Diaphragmatic dysfunction refers to the limited ability of the diaphragm to produce inspiratory pressure. The involved patients include newborns, children performed with cardiopulmonary surgery, mechanical ventilators and children with neuromuscular diseases. Missing a diaphragmatic dysfunction diagnosis is easy due to the lack of specificity in clinical manifestations, resulting in serious consequences in children who are less tolerant to it. Ultrasound, which can observe diaphragmatic movement in two-dimensional and M-mode, has gradually replaced chest X-ray and fluoroscopy in diaphragmatic dysfunction diagnosis. Moreover, diaphragmatic plication is the most used method of surgical treatment, which can improve the affected diaphragm function. The quantitative diaphragm monitoring by ultrasound is of clinical significance for the recovery of critically ill children.
children, critical care medicine, diaphragmatic dysfunction, ultrasound
DD: Diaphragmatic Dysfunction; DP: Diaphragmatic Paralysis; MV: Mechanical Ventilation; NMD: Neuromuscular Diseases; VIDD: Ventilator-Induced Diaphragmatic Dysfunction; Ach: Acetylcholine; NMJ: Neuromuscular Junction; IMS: Intermediate Syndrome; CXR: Chest X-ray; DE: Diaphragmatic Excursion; Tdi: Thickening of the diaphragm; DTF: Diaphragmatic Thickening Fraction
The thoracic diaphragm is a dome-shaped septum, composed of muscle surrounding a central tendon, separating the thoracic and abdominal cavities [1]. In the normal breathing movement, the diaphragmatic dome descends when the diaphragm contracts, which increases the negative pressure in the chest cavity and promotes the expansion of the lung to maintain normal respiratory function.
The capacity of the diaphragm to produce inspiratory pressure is reduced [2] when the diaphragmatic movement of critically ill children decreases or even disappears, which causes diaphragmatic dysfunction (DD) to happen. Diaphragmatic weakness is the partial loss of muscle strength to generate necessary pressure for adequate ventilation, while diaphragmatic paralysis (DP) means the total absence of this capacity [3]. Thus, this paper reviews the application of ultrasound imaging in DD diagnosis to enhance DD awareness.
Aetiology and pathogenesis
Diaphragm innervation is primarily from the phrenic nerves, which arise from the third to the fifth cervical roots and passes on the lateral surface of the pericardium to reach the diaphragm [1]. Each hemidiaphragm is independently innervated from the left and right phrenic nerves (Figure 1). The diaphragm is the major muscle of inspiration, and its action normally accounts for approximately 70% of the inspired tidal volume. The diaphragm moves caudally, and the intrathoracic pressure decreases as it contracts, which leads to a decrease in alveolar pressure. Once atmospheric pressure is lowered inside the alveoli, air enters the alveoli via the airways to maintain gas exchange between the alveolar epithelial cells and the red blood cells in the capillaries [4]. The chest wall tends to collapse as the alveoli expand, and this effect is compensated by an increase in intra-abdominal pressure and contraction of the diaphragm in the lower ribs. Diseases that interfere with diaphragmatic innervation, contractile muscle properties or mechanical coupling to the chest wall can cause DD [3]. Furthermore, the common causes of DD in children mainly include
Figure 1. Anatomy of the phrenic nerve.
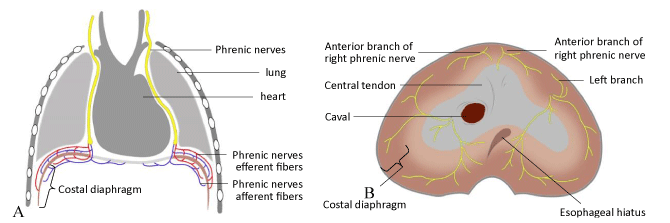
(A): Phrenic nerve and its branches; (B): Branches of phrenic nerve in the abdominal surface of diaphragm.
1. surgery,
2. mechanical ventilation (MV),
3. neuromuscular diseases (NMD),
4. poisoning, drugs and so on.
Thoracic and cardiac surgery is the most common aetiology of phrenic nerve injury in infants with an incidence of approximately 0.3%-12.8% [5-8]. The iatrogenic phrenic nerve injury is usually manifested as unilateral DP. Moreover, phrenic nerve injury can be induced by crushing, amputation, extension, or thermal nerve damage which includes hypothermia caused by contact with cold saline or cauterisation during surgery [9]. Dissection to separate the lungs from the heart increases the risk of phrenic nerve injury due to the adhesion of the lungs to the pericardium, and patients who have a history of prior thoracic surgery are more likely to develop DP [10]. The Fontan procedure, arterial switch operation and Blalock–Taussig shunt surgery are the most frequent cardiac operation types resulting in phrenic nerve injury in children with an incidence of 16.6% [10] (Table 1).
Table 1. Aetiology of DD.
Phrenic nerve injury in neonates is an uncommon but serious condition [11] that is usually associated with shoulder dystocia and breech delivery in the perinatal period. Transvaginal delivery leading to traction and excessive stretching of the third to fifth cervical nerve root usually leads to unilateral disruption or phrenic nerve laceration [11]. The caesarean section is considered a protective factor and reduces the possibility of birth trauma. Furthermore, plexus palsy of the neonates can occur at the same time as phrenic nerve injury. However, whether the prognosis of phrenic nerve injury is related to the severity of plexus palsy is still unknown [12].
