Abstract
Collagen is a key building block protein for bones, skin, and hair. Collagen is naturally produced by the body; however, its production is known to decrease with the onset of intrinsic or extrinsic ageing. Past studies have shown that oral ingestion of collagen hydrolysates promote the growth of fibroblasts and stimulates the production of new collagen type I in the dermis. This study intends to determine the clinical tolerance and efficacy of Nuceutical Skinergy Collagen Shot®, a combination of marine collagen peptides with Polypodium leucotomos and antioxidants, in improving crow’s feet wrinkles and skin hydration on 29 healthy Asian females after 28/37 days of once daily consumption. The results show that Nuceutical Skinergy Collagen Shot ® is effective in improving crow’s feet wrinkles and facial dermal surface hydration. This decrease in the wrinkle volume was confirmed by a decreased clinical visibility of the wrinkle, indicative of the product’s anti-aging efficacy. Self-evaluation of the subjects also revealed that the skin was less dry, softer, more supple, more hydrated. No indication of serious adverse effects following oral consumption was reported.
Keywords
Collagen, wrinkles, collagen supplements, Nuceutical Skinergy Collagen Shot, anti-ageing, skin hydration, clinical efficacy, tolerance.
Introduction
Collagen is the most abundant protein in the human body and is the main structural component of the dermis. It is primarily in charge of providing strength and support to the human skin. Changes in the collagen plays an integral role in the process of ageing [1].
Inherently, the skin is equipped with numerous mechanisms that offers protection against the detrimental effects of ageing, however the effect of these protective systems dwindles substantially over the passage of time. Under genetic and hormonal influences, the skin is subjected to chronological intrinsic ageing as well as extrinsic ageing which is caused by environmental factors [2] leading to a loss of elasticity, increased fragility, and a drop in tensile strength. Ageing has also led to the formation of wrinkles and slowed wound healing [2]. In young skin, collagen I appears as bundles, arranged in a manner that promotes their extension and relaxation. This well-organized structure is facilitated by their interwoven elastic fibers [3]. However, under the process of extrinsic aging, the density of the collagen I bundles increases leading to a loss in their ability to extend and to recover their resting state. Indeed, reactive oxygen species (ROS) primarily arising from the oxidative metabolism of cells, heavily mediates the onset of collagen degradation. Environmental ROS (resulting from UV, oxidative stress, etc.) will lead to photoaged skin with deeper wrinkles, a more significant loss of elasticity compared with intrinsic aging as well as a rough texture [4]. The expression of fibroblast elastase and other matrix-metalloproteases (MMP) is stimulated by UV-B-induced cytokine secretion by keratinocytes. The up-regulated activity of fibroblast elastase damages elastic fibers which makes collagen fragmented, disorganized as well as adopting a less soluble configuration leading to lower biomechanical abilities [5]. This promotes the formation of wrinkles.
Previous investigations have revealed that oral administration of collagen hydrolysates on subjects with dry and rough skin showed that the moisture content, elasticity, and smoothness of the skin was greatly improved while the appearance of wrinkles and skin roughness was decreased [6]. Asserin et al. explored the effects of a daily oral supplement containing collagen peptides on skin hydration and the density of collagen. Four weeks of daily usage revealed that the dermal deposition was significantly increased in test volunteers while eight weeks of usage showed a consequent improvement in skin hydration. These improved skin parameters were proven to persist up to twelve weeks and further ex vivo data was reported to show an induction in the collagen synthesis [7].
A cumulative exposure to different external factors is known to promote photoaging due to increased oxidative stress leading to extrinsic aging. Since many stressors lead to heightened oxidative damage leading to skin aging, products that offer both an antioxidant effect and anti-aging protection are greatly desired.
Nuceutical Skinergy Collagen Shot® is an activated fish collagen peptide supplement enriched with Polypodium leucotomos, vitamin C, astaxanthin and rosemary extract. While traditional sources of collagen for cosmetics are from the hides of pigs and cows, collagen of marine origins are preferred as they are not a cause for lifestyle or religious concerns nor are they associated with potential transmission of pathogens such as prions [8-10]. Astaxanthin (ASX) is known for its powerful antioxidant activity implicated in treating and preventing skin disease [11]. ASX has been shown to inhibit the formation of ROS and affect the expression of enzymes such as heme oxygenase-1 (HO-1) responsible for modulating the oxidative stress [12]. Rosemarinic acid present in rosemary extracts is also known for its beneficial anti-inflammatory and antioxidant properties [13].
