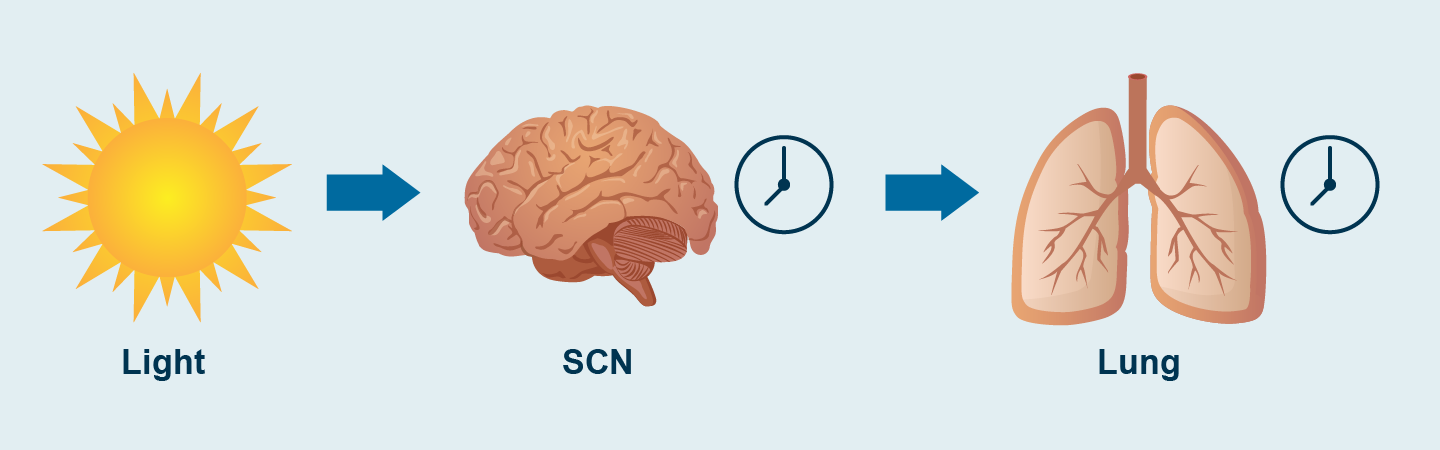
Figure 2. The role of the retina in the circadian rhythm
Light input is received daily by specialized photoreceptor cells in the retina, the intrinsically photosensitive retinal ganglion cells (ipRGCs) and transmitted via the retino-hypothalamic tract to the central clock located in the suprachiasmatic nucleus (SCN) entraining it to the external light dark cycle.
Molecular biology of the circadian rhythm
The core loop consists of basic helix–loop–helix (bHLH) and heterodimeric transcriptional activators (CLOCK [circadian locomotor output cycles kaput] or its paralog, NPAS2 [Per-Arnt-Sim], with BMAL1 [brain and muscle Arnt-like protein 1]) (Figure 3). These factors activate other transcription factors (REV-ERBα and RORα [retinoic acid–related orphan receptor] which are nuclear hormone receptors), which reinforce the core loop. All these factors together activate the expression of hundreds of circadian genes, including the gens that produce their own repressor molecules such as PER1, PER 2, PER 3 and CRY 1 and CRY 2.This occurs because the activators bind to E-box elements in the core clock repressors Period (Per1, Per2, or Per3) and Cryptochrome (Cry1 or Cry2) in mammals, which dimerize and then provide negative feedback to control their own transcription [22]. As protein levels increase, PER and CRY associate and translocate into the nucleus (basically at the night), repressing CLOCK/BMAL1, thereby inhibiting their own transcription. Enzymatic degradation of PERIOD and CRYPTOCHROME proteins provides a delay mechanism prior to the onset of the next transcriptional cycle (the next day). Transcription factors are proteins. Therefore, the loop has transcriptional and translational feedback factors that self-regulate their own activity. That is, it has an excitatory loop and an inhibitory loop. Both are in antiphase with each other, which provides circadian synchronization at the molecular level. Peripheral clocks follow the rhythms or changes of the central pacemaker through neuroendocrine messages. Both the central pacemaker and the peripheral clocks use the same machinery to time the day [23]. The time that the process takes is conditioned by phosphorylation, ubiquitylation or SUMOylation of proteins that induce or degrade information traffic, creating 24-hour feedback loops.
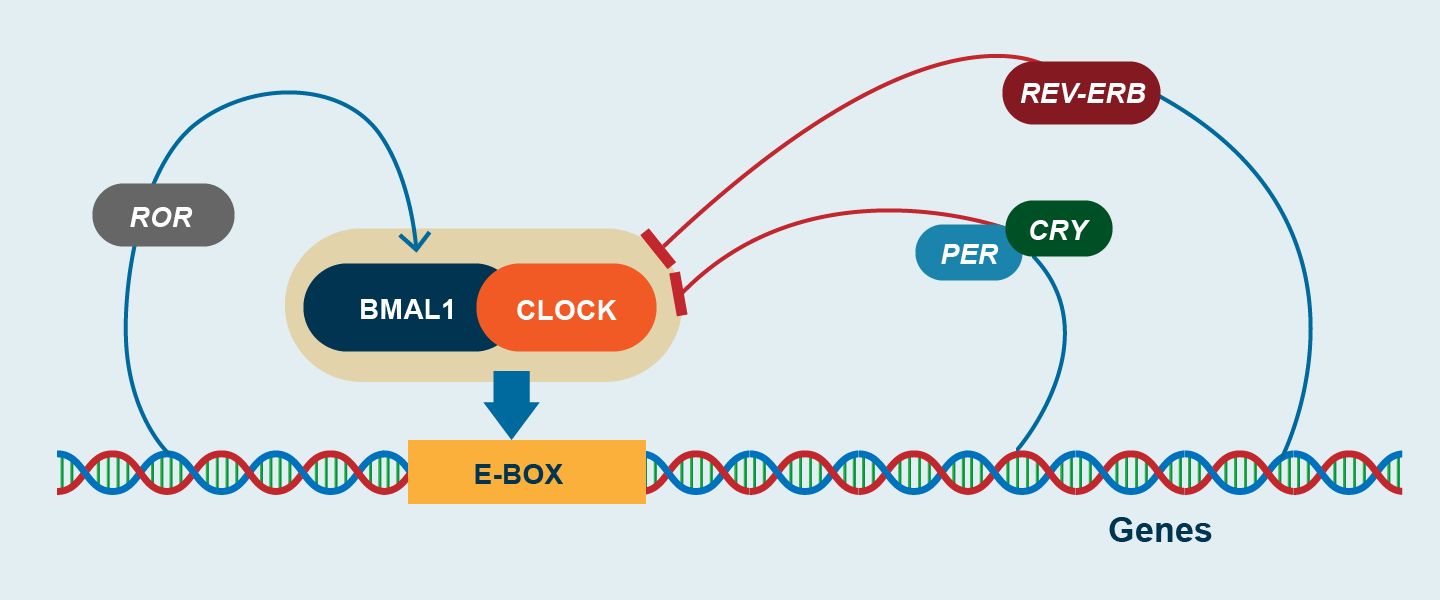
Figure 3. Schematic demonstration of biological clock network
Note the molecular machinery of the circadian rhythm. BMAL1: Brain and Muscle ARNT-Like 1; CLOCK: Circadian Locomotor Output Cycles Kaput; CRY: cryptochrome gene; PER: period gene; ROR: RAR-related orphan receptor.