Other rare causes of phrenic nerve injury include direct phrenic nerve compression with a deeply positioned tube for pleural drainage [13] and indirect injury to the phrenic nerves from continued use of diathermy or direct injury from a suprahepatic caval clamp during liver mobilisation [14]. The factors that may interfere with phrenic innervation also include central lung cancer or mediastinal tumour (can directly invade the phrenic nerves) and cervical spondylosis (which may compress the root of the phrenic nerves). Moreover, vasculitis and pneumonia can lead to inflammation of the phrenic nerves, affecting their function [15].
Assisted MV, such as pressure-support ventilation, is widely used in critically ill patients to unload the respiratory muscles while avoiding muscle atrophy. In such modes, a variable amount of work is generated by the patients’ inspiratory muscles while the remainder is provided by the ventilator [16]. The contractile force reduction of the diaphragm along with the atrophy of the diaphragm muscle fibres due to controlled MV, known as ventilator-induced diaphragmatic dysfunction (VIDD), is one of the major contributors to weaning difficulties and even increased mortality [17]. Prolonged MV significantly reduces active and passive diaphragmatic fibrinogen production by reducing myofibril levels, resulting in reduced diaphragmatic contractility [18]. Meanwhile, muscle fibre atrophy was induced by decreased protein synthesis and increased proteolysis caused by ubiquitin proteasomes, caspases and calpains [19].
Another cause of DD is NMD, especially diffuse muscle disease or motor neuron disease, which usually affects bilateral hemidiaphragm. The most common types of NMD in children include spinal muscular atrophy, Duchenne muscular dystrophy, myotonic dystrophy and so on [20]. Moreover, almost all NMD results in a progressive decline in lung compliance, impaired lung function, decreased vital capacity and maximum inspiratory pressure.
Due to long-term inhibition of cholinesterase activity, a large amount of acetylcholine accumulated in the synaptic gap continues to act on the N2 receptors on the post-synaptic membrane, leading to neuromuscular junction transmission disorder and skeletal muscle paralysis, which is called intermediate syndrome (IMS) [21]. Furthermore, IMS usually occurs 1–4 days after organophosphorus pesticide poisoning. Respiratory muscle palsy is the main IMS risk with varying degrees of dyspnoea, increased respiratory rate, progressive hypoxia leading to consciousness disturbance, coma and even mortality [22].
Skeletal muscle relaxants selectively act on N2 receptors on the post-synaptic membrane, blocking the transmission of nerve impulses to skeletal muscles and leading to muscle relaxation. Furthermore, muscular relaxants commonly used in children mainly include succinylcholine and tubocurarine [23]. All muscle relaxants cause respiratory depression in varying degrees. Its irrational use may cause DD and respiratory depression, resulting in serious consequences. Therefore, children treated with muscle relaxants must be closely observed after the operation. Only when the ventilation capacity, various protective reflexes and muscle tension return to normal can they be extubated back to the ward.
Asthma, chronic obstructive pulmonary disease and other diseases with pulmonary hyperaeration can also worsen the function of the diaphragm because the diaphragm does not have an optimal length for its normal functioning [4].
DD leads to abnormal pulmonary ventilation function, which is mainly manifested as dyspnoea in children. The main mechanisms include impaired diaphragmatic function, compensatory contraction of intercostal and contralateral diaphragm leading to respiratory muscle fatigue and paradoxical respiration, atelectasis or alveolar ventilate-perfusion mismatch, which may cause hypoxemia and increases susceptibility to respiratory infections [24]. Severe cases can quickly lead to respiratory failure. When sedatives, muscle relaxants and MV are not properly combined, respiratory failure can be further aggravated, especially in critically ill children. Lung ultrasound is useful for diagnosing respiratory failure and dynamic monitoring of the recovery of the lung for paediatric patients [25].
Imaging method
Chest X-ray (CXR) is a traditional imaging method that allows the physician to see the shape, and elevation of the diaphragm. Unilateral DD can be identified with a marked elevation of the affected hemidiaphragm on a CXR (Figure 2) [26]. The CXR showed an elevation for both hemidiaphragms with basal areas of dystelectasis when bilateral hemidiaphragms are involved [27]. The DD diagnosis may be ruled out if no diaphragmatic elevation on either side exists. However, the CXR may not be a helpful tool to identify DD early in newborns and infants [28]. The CXR sign is not characteristic and is easily missed when bilateral hemidiaphragms are simultaneously elevated. Thus, CXR has some limitations for differential DD diagnosis [29].
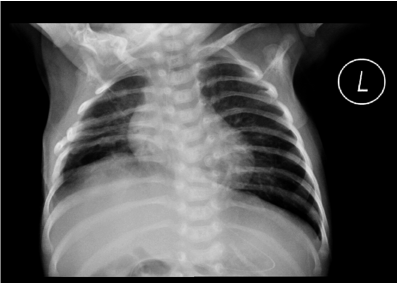
Figure 2. CXR findings of unilateral DP. CXR shows elevation of the right diaphragm.
CXR has favourable interobserver reliability [30], but there are still many limitations, such as radiation exposure, children with poor compliance cannot cooperate with inspiration and expiration [28]. It is not suitable for the evaluation of diaphragmatic movement in critically ill children.
Diaphragmatic ultrasound
Cohen et al. [31] first described the diaphragmatic ultrasound in 1969, and reports exist on the application of M- and classical B-mode ultrasound to quantitatively evaluate the direction and excursion of diaphragmatic movement and compare the hemidiaphragm on both sides [32-34]. Moreover, the diaphragmatic movement can even be indirectly evaluated by breathing movements of the liver, spleen, and kidneys [35].