Polypodium leucotomos (PL) extracts from a fern of the Polypodiaceae family has traditionally been used for treating skin diseases such as psoriasis and atopic dermatitis. A renewed interest in its overall properties has spiked recently owing to elevated content of phenolic compounds [14]. Its potent antioxidant properties and ability to counteract various effects of oxidative stress such as DNA damage whereby PL can significantly inhibit the accumulation of cyclobutane pyrimidine dimers (CPD) [15]. Furthermore, PL has been demonstrated to suppress damages to the ECM by hindering the production of MMP-1 [16]. Its potential to counter immunosuppression and inflammation by inhibiting cell depletion and impede the release of TNF-α, NF-kB and reduce macrophage, neutrophils, and mast cells infiltration has been published [15,17]. In addition to its antioxidant and anti-inflammatory activities, PL demonstrated the prevention of photoaging, the remodeling of the extracellular matrix, the inhibition of matrix metalloproteases (MMPs) and promoted the regeneration of different collagens [18].
In the study reported here, the clinical efficacy and tolerance of the collagen supplement, Nuceutical Skinergy Collagen Shot ® was assessed in improving the appearance of crow’s feet wrinkles in 29 healthy Asian females through instrumental evaluations, standard photography, and self-assessment questionnaire. The efficacy of Nuceutical Skinergy Collagen Shot ® in restoring skin hydration was also assessed in each subject.
Materials and methods
Study design and ethical aspects: This clinical study employed a mono-center, close labelled, and intra-individual study each subject acting as their own control. The study was conducted in compliance with the protocol, current internal procedures and in the spirit of ICH Topic E6 (R2). The investigation was in full compliance with the principles outlined in the Declaration of Helsinki and with national regulations of Singapore. A written informed consent was received from all volunteers.
Study participants: A total of 30 healthy Asian females with healthy skin presenting grade 3-5 crow’s feet wrinkles on both eyes were recruited as subjects for this study. The main exclusion criteria were as follows: pregnancy, breastfeeding or planning a pregnancy, diagnosis with cancer, diabetes, or any major systemic diseases, on antibiotics treatment, presenting allergies towards seafood or fish, subjects with eyeliner tattoos that may interfere with crow’s feet analysis and any onset of skin diseases including atopic dermatitis, contact dermatitis, eczema, chronic urticaria etc. that may affect measurements. The subjects were instructed to maintain their current skincare and cosmetic routine. Subjects were also instructed to maintain their regular lifestyle and dietary intakes. Any other intake of similar products as Nuceutical Skinergy Collagen Shot® was not allowed.
Study schedule: The study duration was of 37 days where subjects attended visits on D0 (before consumption of the investigational product (IP)), on D28 (after 28 days of IP use), and on D37 (after 37 days of IP use). Volunteers were instructed to take one Nuceutical Skinergy Collagen Shot® a day. The evaluation of crow’s feet wrinkles, the skin hydration measurements and ColorFace® images of the full face was also taken on each visit. Subjects were dermatologically examined, and tolerability and efficacy data were collected. At the end of the test duration, the sustainability of the previously observed effects was evaluated through self-assessment questionnaires in the test group of subjects who had taken the test product for 37 days. Each subject was also given a daily log to record IP use, adverse events and/or concomitant medications.
Test product: The test product is classified as a health supplement that was delivered as a single use sachet of 15 mL that is consumed as a liquid. The test product, Nuceutical Skinergy Collagen Shot ® contains a specially developed blend of 5000 mg of fish collagen peptides with 450 mg of Polypodium leucotomos, 11.5g of vitamin C, astaxanthin and rosemarinic acid (rosemary extract). The test product was consumed once daily in the morning before or together with a meal. The supplement was produced following good manufacturing practice (GMP) guidelines.
Evaluation of crow’s feet wrinkle and skin hydration: The volume of crow’s feet wrinkles was evaluated on D0, D28 and D37 by use of a skin profiling instrument PRIMOS 3D Lite (Monaderm, Monaco). The hydration level of the skin surface (stratum corneum) was evaluated using a Corneometer® CM 825 (Courage+ Khazaka electronic GmbH, Koln, Germany) on one side of the cheek of the subjects on each visit on D0, D28, and D37. These measurements were obtained on the suborbital bone (top of the cheekbone), by three repetitions in a row. All measurements were done under rested conditions of the subjects and under stable physical environmental conditions; room temperature of 20oC and a humidity of 40% – 60%.