A large fraction of the human genome is clock-regulated, and more than half of protein-coding genes show circadian oscillations in distinct patterns across tissues, the rhythmicity of which is genetically constrained [24-28]. Epigenetic modifications of circadian gene expression are also influential and usually involve DNA methylation at CpG sites (locations in DNA at which a cytosine precedes a guanosine in the 5′ to 3′ sequence). Epigenetic modifications of circadian genes should be viewed as central to certain disease processes [29]. Orexin A and orexin B represent a pair of excitatory neuropeptides that share a high degree of homology. Levels of these neuropeptides, which are controlled by the circadian clock located in the suprachiasmatic nucleus of the hypothalamus, oscillate over a 24-hour period and peak in the awake phase. Orexins promote arousal and wakefulness by binding to orexin receptors 1 and 2, which are widely distributed. The orally available dual orexin receptor antagonist suvorexant has been approved by the Food and Drug Administration as a treatment for insomnia characterized by difficulty with the onset or maintenance of sleep [30-32].
The circadian rhythm in respiratory diseases
Just as respiratory physiology is subject to circadian rhythm, so are respiratory diseases. Next, we proceed to a systematic review of the most relevant respiratory pathologies that are pathogenically impacted by alterations in the circadian rhythm.
Sleep disorders and obstructive sleep apnea
Sleep is a behavioral and physiological state of the body of reversible unconsciousness with minimal physical movement and no response to usual external stimuli. Sleep helps maintain normal physiological processes such as brain development, plasticity, memory, learning, and immunity [21].
There is evidence that circadian misalignment due to artificial light, shift work, and jet travel is common in modern life and contributes to a wide range of human diseases. Light exposure at the incorrect time of day shifts the phase of pacemaker neuronal clocks and peripheral-tissue clocks and can impair cognitive performance [33]. Irregular sleep and eating schedules can misalign clocks in metabolic organs, leading to obesity and diabetes. General circadian rhythm sleep disorders are characterized by misalignment between intrinsic circadian cycles and the environmental light–dark cycle. These disorders can be due to conditions, such as travel across time zones, exposure to artificial light, intrinsic disorders of clock function, such as those due to mutations in core clock genes or sleep disorder that occurs in persons who are blind because of bilateral enucleation [34]. Because the human central circadian pacemaker can shift by only about 1 hour per day, rapid air travel across multiple time zones results in misalignment between the destination environment and the internal clock. Jet lag is associated with impaired motor performance and symptoms of malaise such as gastrointestinal disorder [35]. Social jet lag refers to a pattern of inconsistent sleep time between workdays and days off from work. This problem can be exacerbated by exposure to phase-delaying blue light in the evening from electronic devices or other artificial lighting. Shift-work sleep disorder is defined by insomnia or excessive sleepiness occurring in relation to work scheduled during normal sleep time (10). Some patients have mutations in the genes that code for the rhythmicity of the clock [36].
OSA is a pathology characterized by recurrent collapse of the pharynx during sleep, partially (hypopnea) or total (apnea), thus producing a decrease or absence of airflow despite the respiratory efforts of the patient [37]. The obstructive events (apneas and hypopneas) cause a progressive asphyxia, which as they increase, stimulate respiratory efforts against a collapsed airway, until the patient wakes up. These respiratory efforts related to awakening are known as RERA (respiratory event-related arousal). These episodes are associated with recurrent oxyhemoglobin desaturation [38]. OSA is the most common type of sleep-disordered breathing (SDB). OSA is commonly associated with excessive daytime sleepiness, which is why it is also called obstructive sleep apnea syndrome or obstructive sleep apnea-hypopnea syndrome (OSAH). The cardinal symptoms of OSA include the "3 S's": Snoring, Sleepiness and Significant-other of sleep apnea episodes. Obesity, OSA and metabolic syndrome have in common denominator an inflammatory phenomenon.
OSA is associated with hypertension, cardiovascular disease, and a change in the 24 h pattern of adverse cardiovascular events and mortality. Adverse cardiovascular events occur more frequently in the middle of the night in people with OSA, earlier than the morning prevalence of these events in the general population. OSA appears to be associated with a phase change (relative to dim light melatonin onset) in the endogenous circadian rhythm of blood pressure during relaxed wakefulness, independent of common daily behaviors. On the other hand, HR, cortisol, and melatonin secretion patterns are minimally affected by OSA [39].
From a molecular point of view, OSA is a hypoxemic disorder associated with increased oxidative stress (ROS) and upregulation of systemic inflammatory responses [40]. TNFα, IL-6 and IL-8 exhibit circadian rhythms in OSA. Upregulation of these systemic inflammatory markers is strongly associated with morbid phenotypic signatures in patients with OSA. HIF-1 (hypoxia-inducible factor-1) has bidirectional interactions with the circadian clock. BMLA1 and CLOCK heterodimerize and regulate the systemic expression of HIF-1a, but in turn, HIF-1a colocalizes with BMLA1 and regulates the rhythmic expression of CRY 1 and PER 2 [41]. The crosstalk between CLOCK and HIF-1α coordinates oxygen sensing and circadian transcription cycles, contributing to day–night differences in exercise capacity. Clock factors also respond to changes in the partial pressure of oxygen through heme, which binds to the REV-ERBs, contributing to rhythmic skeletal-muscle metabolism [42]. Circadian patterns could serve as powerful biomarkers and as therapeutic targets in OSA.