Ultrasonic method
The diaphragmatic movement can be evaluated by the ultrasonic method in two positions: the zone of apposition and the diaphragmatic dome [36]. The zone of apposition is a three-layer structure near the chest wall that can be identified when placing a linear probe on the eighth and ninth intercostal spaces between the anterior axillary and axillary mid-lines, which consists of a hypoechoic muscle layer (diaphragm) surrounded by echogenic membranes of the peritoneum and pleura [37]. The diaphragmatic dome is when the US beam can perpendicularly reach the diaphragmatic dome when placing a convex or sector probe on the midclavicular line in the subcostal area. The liver or spleen can be seen as a window for each hemidiaphragm. The diaphragmatic movement curve can be observed, and the excursion of the diaphragm can be measured in the M-mode line [38] (Figure 3).
Figure 3. Normal diaphragmatic sonography.
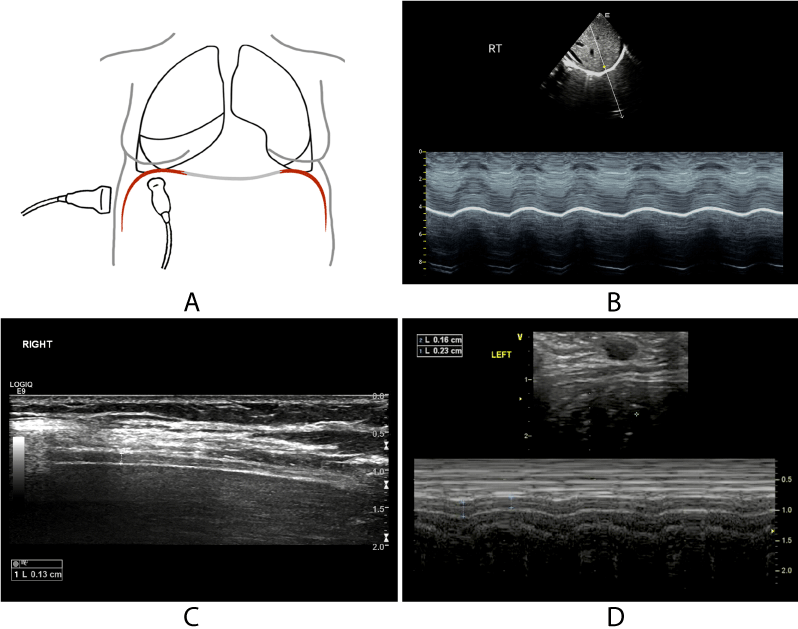
(A): Schematic diagram of operation. (B):M-mode curve of normal diaphragmatic movement. (C): 2D image of the zone of apposition. (D):M-mode curve shows that the thickness of the diaphragm varies with respiration.
Ultrasonic diagnosis
The diaphragmatic excursion (DE) is relatively simple to obtain and can be completed at the bedside for critically ill children [39]. Moreover, diaphragmatic movement can be divided into four categories: normal, decreased, absent or paradoxical. A normal condition is considered when the diaphragm moves toward the transducer in the inspiratory phase, the DE is >4 mm and the excursion difference between each hemidiaphragm is <50%. A reduction in movement should be considered if the DE is >4 mm and the difference between the DE between both hemidiaphragms is >50%. In addition, absent movement is considered if the track is a flat line. A paradoxical movement should be considered when the diaphragm moves away from the transducer in the inspiratory phase [40].
The zone of apposition will be thickened and shortened in a state of quiet inspiration. The thickening of the diaphragm (Tdi) is measured in the zone of apposition at the end of expiration and the end of inspiration through M-mode, respectively. Consequently, the diaphragmatic thickening fraction (DTF) is calculated as the change of diaphragmatic thickness during expiration and inspiration. The calculation formula is (Tdiinsp − Tdiexp) /Tdiexp. The lower limit of normal Tdi at rest in adults is 1.5 mm [38]. Moreover, DP is present on the hemidiaphragm when the Tdi and DTF are <2.0 mm and <20% at the end of expiratory, respectively [41]. This approach could practice without having to disconnect the mechanical ventilator. Consequently, it is less stressful for these critically ill patients and it will not be affected by bowel gases [39]. However, a lack of normal reference values for Tdi and DTF in children still exists.
Diaphragmatic ultrasound can detect DD by evaluating diaphragm movement in critically ill patients. DD diagnosed with ultrasound was found in 29% of mechanically ventilated patients without a history of diaphragmatic or neuromuscular diseases [42]. This finding indicates that DD is probably underestimated in intensive care unit patients. Furthermore, diaphragmatic ultrasound can predict weaning success/failure from MV without interruption, which is conducive to timely extubation and prevent VIDD occurrence [43]. Tdi measurement has high sensitivity and specificity to predict weaning success/failure [44]. Moreover, the DTF value to predict extubating success or failure ranges between 30% and 36 % during spontaneous breathing trials [44,45].
Diaphragmatic ultrasound can also be used to assess the contractility of the diaphragm [16,46]. In contrast to invasive methods such as measuring transdiaphragmatic pressure, ultrasound is non-invasive and can be performed at the bedside. The DTF has shown a significant correlation with respiratory effort in patients on MV [16]. Therefore, ultrasound emerged as a new non-invasive tool to monitor respiratory workload during assisted MV (Figure 4).