Subject self-evaluation questionnaire : Subjects were also requested to complete a self-evaluation questionnaire to assess their facial skin condition on D0, D28 and D37. The questionnaires were designed to collect feedback from the subjects to evaluate the perceived efficacy of the IP after a minimum of 28 days of use.
Illustration of clinical efficacy: Images of the subjects’ face (front as well as left and right profiles) were captured on D0, D28, and D37 using ColorFace© (New Tone Technologies, Lyon, France). The images obtained were labelled with the subject number, study number, the study day the images were taken on, and filter used. The filters used were standard 60 and parallel polarized.
Statistical analysis: SPSS 19.0 (SPSS. Inc, Chicago, USA) program was used for statistical analysis purpose. Qualitative variables were described as number and percentage of the different responses modalities; 95% confidence interval (CI) was calculated to visually assess the evolution across time, by treatment (where applicable). For each parameter, the evolution across time (with respect to the initial state, D0) was investigated by comparing Yt and YD0 using either the Student's Paired t-test or the Wilcoxon Signed Rank Test, depending on the normality of the difference data. All statistical analyses were performed at 5% significance using 2-sided tests, except normality testing at 1% (Shapiro-Wilk test). Quantitative measurements were summarized using mean, median, maximum, and minimum and measures of dispersion, such as standard deviation. The count and percentage of subjects responding to questionnaires were provided for each point. The stars (*) on the graphs represent significance obtained with respect to baseline (Day 0).
Results
Panel description and study follow up: Thirty subjects aged 43-61 years were included in the study. Twenty-nine subjects completed the study and were included in the data and statistical analysis of all parameters measured. Subject EN04 withdrew from the study on Day 10 due to constipation although the event was determined to be unlikely related to the effects of the product. Therefore, data from this subject was excluded in the efficacy analysis but it was included in the tolerance data. Six subjects (EN01, EN02, EN03, EN06, EN08, EN09) completed the study on D28. Twenty-three subjects (EN05, EN07, EN10-EN30) completed the study on D37 ± 1D. No protocol violations occurred throughout the study. No serious adverse events were reported. Four adverse events were reported by four subjects, these isolated events were not directly linked to the product and/or study procedure.
Panel description: The study panel (Table 1) comprised of 29 females aged between 43-61 years old with healthy skin and presenting with grade 3-5 crow’s feet wrinkles on both eyes. 28 subjects (97%) were Chinese and 1 (3%) was Indian. 45% of the subjects had combination skin, 28% had dry skin, 24% had very dry skin and 3% had oily skin. 17% of the subjects had sensitive skin.
Number of subjects |
29 (Female) |
Age (mean + SEM) |
54 + 1 year old |
Ethnicity |
Chinese | 28 (97%) |
Indian | 1 (3%) |
Malay | 0 (0%) |
Skin Type |
Normal | 0 (0%) |
Combination | 13 (45%) |
Dry | 8 (28%) |
Very Dry | 7 (24%) |
Oily | 1 (3%) |
Sensitive skin |
Yes | 5 (17%) |
No | 24 (83%) |
Table 1. Characteristics of the subjects included in the study
Skin hydration levels at D28 and D37: The consumption of Nuceutical Skinergy Collagen Shot ® over the span of 28 and 37 days both presented an improvement in the levels of skin hydration measured by the corneometer®. Although not significant (only 6 subjects), the levels of skin hydration at D28 were noted to be higher than the baseline at D0 (Figure 1 and Table 2). Indeed, a rise from 75.50 + 6.82 arbitrary units (AU) to 83.15 + 8.31 AU was measured between D0 and D28. However, at D37, a significant improvement in the hydration levels of the stratum corneum was measured rising from 76.97 + 6.56 AU at D0 to 80.98 + 5.82 AU at D37 (Figure 2 and Table 2). Overall, the significant improvement in the Corneometer® values is indicative of the product’s efficacy in improving surface hydration levels of the facial skin. Additionally, based on the self-evaluation questionnaires completed at D28, 62% of subjects indicated that they felt their skin was less dry, softer, and more supple while 69% of subjects indicated that their skin felt more hydrated. At D37, 74% of subjects indicated that their skin felt less dry while 70% of them indicated that their skin was softer and more supple. 78% also indicated that their skin was more hydrated.