Lung cancer
Epidemiologic and experimental studies provide evidence that cancer is associated with shift work and circadian disruption. The circadian period proteins form complexes with the cryptochromes and have been implicated as regulators of the cell cycle and of the tumor suppressor p53, which is important in lung cancer [43]. In addition to the connection between circadian disruption and cancer initiation, interference with rhythms may contribute to the DNA damage response and other aspects of cancer progression. For example, a major output of the clock involves the rhythmic control of enzymes involved in the biosynthesis of NAD+, a cofactor for the DNA repair pathway involving poly(ADP-ribose) polymerase (PARP) enzymes and sirtuin deacetylases [10].
Chronic obstructive airway diseases
Patients with chronic obstructive pulmonary disease (COPD) and asthma develop more frequent and severe exacerbations, with an increased rate of emergency room visits and hospitalization, mostly at night and in the early morning hours [44]. Exacerbation in patients with COPD/asthma, with increased lung inflammation and deterioration of the disease state, is associated with a rapid decline in lung function. Air pollutants, cigarette smoke (CS), and respiratory viral (influenza and rhinoviruses and SARS-CoV-2) and bacterial infections can lead to exacerbations of COPD/asthma, with the most severe effects appearing in the early morning hours and affecting lung function. Hence, there is a connection between circadian decline in lung function and exacerbations of COPD/asthma. Many growth factors (ligands), receptors, inflammatory molecules, and airway glands that are involved in lung regulation and homeostasis show circadian influence.
Bronchial asthma’ symptoms are worse around 4 AM and sudden death in asthma occurs predominantly at night [45]. 74% of asthmatic patients wake up at night at least once, and 80% of fatal attacks occur at night or in the early morning. Bronchial asthma is the most common inflammatory chronic disease and affects 339 million patients worldwide [46]. Healthy individuals exhibit variability in pulmonary physiology throughout the 24 hours, but in the asthmatic patient the variability is amplified, in such a way that the FEV1 (flow expiratory volume in first second) and PEF (peak expiratory flow) can be reduced 2-4 times in the early morning with respect to the day [47]. Eosinophils in bronchioloalveolar lavage are 2-3 times higher at 4 hours than at 16 hours in patients with asthma, as are tissue eosinophils in transbronchial lung biopsies and in sputum. Serum Ig-E also shows this pattern as do some volatile organic compounds. The results of studies with exhaled nitric oxide (FENO) have produced very different results [48,49]. Therefore, the worsening of asthma at night is associated with airway inflammation, which correlates not only with the appearance of symptoms, but also with the deterioration in respiratory function and bronchial hyperresponsiveness. And this phenomenon is associated with the expression of clock genes. Investigating the mechanisms that underregulate the genes of the circadian clock in asthma and the molecular causal effects of these genes is a priority [50].
The respiratory system has its own autonomic mechanisms, which are in bronchial epithelial cells. The peripheral pulmonary clock is present in many Club cells (formerly Clara cells) in the bronchial epithelium and is responsible for the recruitment of inflammatory cells [51]. REV-ERBα plays a key role in repressing inflammation [52]. The expression of BMAL 1 is reduced at night, which also explains the increased nocturnal inflammation in asthma. Children with respiratory syncytial virus infection, bronchiolitis, and wheezing also show deficient expression of BMAL1 in nasal washes in the night [53]. In the context of allergic diseases, the biology of eosinophils, basophils, and mast cells is under circadian regulation. Sleep deprivation alone does not influence circadian variation of lung function, suggesting that an endogenous circadian pacemaker is responsible for the diurnal variations [54]. Many other factors are associated with the nocturnal exacerbation of asthma. The robust circadian rhythms of cortisol dip during the night and the vagal tone increase during sleep are believed to contribute significantly to the diurnal variation in airway inflammation and reactivity [55,56]. Cortisol binding and steroid responsiveness appear impaired in nocturnal asthma, resulting in impaired endogenous anti-inflammatory processes [57]. Other factors, such as late phase response to allergen exposure, nighttime predominance of gastroesophageal reflux, sleep apnea, and lung volume changes during sleep, may also play a role in nocturnal asthma symptoms [58-60].
Symptoms in Chronic Obstructive Pulmonary Disease (COPD) occur at night in 50% of patients and 80% have symptoms in the early morning and/or later in the day. Nocturnal symptoms predominate in the chronic bronchitis phenotype rather than in emphysema. In both phenotypes, the presence of nocturnal symptoms is associated with poor quality of life, poor quality of sleep, and high levels of anxiety and depression. The accumulation of nocturnal secretions and bronchial vagal hypertonus at night partly explain the nocturnal cough, which awakens the patient by interrupting the sleep cycle [61,62]. Comparing with bronchial asthma, the link between the circadian rhythm and COPD is less well established. The circadian rhythm in FEV1, in stable COPD, is like that in asthma in that it increases around 16 hours and decreases around 4 hours, but the amplitude of the variation is substantially less than in bronchial asthma. Chronic smoking, viral and bacterial infections (the most frequent cause of COPD exacerbations), and environmental stress are factors that affect the function of the circadian clock in COPD [63]. Chronic smoking reduces the expression of Sirtuin 1 (SIRT1, which is an anti-senescence molecule). Monocytes, airway cells and lung tissue from chronic smokers and COPD patients (compared to non-smokers) have reduced not only the expression but also the activity of SIRT 1 produced by the oxidative stress generated by smoking. SIRT 1 promotes the deacetylation of BMAL1 (at amino acid residue lys537 with proteasome degradation), and PER 2 by regulating the transcription of clock genes. This reduced activity facilitates the hyperacetylation of the histones of the clock genes, culminating in abnormal proinflammatory rhythms of the clock molecules [64]. It should be remembered that COPD is an inflammatory disease and SIRT 1 an anti-inflammatory molecule [65]. Therefore, future therapies that aim to increase SIRT 1 activity could be a new form of treatment for senescence-associated diseases such as COPD. Leptin is a hormone derived from adipocytes that conditions the energy deposited in adipose tissue. Whether free or conjugated, it acts on specific receptors in the hypothalamus to regulate food intake. It has undulating values in a circadian and ultradian cycle in healthy controls and in non-cachectic COPD, but in cachectic COPD leptin levels are practically absent [66].
There are many gaps in the knowledge of the mechanisms that operate in the molecular dysfunction of the clock and that contribute to the pathogenesis of chronic airway diseases.