Figure 4. Diaphragmatic sonography under different situations.
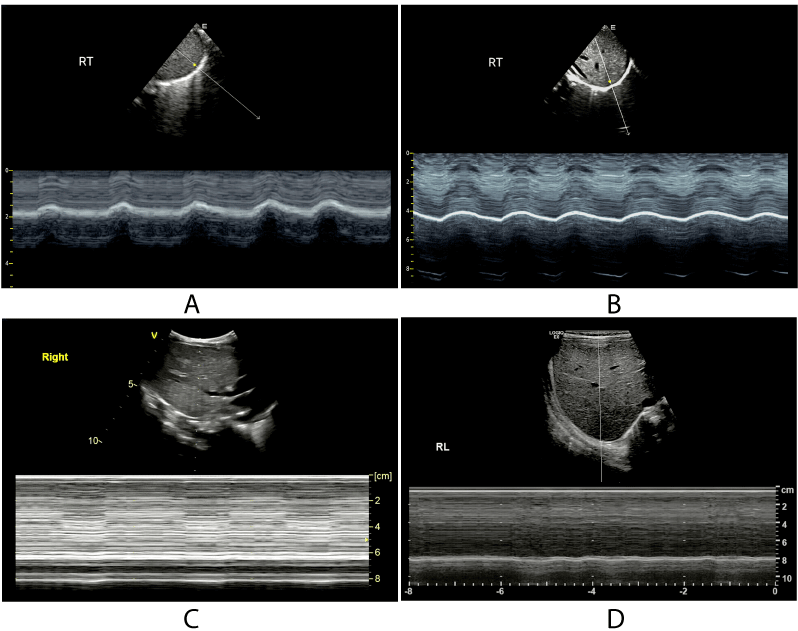
(A):M-mode curve of normal diaphragmatic movement. (B): M-mode curve of diaphragmatic thickness of a patient on MV. (C): M-mode curve of the diaphragm of a patient with DP. (D):M-mode curve of the diaphragm of a patient after diaphragmatic plication.
Diaphragmatic ultrasound in treatment
Common treatment methods in critically ill children include conservative treatment, diaphragmatic pacing and diaphragmatic plication. The choice of treatment depends on the aetiology of the child and the severity of the symptoms. The function of diaphragm may spontaneously recover after DD in some children conservative treatment may be a reasonable approach for non-critical children to avoid complications resulting from surgery. ventilation a preferred therapy to maintain respiratory function and allow the diaphragmatic function to recover 14. Other conservative management mainly includes spirometry, chest physiotherapy, daily walking, taking deep breaths and keeping a semi-sitting position [47]. One-year inspiratory muscle training after cardiac surgery can improve diaphragmatic mobility and the inspiratory muscle strength of children with DD [48]. Moreover, using tracheostomy and invasive ventilation is recommended if no recovery after 3 months of conservative treatment or a recurrent lung infection is noted in patients with bilateral DD after cardiac surgery [49]. Thus, ultrasound examination of diaphragmatic movement is helpful to quantitatively and dynamically evaluate the response to treatment.
Diaphragmatic pacing works by stimulating the diaphragm with electrical signals to restore normal breathing mechanisms and reduce MV dependence in patients with DD [50]. A diaphragmatic pacer can increase tidal volume as much as possible by changing different characteristics of the breath cycle in children with DD [51]. Only when the patient has an intact phrenic nerve can diaphragmatic pacing be processed [15]. Moreover, diaphragmatic pacing is usually not an option in bilateral paralysis. Diaphragm pacing can be used as a transitional means before extubating for DD children undergoing MV. Gradually increasing the duration of diaphragmatic pacing and shortening the duration of MV facilitates MV weaning and improves the quality of life in paediatric patients [52].
Diaphragmatic plication is the most used surgical treatment method. Children with DD who require longer ventilatory support after heart surgery should be operated on early, especially in children <1 year old [53]. Diaphragmatic plication avoids the risk of long-term ventilation and the possibility of long-term stays in the intensive care unit so that it reduces VIDD incidence and pulmonary infection. Moreover, it has a low medium-term risk of restoring phrenic nerve function, lung function and motor capacity [7]. The operation aims to fix the diaphragm in a relatively flat and low position to improve ipsilateral lung expansion [54]. Diaphragmatic plication performed via a thoracotomy may need to make an incision in the inferior intercostal muscle, which is involved in breathing movements [55]. This situation may make respiration worse by causing decreased ventilatory function and reduced ventilation efficiency [24]. The acoustic window is complete after the operation, and the quantitative evaluation of the diaphragmatic movement can be carried out with ultrasound as soon as possible. Furthermore, diaphragmatic ultrasound can be used to evaluate the recovery of diaphragmatic function after diaphragmatic plication.
Case 1 was male (3 h old) and admitted due to dyspnoea for 3 h. The imaging examination suggested that the child had aesophageal atresia and ventricular septal defect. He underwent aesophageal reconstruction with ligation of tracheoesophageal fistula and ventricular septal defect repair 2 and 19 days after birth, respectively. The patient was extubated 3 days after cardiac surgery. However, carbon dioxide retention occurred, and auscultation suggested a low respiratory sound in the right lung. CXR showed a disappearance of the right costophrenic angle and consolidation of the right lung. Diaphragmatic ultrasound indicated that the amplitude of excursion of the diaphragm was 7.0 on the left side and there was a flat line on the right side. Thus, a DP diagnosis was considered. The right diaphragmatic plication was performed on the 7th day after a heart operation. Post-operative CXR revealed right diaphragmatic elevation and sharp costophrenic angle. Diaphragmatic ultrasound indicated that the amplitude of excursion of the diaphragm was 1.0 mm on the right side, which was increased compared with pre-operative (Figure 5). The child was successfully weaned from MV 2 days after diaphragmatic plication and was discharged 2 weeks later.