Statistic |
D0 |
D28 |
D28 - D0 |
D0 |
D37 |
D37-D0 |
n |
6 |
6 |
6 |
23 |
23 |
23 |
Mean |
75.50 |
83.15 |
7.65 |
76.97 |
80.98 |
4.01 |
Median |
74.77 |
79.97 |
4.62 |
76.67 |
79.57 |
3.47 |
Minimum |
68.20 |
76.90 |
-3.80 |
64.77 |
70.33 |
-6.77 |
Maximum |
83.87 |
98.70 |
30.50 |
88.80 |
89.73 |
13.83 |
SD |
6.82 |
8.31 |
12.30 |
6.56 |
5.82 |
4.91 |
% Variation |
|
|
10.13 |
|
|
5.20 |
t or z statistic |
|
|
1.523 |
|
|
3.912 |
p-value |
|
|
0.188 |
|
|
0.001 |
Significance |
|
|
NS |
|
|
S |
Statistical test used |
|
|
Paired t-test |
|
|
Paired t-test |
Table 2. Evolution across time for ‘Corneometer® readings’ on D0, D28 and D37
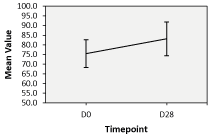
Figure 1. Skin hydration levels at D28 and D37. ‘Corneometer ® readings’ by treatment at Day 28 (D28), Error bars: 95% CI, percentages indicate difference between sides vs. baseline (D0)
Combined evaluation of skin hydration at both D28 and D37: A compilation of measurements of all 29 subjects revealed a significant improvement of 6.94% compared to measurements recorded at the baseline of D0 (Figure 3 and Table 3). Indeed, at D0 the hydration level in the skin surface was 76.67 + 6.52 AU while at D28 and D37, it was 81.43 + 6.31 AU a very significant improvement (p<0.001).
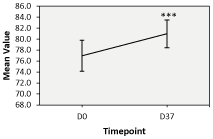
Figure 2. ‘Corneometer ® readings’ by treatment at Day 37 (D37), Error bars: 95% CI, percentages indicate difference between sides vs. baseline (D0), *** p-value ≤ 0.001.
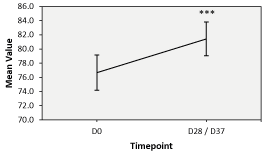
Figure 3. Combined evaluation of skin hydration at both D28 and D37. The skin hydration levels evaluated on D0, and D28/D37. The error bars were calculated at a 95% confidence interval (CI) and the percentages indicate difference between sides with respect to the baseline at D0. * represents p-value≤0.050 and *** represents p-value≤0.001.
Statistic |
D0 |
D28 / D37 |
D28 / D37 - D0 |
n |
29 |
29 |
29 |
Mean |
76.67 |
81.43 |
4.76 |
Median |
76.67 |
79.87 |
3.77 |
Minimum |
64.77 |
70.33 |
-6.77 |
Maximum |
88.80 |
98.70 |
30.50 |
SD |
6.52 |
6.31 |
6.94 |
% Variation |
|
|
6.21 |
t or z statistic |
|
|
-3.492 |
p-value |
|
|
<0.001 |
Significance |
|
|
S |
Statistical test used |
|
|
Wilcoxon |
Table 3. Evolution across time for ‘Corneometer® readings’ (on 29 subjects)
Anti-aging efficacy of Nuceutical Skinergy Collagen Shot®: The volume of wrinkles was measured via PRIMOS® on D28 for 29 subjects followed by D37 for 23 subjects. Both timepoints were evaluated against the baseline of D0 and reported to be significantly lower and clinically visible on the skin (Figure 4). Indeed, at D0 the volume of crow’s feet wrinkles was 0.19 + 0.14 onsets of fine lines and wrinkles. At D28, it had significantly dropped to 0.16 + 0.10 in 29 subjects while at D37, it had further dropped to 0.13 + 0.08 (Table 4).