Infection, inflammation, and immunity
Response to pathogens shows circadian variation in circulating cells of the innate immune system. Expression of the clock in pulmonary epithelial cells generates rhythmic variations in Streptococcus pneumoniae infection and rhythms of the sympathetic nervous system also generates rhythmic variations in the response to endotoxins [67]. In the epidermis, mast cells show circadian variations in the Ig-E-mediated cutaneous anaphylactic reaction. It is possible that the same thing happens in the airway, in anaphylaxis. The molecular clock also regulates fundamental inflammatory aspects such as the expression of TLR-9 (Toll-like receptor 9), CCL2 (chemokine ligand 2), Il-6 (interleukin), and activation and transcription of NF-kβ (nuclear factor kappa-beta) and AP1 (activating protein 1) in response to oxidative stress. NF-kβ is a primary mediator of immune cell activation and inhibits the PER 2 clock repressor. Likewise, the activation of the Nrf2/glutathione axis to counteract damage in pulmonary fibrosis. Nrf2 (factor-erythroid 2-related factor 2). This implies that bacterial infection alters the cycling time and the amplitude of the gene expression of the clock. These mechanisms are likely to play a pathogenic role in respiratory infections [68]. Molecular clock dysfunction can amplify the response to environmental stressors. Multiple studies suggest that pro-inflammatory activity is elevated during rest and induces sleep, while anti-inflammatory mediators induce wakefulness and inhibit sleep.
The innate and adaptive immune systems oscillate in a circadian manner. Immune cell trafficking, susceptibility to bacterial infections, septic shock, receptor pattern recognition, phagocytosis, cytokine, and chemokine secretion, is under rhythmic control [23]. During the resting period, the synthesis of growth hormone, melatonin, leptin, and prolactin is increased, which leads to immune activation, proliferation, and production of proinflammatory cytokines. Sleep deprivation affects response to vaccines or viral infections. Exposure to light during sleeping hours alters sleep and is detrimental to the immune system.
Sepsis, allergic and immune diseases are more likely to occur during the last period of rest or early in the onset of daytime activity. Also, exacerbations and mortality are higher in these time tracts. Therapies that reduce inflammation during the night and early in the morning have more opportunities to see their effectiveness increased, than if this cycling is not considered. It should be remembered that BMLA 1 and REV/ERBα have anti-inflammatory effects [1,9].
Molecular clock dysfunction is also involved in response to DNA damage. Chronic smoking and exposure to environmental stressors, in addition to causing molecular clock dysfunction, can cause DNA damage. Persistent DNA damage induces SIPS (stress-induced premature senescence) and SASP (senescence-associated secretory phenotype). The most dramatic damage to DNA is DSB (double strand break). The response to damaged DNA can generate an inflammatory phenotype and accelerate senescence. In COPD, for example, both damage and repair capacity are involved, with an inflammatory process and accelerated aging of the lung occurring [64]. Recent studies have shown that age affects the re-entry rates of central and peripheral circadian oscillations and rhythm is particularly affected at older ages making this population more susceptible to frequent exacerbations.
Chronotherapy
Chronotherapy is the synchronizing of drug concentrations to rhythms in disease activity, increasing efficacy as well as reducing adverse effects. Some options in respiratory diseases are discussed.
Corticosteroids
Asthma and COPD (lesser extent) exhibit a marked time of day variation in symptoms, airway physiology, and airway inflammation. They might be thought of as a good model to illustrate the need for chronotherapy. However, neither GINA 2021 nor GOLD 2021 take this factor into account. Both tools recommend in crisis, oral steroids (a single dose) in the morning, 5-7 days without the need for progressive reduction. For maintenance, inhaled steroids are recommended once or twice a day and there is no single line that explains the chronotherapeutic reason for this indication [69,70]. However, several studies investigating systemic and inhaled corticosteroids have consistently shown that the best time of day to take these medications for treating asthma is in the afternoon or early evening and not in the morning, when these medications are often prescribed. Future, large, randomized, placebo-controlled studies of systemic and inhaled corticosteroids in asthma and COPD are needed to inform clinical practice. There is a well-recognized endogenous circadian variation in cortisol levels, with levels of cortisol highest in the morning and lowest during the night. Infusion of methylprednisolone between 8:00 am and 4:00 pm caused no adrenal suppression; yet an infusion administered between 12:00 and 4:00 am caused severe adrenocortical suppression. Infusion during 4:00 and 8:00 pm and between 4:00 am and 8:00 am resulted in moderate adrenocortical suppression [71]. In several studies, the use of 50 mg of prednisolone at 3 pm has been shown to significantly reduce the decline in FEV1 in nocturnal asthma and cause less disruption to endogenous circadian cortisol rhythm [72]. In addition, direct circadian control of glucocorticoid signaling may point to new therapeutics for inflammatory disease [73]. For example, the GC-GCR complex (glucocorticoid bound to its receptor) in the cytoplasm translocate to the nucleus and binds to the GRE (glucocorticoid response element) of the Per gene. Therefore, compounds designed to increase the amplitude and normalization of the expression of circadian clock-dependent genes and phase said expression, could be promising in chronic airway inflammation and an alternative to glucocorticoids and β-2 agonists [74].
In COVID-19, corticosteroids suppress the activation of the immune system, as anti-inflammatories, preventing the cytokine storm in patients with a hyperactive immune response, responsible for death in patients with the infection [75]. Dexamethasone, prednisone, and methylprednisolone reduce the risk of death compared to placebo in COVID-19, but only in patients with hyperinflammation [76]. No studies with corticosteroids in COVID-19 impacting circadian rhythm.
β-2 agonists
Plasma adrenaline fluctuates in a circadian manner, with "valley" (reduced) levels at 4 am and "peak" (elevated) levels at 4 pm, both in healthy individuals and in asthmatic patients [77]. SABA (short-acting beta-2 agonist) chronotherapy appears to induce the clock gene hPer 1 in epithelial cells in vitro, but inhaled LABA (long-acting beta-2 agonist) chronotherapy has not been extensively investigated. It would be of great therapeutic interest to establish the difference between morning and night doses of these agents. Vilanterol (65 K) is an Ultra-laba that has a duration of action of 24 hours. No differences in efficacy have been demonstrated when dispensing it in the morning vs at night, associated with fluticasone (25 ug/100 ug, respectively), which suggests that in drugs with this duration of action, the circadian effect is not important or may be masked [78]. Another randomized control trial conducted on 20 mild–moderate asthma patients studied whether a once-daily inhaled formoterol in the evening, administered from the combination budesonide/formoterol (BUD/F) Turbuhaler, notably ameliorated the circadian rhythm in airway tone for over 24 h. Patients received either inhaled BUD/F (2 × 100/6 microg) or a placebo at 8 p.m. on two separate occasions followed by measurement of lung function parameters, such as FEV1, airways conductance and maximum expiratory flow at baseline, at 1 h after administration and every 4 h post-administration for the next 24 h. The results showed that BUD/F remarkably ameliorated all three lung function parameters throughout the 24 h period, with a difference in FEV1 at 24 h of 0.20 L (0.04–0.35 L) as compared to the placebo control. BUD/F also ameliorated the biphasic pattern of the circadian rhythm in the airway, suggesting that a once daily evening dose of formoterol showed prolonged bronchodilation for at least 24 h [79].