Figure 5. Examination image and schematic diagram of the patient.
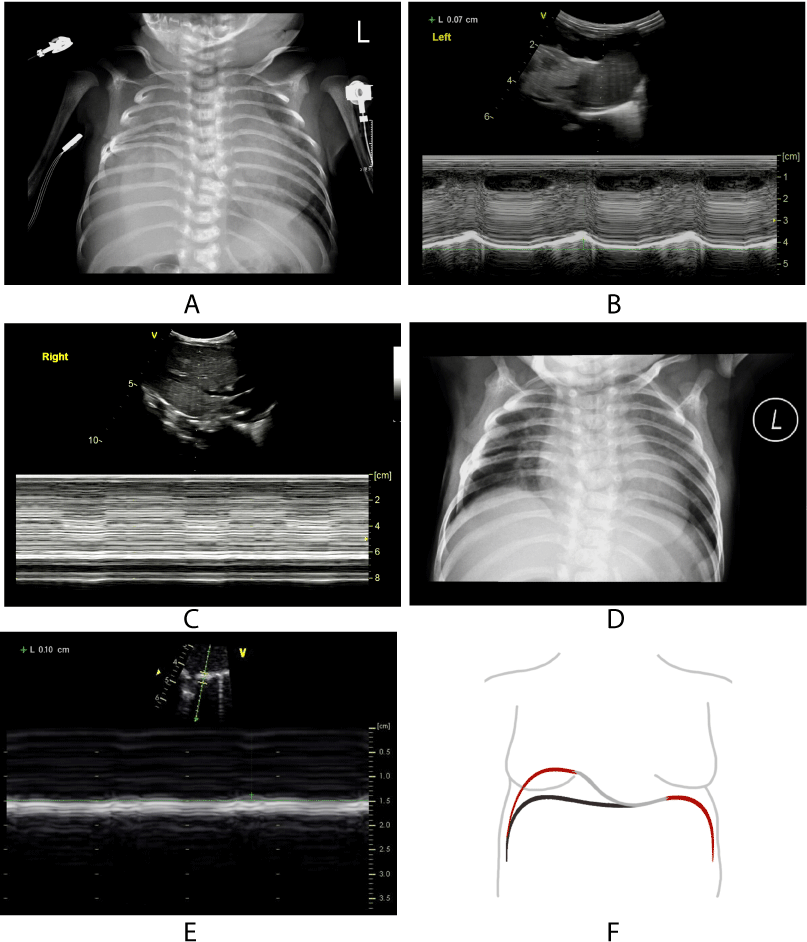
(A): CXR after heart surgery showed disappearance of right costophrenic angle and consolidation of right lung. (B): M-mode curve of left hemidiaphragm after heart surgery shows normal displacement (7.0mm). (C): M-mode curve of right hemidiaphragm after heart surgery shows decreased displacement (0mm). (D): CXR after diaphragmatic plication revealed right diaphragmatic elevation and sharp costophrenic angle. (E): M-mode curve of right hemidiaphragm after diaphragmatic plication shows amplitude of excursion of the diaphragm was increased compared with preoperative (1.0mm). (F): Schematic diagram of DP.
Case 2 was female (15 months old) and was delivered vaginally with forceps at the gestational age of 39 + 6 weeks and presented with clinical manifestations (e.g., cyanosis, weakened breathing and low muscle tension). After mild hypothermia therapy, invasive MV and anti-infection treatment, the patient's condition improved. However, many attempts to change to non-invasive ventilation failed. Ultrasound examination suggested that the left diaphragm movement was weakened, and the left DP diagnosis was considered. The clinical DP diagnosis is caused by birth trauma. The left diaphragmatic plication was performed 55 days after birth, and the left diaphragmatic dome was significantly raised, and the diaphragm was thin during opening thoracic exploration. The ventilator parameters were gradually reduced after the operation. However, the oxygen saturation of the child decreased after the disconnection of the ventilator, and the child could only be treated with ventilator-assisted ventilation again. The child was changed to non-invasive-assisted ventilation on day 9 after diaphragm operation and was discharged 2 months after the operation. Diaphragmatic ultrasound was re-examined >1 year after the operation, indicating that the excursion amplitude of the diaphragm was 6.7 and 5.2 mm on the right and left sides, respectively. Moreover, the left diaphragm movement was weaker compared with that of the opposite side (Figure 6).
Figure 6. Postoperative diaphragmatic plication.