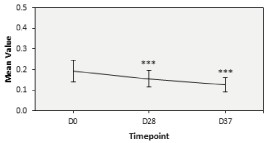
Figure 4. Anti-aging efficacy of Nuceutical Skinergy Collagen Shot ® ‘Skin Primos- Wrinkle volume’, Error bars: 95% CI, p-value≤0.001
Statistic |
D0 |
D28 |
D37 |
D28 - D0 |
D37 - D0 |
n |
29 |
29 |
23 |
29 |
23 |
Mean |
0.19 |
0.16 |
0.13 |
-0.04 |
-0.06 |
Median |
0.14 |
0.13 |
0.11 |
-0.03 |
-0.05 |
Minimum |
0.05 |
0.06 |
0.04 |
-0.26 |
-0.27 |
Maximum |
0.64 |
0.47 |
0.31 |
0.04 |
-0.01 |
SD |
0.14 |
0.10 |
0.08 |
0.05 |
0.05 |
% Variation |
|
|
|
-18.87 |
-34.70 |
t or z statistic |
|
|
|
-4.08 |
-4.203 |
p-value |
|
|
|
<0.001 |
<0.001 |
Significance |
|
|
|
S |
S |
Statistical test used |
|
|
|
Wilcoxon |
Wilcoxon |
Table 4. Evolution across time for ‘Skin Primos- Wrinkle volume’
Through the self-evaluation questionnaire, 57% of respondents noticed an improvement in the appearance of fine lines and wrinkles at D28 while 75% of respondents reported a decrease in the clinical visibility of fine lines and wrinkles. Additionally, at both D28 and D37, 52% of subjects reported that the appearance of wrinkles was less visible. Increased smoothness in the skin was equally reported to be improved by 62% and 70% of respondents at D28 and D37, respectively.
Illustration of clinical efficacy: An illustrative picture of the volume of wrinkles was captured via ColorFace® on D28 and D37. Both photographs were compared against the baseline of D0 (Figure 5). It is confirmed through the 3D skin profiling that there is a visible clinical improvement on the volume of the wrinkles. Indeed, the crow’s feet wrinkles that were clearly visible on D0 were markedly less visible at D28 and progressed to display very little visibility at D37. The overall appearance of EN20 also appears to be more radiant after 37 days of Nuceutical Skinergy Collagen Shot ® compared to D0. The wrinkle volume at D0 for EN01 was reported to be 0.91 AU followed by 0.75 AU at D28 and 0.47 AU at D37 (Data not shown).
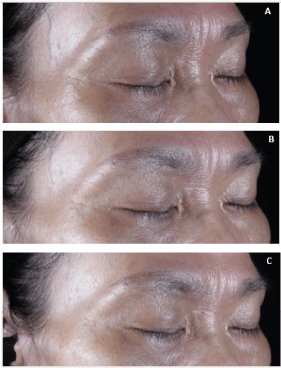
Figure 5. Illustration of clinical efficacy. Skin PRIMOS 3D Illustrative photo of subject (EN20) on the volume of crow’s feet wrinkles in parallel polarized light at (A) D0, (B) D28 and (C) D37.
Overview of self-evaluation ratings: Overall, Nuceutical Skinergy Collagen Shot ® was well received by the subjects, appreciating the product for its pleasant taste and convenient packaging. The factors that were most reported by subjects to have had an improvement at both D28 and D37 were skin hydration, dryness, softness, suppleness, and smoothness (Table 5). 72.7% of respondents reported an improvement in skin dryness and sagging. 66.7% of respondents also reported an improvement in skin fairness while 57.1% reported an improvement in lines and wrinkles. At D37, 75.0% of subjects reported an improvement in the onset of lines and wrinkles while 60% reported an improvement in the appearance of rough and dull skin. 55.0% of respondents also indicated that an improvement in dry and sagging skin was noticeable after 37 days.
Table 5. Self-perception rating on D28 and D37 concerning test product effects reported as a percentage
After taking the product |
(%) Test group at D28 relative to the baseline (D0) |
(%) Test group at D37 relative to the baseline (D0) |
Lines or Wrinkles |
57.1 |
75.0 |
Dry / Sagging skin |
72.7 |
55.0 |
Rough / Dull Skin |
56.5 |
60.0 |
Dark Circles on Eyes |
60.0 |
43.8 |
Skin fairness |
66.7 |
50.0 |
Discussion
The complex architecture of the skin can be distinguished by its different layers, and these undergo gradual degradation with the onset of ageing. The skin holds 70% of collagen which provides skin tissues with considerable tensile strength. However, as ageing progresses, the decline of collagen accelerates particularly in women [6].
Nuceutical Skinergy Collagen Shot® was developed as a unique drinkable blend of active fish collagen peptide with Polypodium leucotomos and antioxidants such as Vitamin C, astaxanthin and rosemary extract. In a recent study Polonini et al., conducted a literary investigation on the benefits of a bovine collagen peptide supplemented with green tea extracts and Vitamin C. Relying on previous existing studies for the benefits of each component individually, the study gave an insight on how the supplement might be beneficial over the course of a month. It was theorized that the collagen supplement would induce a beneficial effect on the skin and affect the health of individuals in a positive manner when consumed orally for at least a month [19]. The publication did not report on any clinical test to support the claims.