Anticholinergics
The vagus nerve is one of the most important pathways for transmitting circadian signals from the central pacemaker (central clock) to the peripheral clocks of the respiratory tract and increased cholinergic tone during the night can cause nocturnal bronchoconstriction and mucus hypersecretion [80]. Of the LAMAs (long-acting muscarinic antagonists), tiotropium bromide has not shown significant differences when administered once daily, either in the morning or in the afternoon, but the long duration of action could mask a circadian time-lapse effect-dependent [81]. In other papers, with tiotropium, its use at night reduces nocturnal symptoms, the use of rescue SABA and offers less variability in FEV1 [82]. In such a way that there is no clarity in which is the optimal time of day to dispense it. It is likely that this fact partly conditions the morning use of LABAs in the GOLD guides. The currently prevailing idea is that LAMAs in general produce prolonged bronchodilation for 24 hours without being impacted by the circadian rhythm [83].
LTRAs (leukotriene receptor antagonists) are recommended to be dispensed at night, since the improvement in FEV1 is greater than if they are ingested in the morning [84].
Antiviral drugs
Host clock components help viruses replicate in a direct or indirect manner. However, timed administration of antivirals in patients has rarely been taken into consideration. In October 2020, 7 months since the declaration of the COVID-19 pandemic, the United States FDA authorized the antiviral drug, remdesivir to treat COVID-19. Studies have observed that remdesivir to have a superior effect over placebo in shortening the recovery time in hospitalized COVID-19 patients [85]. This facilitated the use of remdesivir by clinicians among hospitalized COVID-19 patients. However, a recent WHO solidarity trial published, taking 11,330 adult COVID-19 patients from 30 countries worldwide, showed that antiviral drugs like remdesivir and lopinavir have little to no effect on the outcome such as overall mortality, the need for ventilation support, or hospital recovery time [86]. Very recently, among no hospitalized patients who were at high risk for Covid-19 progression, a 3-day course of remdesivir had an acceptable safety profile and resulted in an 87% lower risk of hospitalization or death than placebo [87]. However, in COVID-19 there are no studies that consider the circadian rhythm for the administration of remdesivir.
Anticoagulants
SARS-CoV-2 increases the risk of blood clots and therefore of stroke, pulmonary thromboembolism, respiratory failure, and acute myocardial infarction. Although the exact cause of this increased incidence of blood clots among COVID-19 patients remains unclear, evidence suggests that severe inflammatory response, like that of COVID-19 patients with a hyperactive immune system, can trigger coagulation, decrease the activity of natural anticoagulants, and impair the fibrinolytic system [88]. Hyper-coagulatory and hypofibrinolytic conditions are more frequent in the morning than any other time during the day because of increased platelet activity. This partly explains the increased incidence of thromboembolic events during the early hours of the day [89]. Rivaroxaban should be taken at night as concentrations are highest 12 hours after the nighttime dose, making it more effective. For the same reason, acetylsalicylic acid should be taken at night as a platelet antiaggregant [90]. There is sufficient evidence that proves circadian variation in coagulation and fibrinolysis exists, and certain anticoagulatory or antiplatelet drugs work best only when administered at a specific time of the day. Therefore, timed-administration of these drugs can tremendously prove to be a cost-effective way to improve patient care during these times with the added benefit of reducing drug toxicity while maximizing the benefit in critically ill COVID-19 patients. Future studies should therefore aim to further evaluate the effect of timed-administration of full doses of blood thinners, once a day, on COVID-19 patients.
Lung cancer
To date, the in-depth mechanisms that reveal the link between circadian biology and the molecular action of anti-cancer treatments are not fully understood. This has led to limitations in the potential use of CR-based therapy (chronotherapy) in clinical settings. The metabolism of various chemotherapeutic drugs, such as cisplatin, epirubicin, docetaxel, doxorubicin, paclitaxel, vinblastine, and methotrexate, are associated with ABC family transporters because abcc2 transporters are responsible for effluxes of these chemotherapeutic agents. As these transporters are present locally in the pulmonary system, there is a clear role of influence of the circadian clock gene on the efficacy of these chemotherapeutic agents. In this context, the time of administration of chemotherapeutics to patients also plays a key role in determining the efficacy of the treatment. For example, it was found that the efficiency of 5-flouoracil was augmented when it was administered early in the morning at 4 a.m. Similar results were found regarding irinotecan at 5 am and oxaliplatin at 4 p.m. [91,92]. The efficiency of 5-fluoracil (5-FU) was related with increased intracellular dehydropyrimide dehydrogenase (DPD) enzyme activity in healthy cells as well as low DNA synthesis in healthy tissues.
Conclusions and Future Directions
The past few years have been very exciting for circadian research, making it clear that circadian biology is at the core of human physiology. A multitude of additional layers of circadian clock regulatory mechanisms have been demonstrated recently. The growing body of evidence in mammalian circadian rhythms research is revealing an undisputable link between circadian rhythms and human health.
Nevertheless, we are far from understanding the complexity of circadian biology, but the scientific information establishes a clear relationship not only between physiology and the circadian rhythm, but also between it and various pathologies of the human economy. Some respiratory diseases have been used in this article to exemplify this relationship. In respiratory diseases, signs, and symptoms as well as severity show circadian variability across the 24-h cycle. Specifically, obstructive airways diseases demonstrate increased inflammation and disease severity at night. OSA (and other sleep diseases), lung cancer, sepsis, allergy, inflammatory and immune diseases are more likely to occur during the last period of rest or early in the onset of daytime activity or are subjects to circadian control. Circadian medicine is clearly an interdisciplinary field that requires complementary expertise. Advances in technology have shaped circadian research in recent years and will continue to be crucial going forward. Defining the influence of the core circadian clock in the cell signaling pathways leading to lung disorders could unlock novel therapeutic avenues. The efficacy and side effects of a therapy may vary when administration occurs at 8 a.m. compared to 8 p.m.; therefore, elucidating the circadian oscillation (time of peak and trough) of various disease-related proteins, genes and enzymes will facilitate the selection of the timing of drug administration. For various diseases including respiratory diseases, chronotherapy could be superior to routine therapy in terms of efficacy and the control of side effects. However, more scientific studies that support chronotherapy as a choice of therapeutic management over conventional therapy are essential for the validation of its effectiveness.