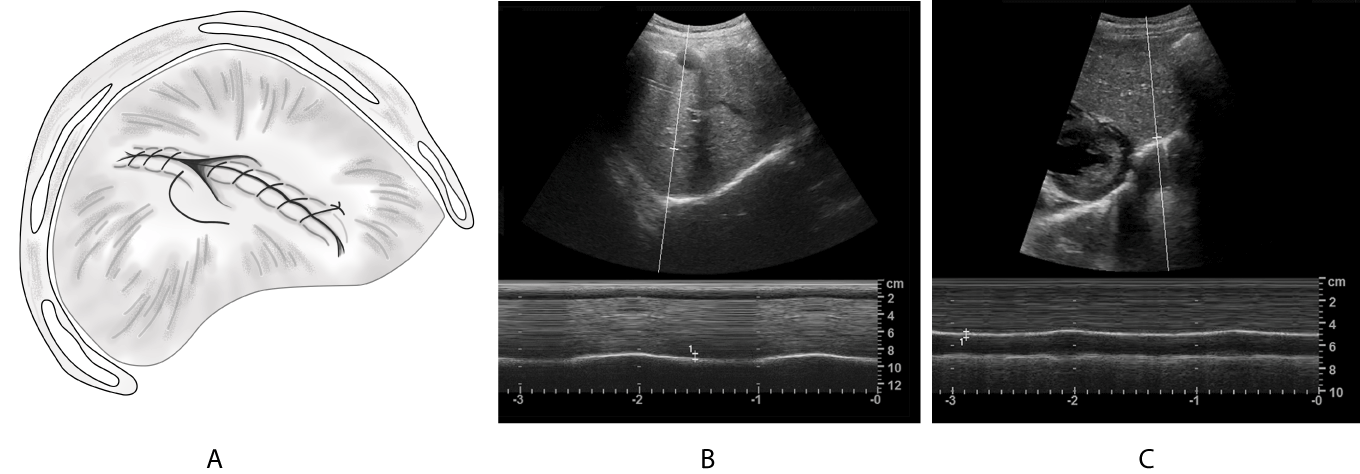
(A): Schematic diagram of the diaphragmatic plication; (B): M-mode curve of right diaphragm shows normal displacement(6.7mm); (C): The left image postoperatively shows that movement displacement of left diaphragm decreased(5.2mm).
For those critically ill children at the bedside or in the perioperative period, ultrasound is useful to dynamically evaluate the variety of diaphragm movement; quantitatively display the excursion, direction of movement and thickening fraction of the diaphragm by transabdominal and transthoracic approaches and display early cardiac surgery complications. It can also predict weaning success/failure from MV and assess diaphragmatic contractility [44,56]. For patients in the paediatric intensive care unit, ultrasound can be performed at the bedside, at any age, and is a very important examination technique [34,37]. No normal reference value of Tdi and DTF in children currently exists. Thus, the clinical application of diaphragmatic ultrasound is still limited. The correlations between sonographic measurements and anthropometric data such as age, weight and head circumference and the correlation between the diaphragmatic mass and body weight need further study [57,58].
We would like to acknowledge and thank the members of the department of ultrasound for their assistance.
- Downey R (2011) Anatomy of the normal diaphragm. Thor Surg Clin 21: 273-279. [Crossref]
- Demoule A, Jung B, Prodanovic H, Molinari N, Chanques G, et al. (2013) Diaphragm dysfunction on admission to the intensive care unit. Prevalence, risk factors, and prognostic impact-a prospective study. Am J Respir Crit Care Med 188: 213-219. [Crossref]
- Ricoy J, Rodríguez-Núñez N, Álvarez-Dobaño JM, Toubes ME, Riveiro V, et al. (2019) Diaphragmatic dysfunction. Pulmonol 25: 223-235. [Crossref]
- McCool FD, Manzoor K, Minami T (2018) Disorders of the Diaphragm. Clin Chest Med 39: 345-360. [Crossref]
- Sanchez de TJ, Munoz R, Landsittel D, Shiderly D, Yoshida M, et al. (2010) Diagnosis of abnormal diaphragm motion after cardiothoracic surgery: ultrasound performed by a cardiac intensivist vs. fluoroscopy. Congenit Heart Dis 56: 565-572. [Crossref]
- Joho-Arreola AL, Bauersfeld U, Stauffer UG, Baenziger O, Bernet V (2005) Incidence and treatment of diaphragmatic paralysis after cardiac surgery in children. Eur J Cardiothorac Surg 27: 53-57. [Crossref]
- Lemmer J, Stiller B, Heise G, Hübler M, Alexi-Meskishvili V, et al. (2006) Postoperative phrenic nerve palsy: early clinical implications and management. Intensive Care Med 32: 1227-1233. [Crossref]
- Akay TH, Ozkan S, Gultekin B, Uguz E, Varan B, et al. (2006) Diaphragmatic paralysis after cardiac surgery in children: incidence, prognosis and surgical management. Pediatr Surg Int 22: 341-346. [Crossref]
- Baker CJ, Boulom V, Reemtsen BL, Rollins RC, Starnes VA, et al. (2008) Hemidiaphragm plication after repair of congenital heart defects in children: quantitative return of diaphragm function over time. J Thorac Cardiovasc Surg 135: 56-61. [Crossref]
- Akbariasbagh P, Mirzaghayan MR, Akbariasbagh N, Shariat M, Ebrahim B (2015) Risk Factors for post-Cardiac Surgery Diaphragmatic Paralysis in Children with Congenital Heart Disease. J Tehran Heart Cent 10: 134-139. [Crossref]
- Stramrood CA, Blok CA, van der Zee DC, Gerards LJ (2009) Neonatal phrenic nerve injury due to traumatic delivery. J Perinat Med 37: 293-296. [Crossref]
- Bowerson M, Nelson VS, Yang LJ (2010) Diaphragmatic paralysis associated with neonatal brachial plexus palsy. Pediatr Neurol 42: 234-236. [Crossref]