Authorship
This work was only carried out by the author. Author AA contributed on the planning, data collection, data analysis, writing and critical review. AA read and approved the final manuscript
Source of economic support
None.
Conflict of interest
None.
References
- Comas M, Gordon CJ, Oliver B, Stow NW, King G, et al (2017) A circadian based inflammatory response-implications for respiratory disease and treatment. Sleep Sci Pract 1: 18.
- Truong KK, Lam MT, Grandner MA, Sassoon CS, Malhotra A (2016) Timing matters: circadian rhythm in sepsis, obstructive lung disease, obstructive sleep apnea, and cancer. Ann Am Thorac Soc 13: 1144-1154. [Crossref]
- Konopka RJ, Benzer S (1971) Clock mutants of Drosophila melanogaster. Proc Natl Acad Sci U S A 68: 2112-2116. [Crossref]
- Hardin PE, Hall JC, Rosbash M (1990) Feedback of the Drosophila period gene product on circadian cycling of its messenger RNA levels. Nature 343: 536-540.
- King DP, Zhao Y, Sangoram AM, Wilsbacher LD, Tanaka M, et al (1997) Positional cloning of the mouse circadian clock gene. Cell 89: 641-653. [Crossref]
- Huang RC (2018) The discoveries of molecular mechanisms for the circadian rhythm: The 2017 Nobel Prize in Physiology or Medicine. Biomed J 241: 5-8. [Crossref]
- Callaway E, Ledford H (2017) Medicine Nobel awarded for work on circadian clocks. Nature 550: 18. [Crossref]
- Burki T (2017) Nobel Prize awarded for discoveries in circadian rhythm. Lancet 390: e25. [Crossref]
- Rijo-Ferreira F, Takahashi JS (2019) Genomics of circadian rhythms in health and disease. Genome Med 11: 82.
- Allada R, Bass J (2021) Circadian mechanisms in Medicine. N Engl J Med 384: 550-561. [Crossref]
- Gehring W, Rosbash M (2003) The coevolution of blue-light photoreception and circadian rhythms. J Mol Evol 57: S286-S289. [Crossref]
- Ralph MR, Foster RG, Davis FC, Menaker M (1990) Transplanted suprachiasmatic nucleus determines circadian period. Science 247: 975-978. [Crossref]
- Paul JR, Davis JA, Goode LK, Becker BK, Fusiller A, et al (2019) Circadian regulation of membrane physiology in neural oscillators through the brain. Eur J Nerurosci 51: 109-138. [Crossref]
- Flourakis M, Kula-Eversole E, Hutchinson AL, Diekman CO, Raman IM, et al (2015) A conserved bicycle model for circadian clock control of membranes excitability. Cell 162: 836-848. [Crossref]
- Bass J, Lazar MA (2016) Circadian time signatures of fitness and disease. Science 354: 994-999. [Crossref]
- Izumo M, Pejchal M, Schook AC, Lange RP, Wallisser JA, et al (2014) Differential effects of light and feeding on circadian organization of peripheral clocks in a forebrain B mal1 mutant. Elife 3: e04617. [Crossref]
- LeGates TA, Fernández DC, Hattar S (2014) Light and a central modulator of circadian rhythms sleep and affect. Nat Rev Neurosci 15: 443-454. [Crossref]
- Foster RG, Hughes S, Pierson SN (2020) Circadian photoentrainment in mice and humans. Biology (Basel) 9: 180. [Crossref]
- Kim P, Oster H, Lehnert H, Schmid SM, Salamat N, et al (2019) The role of Period in timings circadian clock. Endocrinol Rev 40: 66-95.
- Scheuermann C, Gibbs J, Ince l, Loudon A (2018) Clocking into immunity. Nat Rev Immunol 18: 423-437. [Crossref]
- Giri A, Srinivasan A, Sundar IK (2021) COVID-19: sleep, circadian rhythms, and Immunity-repurposing drugs and chrono therapeutics for SARS-CoV-2. Front Neurosci 15: 674204. [Crossref]
- King DP, Zhao Y, Sangoram AM, Wilsbacher LD, Tanakaet M (1997) Positional cloning of the mouse circadian clock gene. Cell 89: 641-653. [Crossref]
- Krakowia KK, Durrington HJ (2018) The role of the Body Clock in asthma and COPD. Implications for treatment. Pulm Ther 4: 29-43. [Crossref]
- Fang B, Everett LJ, Jager J, Gerhart-Hines Z, Sung Z, et al (2014) Circadian enhancers coordinate multiples phases of rhythmic gene transcription in vivo. Cell 159:1140-1152. [Crossref]
- Koike N, Yoon SH, Huang HC, Kumar V, Lee C, et al (2012) Transcriptional architecture and chromatin landscape of the core circadian clock in mammals. Science 338: 349-354. [Crossref]
- Trump RP, Bresciani S, Cooper AW, Telamm JP, Wojno J, et al. (2013) Optimized chemical probes for REV-ERBα. J Med Chem 56:4729-4737. [Crossref]
- Green CB, Takahashi JS, Bass J (2008) The meter of metabolism. Cell 134:728-742. [Crossref]
- Morawk JA, Green CB, Takahashi JS (2012) Central and peripheral circadian clock in mammal. Ann Rev Neurosci 35: 445-462. [Crossref]
- Lahtinen A, Häkkinen A, Puttonen S, Vanttola P, Viitasalo K, et al (2021) Differential DNA methylation in recovery from shift work disorder. Sci Rep 11: 2895. [Crossref]
- Ruan W, Yuan X, Eltzschig HK (2021) Circadian rhythm as a therapeutic target. Nat Rev Drug Discov 20:287-307. [Crossref]
- Coleman PJ, Gotter AL, Herring WJ, Winrow CJ, Renger JJ (2017) The discovery of suvorexant, the first orexin receptor drug for insomnia. Annu Rev Pharmacol Toxicol 57: 509-533. [Crossref]
- Ruan W, Yuan X, Eltzschig H (2021) Circadian rhythm. N Engl J Med 384: e76. [Crossref]
- LeGates TA, Altimus CM, Wang H, Lee HK, Yang S, et al (2012) Aberrant light directly impairs mood and learning through melanopsin-expressing neurons. Nature 491: 594-598.
- Skene DJ, Arendt J (2007) Circadian rhythm sleep disorders in the blind and their treatment with melatonin. Sleep Med 8: 651-655. [Crossref]
- Duffy JF, Cain SW, Chang AM, Phillips AJK, Münch MJ, et al (2011) Sex difference in the near-24-hour intrinsic period of the human circadian timing system. Proc Natl Acad Sci U S A 108: 15602-15608. [Crossref]
- Patke A, Murphy PJ, Onat OE, Özşelik T, Campells S (2017) Mutation of the human circadian clock gene CRY1 in familial delayed sleep phase disorders. Cell 169: 203-215.e13. [Crossref]
- Eastwood PR, Malhotra A, Palmer RL, Horner RL, Thurnheer R, et al (2010) Obstructive sleep apnoea: from pathogenesis to treatment. Current controversies and future directions. Respirology 15: 587-595. [Crossref]
- Spicuzza L, Caruso D, Di Maria G (2015) Obstructive sleep apnoea syndrome and its management. Ther Adv Chronic Dis 6: 273-285. [Crossref]
- Butler MP, Thosar SS, Smales C, DeYoung PN, Wu H, et al (2020) Effects of obstructive sleep apnea on endogenous circadian rhythms assessed during relaxed wakefulness: an exploratory analysis. Chronobiol Int 37: 856-866. [Crossref]
- Tasali E, Ip M (2008) Obstructive sleep apnea and metabolic syndrome: alterations in glucose metabolism and inflammation. Proc Am Thorac Soc 153: 207-217. [Crossref]
- Wu Y, Tang D, Liv N, Xiong W, Li Y, et al (2014) Reciprocal regulation between the circadian clock and hypoxia signaling at the genome level in mammals. Cell Metab 25: 73-85. [Crossref]
- Yin L, Wu N, Curtin JC, Qatanani M, Szwergold NR, et al (2007) Rev-erbalpha, a heme sensor that coordinates metabolic and circadian pathways. Science 318: 1786-1789. [Crossref]
- Papagiannakopoulus T, Baver MR, Davidson SM, Bartlebauh J, Vander Heiden, MG, et al (2016) Circadian rhythm disruption promotes lung tumorigenesis. Cell Metab 24: 324-331. [Crossref]
- Tsai CL, Brenner BE, Camargo CA (2007) Circadian-rhythm differences among emergency department patients with chronic obstructive pulmonary disease exacerbation. Chronobiol Int 24: 699-713. [Crossref]
- Sutherland ER (2005) Nocturnal Asthma. J Allergy Clin Immunol 6: 1179-1186. [Crossref]
- Wang R, Murray CS, Fowler SJ, Simpson A, Durrington HJ (2021) Asthma diagnosis: into the fourth dimension. Thorax 76: 624-631. [Crossref]
- Zhou D, Li H, Wang Y, Hochaus G, Sinha V, et al (2015) Quantitative characterization of circadian rhythm of pulmonary function in asthmatic patients treated with inhaled corticosteroids. Pharmacodyn 46: 391-399. [Crossref]
- Wilkinson M, Maidstone R, Loudon A, Blaikleya J, Laine R, et al (2019) Circadian rhythm of exhaled biomarkers in health and asthma. Eur Respir J 54: 1-4. [Crossref]
- Green RH, Brightling CE, McKenna S, Hargadon B, Parker D, et al (2002) Asthma exacerbations and sputum eosinophils count: a randomized controlled trial. Lancet 360: 1715-1721. [Crossref]
- Chen H-C, Chen Y-C, Wang T-N, Fang W-F, Chang Y-C, et al (2021) Disrupted expression of circadian clock genes in patients with bronchial asthma. J Asthma Allergy 14: 371-380. [Crossref]
- Hadden H, Soldin SJ, Massaro D (2012) Circadian disruption alters mouse lung clock gene expression and lung mechanics. J Appl Physiol 113: 385-392. [Crossref]
- Parriollaud M, Gibbs JE, Hopwood TW, Brown S, Begley N, et al (2018) Circadian clock component 1 rev-erbα controls homeostatic regulation of pulmonary inflammation. J Clin Invest 128: 2281-2296. [Crossref]
- Ehlers A, Xie W, Agapov E, Brown S, Steinberg D, et al (2018) Bmal 1 links the circadian clock to viral airway pathology and asthma phenotypes. Mucosal Immunol 11: 97-11. [Crossref]
- Spengler CM, Shea SA (2000) Endogenous circadian rhythm of pulmonary function in healthy humans. Am J Respir Crit Care Med 162: 1038-1046. [Crossref]
- Soutar CA, Carruthers M, Pickering CA (1977) Nocturnal asthma and urinary adrenaline and noradrenaline excretion. Thorax 32: 677-683.
- Soutar CA, Costello J, Ijaduola O, Turner-Warwick M (1975) Nocturnal and morning asthma: relationship to plasma corticosteroids and response to cortisol infusion. Thorax 30: 436-440. [Crossref]
- Kraft M, Hamid Q, Chrousos GP, Martin RJ, Leung DY (2001) Decreased steroid responsiveness at night in nocturnal asthma: is the macrophage responsible? Am J Respir Crit Care Med 163: 1219-1225. [Crossref]
- Ballard RD, Irvin CG, Martin RJ, Pak J, Pandey R, et al (1985) Influence of sleep on lung volume in asthmatic patients and normal subjects. J Appl Physiol 68: 2034-2041. [Crossref]
- Mohiuddin AA, Martin RJ (1990) Circadian basis of the late asthmatic response. Am Rev Respir Dis 142: 1153-1157. [Crossref]
- Yigla M, Tov N, Solomonov A, Rubin AHE, Harlev D (2003) Difficult-to control asthma and obstructive sleep apnea. J Asthma 40: 865-871. [Crossref]
- Scichilone N, Zedda A, Castellani W, Triolo N, Cuttitta G, et al (2019) Circadian rhythm of COPD symptoms in clinically based phenotypes. Results from the STORICO Italian observational study. BMC Pulm Med 19: 171. [Crossref]
- Stephenson JJ, Cai Q, Mocarski M, Tan H, Doshi JA, et al (2019) Impact and factors associated with nighttime and early morning symptoms among patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis 10: 577. [Crossref]
- Sundar IK, Rahid K, Sellix MT, Rahman I (2019) The nuclear receptor and clock gene REV-ERBα regulates cigarette smoke-induced lung inflammation. Biochem Bhiophys Res Commun 493: 1390-1395. [Crossref]
- Yao H, Sundar IK, Huang Y, Gerloff T, Sellix MT, et al (2015) Disruption of Sirtuin 1-mediated control of circadian molecular clock and inflammation in chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol 53: 782-792. [Crossref]
- Alvarado A, Arce I (2015) Molecular Biology of Chronic Obstructive Pulmonary Disease from the Bases to the Therapeutic Decision: A Review. BJMMR 10: 1-14.