- Nakagama Y, Kaneko Y, Ono H (2015) Reversible diaphragmatic paralysis caused by a malpositioned chest tube. Cardiol Young 25: 1382-1384. [Crossref]
- Olmscheid J, Molero H, Gershan W, Demirel N (2017) Bilateral diaphragmatic paresis following pediatric liver transplantation. SAGE Open Med Case Rep 5: 2050313X17719214. [Crossref]
- Qureshi A (2009) Diaphragm paralysis. Semin Respir Crit Care Med 30: 315-320. [Crossref]
- Umbrello M, Formenti P, Longhi D, Galimberti A, Piva I, et al. (2015) Diaphragm ultrasound as indicator of respiratory effort in critically ill patients undergoing assisted mechanical ventilation: a pilot clinical study. Crit Care 19: 161. [Crossref]
- Kim WY, Lim CM (2017) Ventilator-Induced Diaphragmatic Dysfunction: Diagnosis and Role of Pharmacological Agents. Respir Care 62: 1485-1491. [Crossref]
- Hussain SN, Cornachione AS, Guichon C, Al Khunaizi A, Leite Fde S, et al. (2016) Prolonged controlled mechanical ventilation in humans triggers myofibrillar contractile dysfunction and myofilament protein loss in the diaphragm. Thorax 71: 436-445. [Crossref]
- McClung JM, Kavazis AN, DeRuisseau KC, Falk DJ, Deering MA, et al. (2007) Caspase-3 regulation of diaphragm myonuclear domain during mechanical ventilation-induced atrophy. Am J Respir Crit Care Med 175: 150-159. [Crossref]
- Benditt JO (2018) Pathophysiology of Neuromuscular Respiratory Diseases. Clin Chest Med 39: 297-308. [Crossref]
- Bird SB, Krajacic P, Sawamoto K, Bunya N, Loro E, et al. (2016) Pharmacotherapy to protect the neuromuscular junction after acute organophosphorus pesticide poisoning. Ann N Y Acad Sci 1374: 86-93. [Crossref]
- Hulse EJ, Davies JO, Simpson AJ, Sciuto AM, Eddleston M (2014) Respiratory complications of organophosphorus nerve agent and insecticide poisoning. Implications for respiratory and critical care. Am J Respir Crit Care Med 190: 1342-1354. [Crossref]
- Nugent SK, Laravuso R, Rogers MC (1979) Pharmacology and use of muscle relaxants in infants and children. J Pediatr 94: 481-487. [Crossref]
- Shiohama T, Fujii K, Hayashi M, Hishiki T, Suyama M, et al. (2013) Phrenic nerve palsy associated with birth trauma--case reports and a literature review. Brain Dev 35: 363-366. [Crossref]
- Weiling Chen, Bei Xia, Lan Wang, Lixue Yin, et al. (2021) Recommendations for Operation, Measurement, Reporting and Application of Pediatric Lung Ultrasound: Chinese Experts Consensus. AUDT 5: 1-11.
- Bhaskar P, Lone RA, Sallehuddin A, John J, Bhat AN, et al. (2016) Bilateral diaphragmatic palsy after congenital heart surgery: management options. Cardiol Young 26: 927-930. [Crossref]
- Sozzo S, Carratù P, Damiani MF, Falcone VA, Palumbo A, et al. (2012) Bilateral diaphragmatic paralysis after kidney surgery. Monaldi Arch Chest Dis 77: 102-104. [Crossref]
- Epelman M, Navarro OM, Daneman A, Miller SF (2005) M-mode sonography of diaphragmatic motion: description of technique and experience in 278 pediatric patients. Pediatr Radiol 35: 661-667. [Crossref]
- Verhey PT, Gosselin MV, Primack SL, Kraemer AC (2007) Differentiating diaphragmatic paralysis and eventration. Acad Radiol 14: 420-425. [Crossref]
- Yi LC, Nascimento OA, Jardim JR (2011) Reliability of an analysis method for measuring diaphragm excursion by means of direct visualization with videofluoroscopy. Arch Bronconeumol 47: 310-314. [Crossref]
- Cohen W (1969) Evaluation of the diaphragm by a subcostal B-scan technique. Proceeding of the First World Congress on Ultrasound Diagnostics in Medicine and SIDUO III. Vienna.
- Lee EP, Hsia SH, Hsiao HF, Chen MC, Lin JJ, et al. (2017) Evaluation of diaphragmatic function in mechanically ventilated children: An ultrasound study. PLoS One 12: e0183560. [Crossref]
- Hamadah HK, Kabbani MS, Elbarbary M, Hijazi O, Shaath G, et al. (2017) Ultrasound for diaphragmatic dysfunction in postoperative cardiac children. Cardiol Young 27: 452-458. [Crossref]
- Gil-Juanmiquel L, Gratacós M, Castilla-Fernández Y, Piqueras J, Baust T, et al. (2017) Bedside Ultrasound for the Diagnosis of Abnormal Diaphragmatic Motion in Children After Heart Surgery. Pediatr Crit Care Med 18: 159-164. [Crossref]
- Toledo NS, Kodaira SK, Massarollo PC, Pereira OI, Dalmas JC, et al. (2006) Left hemidiaphragmatic mobility: assessment with ultrasonographic measurement of the craniocaudal displacement of the splenic hilum and the inferior pole of the spleen. J Ultrasound Med 25:41-49. [Crossref]
- Sferrazza Papa GF, Pellegrino GM, Di Marco F, Imeri G, Brochard L, et al. (2016) A Review of the Ultrasound Assessment of Diaphragmatic Function in Clinical Practice. Respiration 91: 403-411. [Crossref]
- Boon AJ, Harper CJ, Ghahfarokhi LS, Strommen JA, Watson JC, et al. (2013) Two-dimensional ultrasound imaging of the diaphragm: quantitative values in normal subjects. Muscle Nerve 47: 884-889. [Crossref]
- Vivier E, Mekontso Dessap A, Dimassi S, Vargas F, Lyazidi A, et al. (2012) Diaphragm ultrasonography to estimate the work of breathing during non-invasive ventilation. Intensive Care Med 38:796-803. [Crossref]
- Nozaki Y, Lin LS, Kato Y (2018) Ultrasonographic diagnosis of diaphragm paralysis in a neonate during mechanical ventilation after cardiac surgery. Cardiol Young 28: 776-778. [Crossref]
- Urvoas E, Pariente D, Fausser C, Lipsich J, Taleb R et al. (1994) Diaphragmatic paralysis in children: diagnosis by TM-mode ultrasound. Pediatr Radiol 24: 564-568. [Crossref]
- Gottesman E, McCool FD (1997) Ultrasound evaluation of the paralyzed diaphragm. Am J Respir Crit Care Med 155: 1570-1574. [Crossref]
- Kim WY, Suh HJ, Hong SB, Koh Y, Lim CM (2011) Diaphragm dysfunction assessed by ultrasonography: influence on weaning from mechanical ventilation. Crit Care Med 39: 2627-2630. [Crossref]
- Zambon M, Greco M, Bocchino S, Cabrini L, Beccaria PF et al. (2017) Assessment of diaphragmatic dysfunction in the critically ill patient with ultrasound: a systematic review. Intensive Care Med 43: 29-38. [Crossref]
- DiNino E, Gartman EJ, Sethi JM, McCool FD (2014) Diaphragm ultrasound as a predictor of successful extubation from mechanical ventilation. Thorax 69: 423-427. [Crossref]
- Ferrari G, De Filippi G, Elia F, Panero F, Volpicelli G, et al. (2014) Diaphragm ultrasound as a new index of discontinuation from mechanical ventilation. Crit Ultrasound J 6: 8. [Crossref]
- Goligher EC, Laghi F, Detsky ME, Farias P, Murray A, et al. (2015) Measuring diaphragm thickness with ultrasound in mechanically ventilated patients: feasibility, reproducibility and validity. Intensive Care Med 41: 642-649. [Crossref]
- Al-Ebrahim KE, Elassal AA, Eldib OS, Abdalla AHA, Allam ARA, et al. (2019) Diaphragmatic palsy after cardiac surgery in adult and pediatric patients. Asian Cardiovasc Thorac Ann 27: 481-485. [Crossref]
- Kodric M, Trevisan R, Torregiani C, Cifaldi R, Longo C, et al. (2013) Inspiratory muscle training for diaphragm dysfunction after cardiac surgery. J Thorac Cardiovasc Surg 145: 819-823. [Crossref]
- Bhaskar P, Lone RA, Sallehuddin A, John J, Bhat AN, et al. (2016) Bilateral diaphragmatic palsy after congenital heart surgery: management options. Cardiol Young 26: 927-930. [Crossref]
- Filho Pinto DR, Tedde ML, Avino AJ, Brandão SL, Zanatta I, et al. (2015) Video-assisted thoracoscopic implantation of a diaphragmatic pacemaker in a child with tetraplegia: indications, technique, and results. J Bras Pneumol 41: 90-94. [Crossref]
- Onders RP, Ponsky TA, Elmo M, Lidsky K, Barksdale E (2011) First reported experience with intramuscular diaphragm pacing in replacing positive pressure mechanical ventilators in children. J Pediatr Surg 46: 72-76. [Crossref]
- Hazwani TR, Alotaibi B, Alqahtani W, Awadalla A, Shehri AA (2019) Pediatric diaphragmatic pacing. Pediatr Rep 11: 7973. [Crossref]
- Floh AA, Zafurallah I, MacDonald C, Honjo O, Fan CS, et al. (2017) The advantage of early plication in children diagnosed with diaphragm paresis. J Thorac Cardiovasc Surg 154: 1715-1721. [Crossref]
- Taberham RJ, Raza A, Alzetani A, Woo EB, Chamberlain MH, et al. (2017) VATS Plication of the Diaphragm: A Descriptive Observational 10-Year Southampton Experience. Innovations (Phila) 12: 398-405. [Crossref]
- Shimizu M (2003) Bilateral phrenic-nerve paralysis treated by thoracoscopic diaphragmatic plication in a neonate. Pediatr Surg Int 19: 79-81. [Crossref]
- Xue Y, Zhang Z, Sheng CQ, Li YM, Jia FY (2019) The predictive value of diaphragm ultrasound for weaning outcomes in critically ill children. BMC Pulm Med 19: 270. [Crossref]
- El-Halaby H, Abdel-Hady H, Alsawah G, Abdelrahman A, El-Tahan H (2016) Sonographic Evaluation of Diaphragmatic Excursion and Thickness in Healthy Infants and Children. J Ultrasound Med 35: 167-175. [Crossref]
- Rehan VK, McCool FD (2003) Diaphragm dimensions of the healthy term infant. Acta Paediatr 92: 1062-1067. [Crossref]