- Takabatake N, Nakamura H, Minamihaba O, Inage M, Inove S, et al (2001) A novel pathophysiologic phenomenon in cachexic patients with chronic obstructive pulmonary disease. The relationship between the circadian rhythm of circulatory leptin of the very low-frequency component of hearth rate variability. Am J Respir Crit Care Med 165: 1314-1319. [Crossref]
- Gibbs J, Ince L, Matthew L, Mei J, Bell T, et al (2014) An epithelial circadian clock controls pulmonary inflammation and glucocorticoid action. Nat Med 20: 919-926. [Crossref]
- Burioka N, Fukuoka Y, Takata M, Endo M, Miyata M, et al (2007) Circadian rhythm in the CNS and peripheral clock disorders: function of clock genes: influence of medication for bronchial asthma on circadian gene. J Pharmacol Sci 103:144-149. [Crossref]
- GINA (2021) Global Strategy for Asthma Management and Prevention. Global Initiative for Asthma.
- GOLD (2021) Global Strategy for the Diagnosis, Management and Prevention of COPD. Global Initiative for Chronic Obstructive Lung Disease.
- Ceresa F, Angeli G, Buccuzzi G, Molino G (1969) Once-a-day neutrally stimulated and basal ACTH secretion phases in man and their response to corticoid inhibition. J Clin Endocrinol 29: 1074-1082. [Crossref]
- Beam WR, Weiner DE, Martin RJ (1992) Timing of prednisolone and alteration of airways inflammation in nocturnal asthma. Am Rev Respir Dis 146: 1524-1530. [Crossref]
- Lamia KA, Papp SJ, Yu RT, Baresh GD, Lehlenhaut H, et al (2011) Cryptochromes mediate rhythmic repression of the glucocorticoid receptor. Nature 480: 552-556. [Crossref]
- Chen Z, Yoo H (2013) Small molecules modifiers of circadian clocks. Cell Mol Life Sci 70: 2985-2998. [Crossref]
- Ruan Q, Yang K, Wang W, Jiang L, Song J (2020) Clinical predictors of mortality due to COVID-19, based on an analysis of 150 patients from Wuhan, China. Intensive Care Med 46: 846-848. [Crossref]
- RECOVERY collaborative group, Horby P, Lim WS, Emberson JR, Mofhaw M, et al (2021) Dexamethasone in hospitalized patients with COVID-19. N Engl J Med 380: 691-704. [Crossref]
- Maneechosuman K, Essilfie-Quaye S, Mean S, Claine K, Kharitonou SA, et al (2005) Formoterol attenuates neutrophilic airway inflammation in asthma. Chest 128: 1936-1942. [Crossref]
- Kempsford RD, Oliver A, Bal J, Tombs L, Quinn O (2013) The efficacy of once-daily fluticasone furoate/vilanterol in asthma is comparable with morning or evening dosing. Respir Med 107: 1873-1880. [Crossref]
- Masoli M, Williams M, Weatherall M, Beasley R (2006) The 24 h duration of bronchodilator action of the budesonide/formoterol combination inhaler. Respir Med 100: 20-25. [Crossref]
- Smolensky MH, Lemmer AE, Reinberg AE (2007) Chronobiology and chronotherapy of allergic rhinitis and bronchial asthma. Adv Drug Deliv Rev 59: 852-882. [Crossref]
- Calveiley PM, Lee A, Touse L, van Noord J, Witek TJ, et al (2003) Effect of tiotropium bromide on circadian variation in airflow limitation in chronic obstructive pulmonary disease. Thorax 58: 855-860. [Crossref]
- Terzano S, Petroianni A, Conti V, Ceccarelli S, Grazini E, et al (2008) Rational timing of combination therapy with tiotropium and formoterol in moderate and severe COPD. Respir Med 102: 1701-1707. [Crossref]
- Paudel RR, Jha SK, Allaw VSRR, Prahser P, Gupta RK, et al (2021) Recent advances chronotherapy targeting respiratory diseases. Pharmaceutics 13: 2008. [Crossref]
- Noonar MJ, Chervinsky P, Brandon PM, Zhang J, Kundus S, et al (1998) Montelukast, a potent antagonist, causes dose-related improvement in chronic asthma. Montelukast Asthma Study Group. Eur Respir J 11: 1232-1239. [Crossref]
- Madsen LW (2020) Remdesivir for the treatment of Covid-19-final report. N Engl J Med 338: 1813-1826. [Crossref]
- WHO Solidarity Trial Consortium (2020) Repurposed antiviral drugs for COVID-19—interim WHO SOLIDARITY trial results. N Engl J Med 384: 497-511. [Crossref]
- Gottlieb RL, Vaca CE, Paredes R, Mera J, Webb BJ, et al (2022) Early Remdesivir to Prevent Progression to Severe Covid-19 in Outpatients. N Engl J Med 386: 305-315. [Crossref]
- Morrone D, Morrone V (2018) Acute pulmonary embolism: focus on the clinical picture. Korean Circ J 48: 365-381. [Crossref]
- Angleton P, Chandler WL, Schmer G (1989) Diurnal variation of tissue-type plasminogen activator and its rapid inhibitor (PAI-1) Circulation 79: 101-106.
- Brunner-Ziegler S, Jilma B, Schörgenhofer C, Winkler F, Jilma-Stohlawetz P, et al. (2016) Comparison between the impact of morning and evening doses of rivaroxaban on the circadian endogenous coagulation rhythm in healthy subjects. J Thromb Haemost 14: 316-323. [Crossref]
- Bouchahda M, Adam R, Giacchetti S, Castaing D, Brezault-Bonnet C, et al (2009) Rescue chemotherapy using multidrug chrono modulated hepatic arterial infusion for patients with heavily pretreated metastatic colorectal cancer. Cancer 115: 4990-4999. [Crossref]
- Innominato PF, Focan C, Gorlia T, Moreau T, Garufi C, et al (2009) Circadian rhythm in rest and activity: A biological correlate of quality of life and a predictor of survival in patients with metastatic colorectal cancer. Cancer Res 69: 4700-4707. [Crossref]