In this study, we concluded that Nuceutical Skinergy Collagen Shot ® has excellent anti-ageing efficacy through clinical data collected on 29 subjects. The oral collagen supplement Neuceutical Skinergy Collagen Shot® was effective in increasing skin hydration and reducing crow’s feet wrinkles in as fast as 28 days.
As reported here, the decrease in the wrinkle volume corelated to a decrease in the clinical visibility of wrinkles on the face of subjects. This improvement in crow’s feet wrinkles demonstrated the anti-ageing capabilities of the collagen supplement. Moreover, given that the collagen supplement is taken orally, it is hypothesized that its effects would be able to reach the deeper layers of the skin and subsequently improve the skin physiology and appearance. In this connection, the beneficial effects of the collagen may extend beyond the skin of the face and affect the skin of the entire body as well.
Collagen supplements are deemed generally safe and previous scientific evidence has corroborated these claims [20]. In a review study compiling eleven different investigations with a total of 805 patients in the treatment of pressure ulcers, xerosis, skin ageing and cellulite, it was shown that oral collagen supplements increased the skin elasticity, skin hydration and dermal collagen density [21].
In this study, we investigated the skin hydration and reduction in wrinkles formation on a homogenous Asian population by way of a bio-optimized marine collagen peptide as the key active ingredient. The excellent anti-ageing efficacy and improved skin hydration obtained attests to the positive benefits of using collagen peptides of marine origins. Indeed, a previous placebo-controlled study carried out by Proksch et al., 2020 investigated the effects of a fish-derived Bioactive Collagen Peptides on skin elasticity and wrinkles in a mixed population of Asian and Caucasian women. Their data revealed a significant improvement in skin elasticity following four weeks of oral supplementation which persisted up to eight weeks after the trial. Likewise, a pronounced 15% steady reduction was observed in the eye wrinkle volume following four weeks of usage. Despite the variations in the biophysical parameters of the skin of subjects, there was no difference in the beneficial effects of the supplement between the subjects [22]. This confirmed the positive impact collagen derived from fish on the skin barrier. However, to further elucidate any impacts of the collagen supplements on other skin barrier problems such as atopic dermatitis, more in depth investigations would be required.
The water content of the skin is dependent on the rate of cutaneous evaporation and the levels of hydration of the epidermis. These contribute towards maintaining skin moisture and giving a youthful appearance to the skin [23]. The reported clinical trial has revealed a significant increase in skin moisture level after Nuceutical Skinergy Collagen Shot® supplementation, as such it can give a youthful appearance to the skin. Furthermore, the improvement in physical and functional parameters was likely linked to an increase in collagen and glycosaminoglycan synthesis in the layers of the skin.
Our study, while preliminary, demonstrated that oral supplementation of collagen peptide, together with antioxidants and Polypodium leucotomos, can improve the structure of the skin and its health from within, as well as display significant anti-ageing potential.
In this study, the use of the collagen supplement was very well tolerated by the subjects and. the four adverse events (AEs) reported by four subjects were considered as isolated events with an unlikely relationship to the product and study procedure. As there were no AEs presenting any physical symptoms considered as serious intolerance reactions, the product presented a good tolerance profile over the course of this study.
Conclusion
In conclusion, we provide clinical evidence for the efficacy of Nuceutical Skinergy Collagen Shot ® on decreasing the clinical visibility of crow’s feet wrinkles and improving the skin hydration of Asian female adults after 28/37 days of consumption. Skin hydration and the clinical visibility of crow’s feet wrinkles were significantly improved. These promising results provide scientific evidence to prove the counteracting effect of the collagen supplement against two of the hallmarks of skin ageing.
Authors contribution
All authors have read and agreed to the published version of the manuscript.
The study was conducted according to the guidelines of the Declaration of Helsinki, OECD Good laboratory Practices (GLP) and within the national guidelines of Singapore.
Informed consent statement
Informed consent was acquired from all patients involved in this study.
Conflicts of interest
The authors declare no conflict of interest.
References
- Veysey EC, Finlay AY (2010) Aging and the skin. ‘Brocklehurst's Textbook of Geriatric Medicine and Gerontology (Seventh Edition): 133-137.
- Kohl E, Steinbauer J, Landholder M, Szeimies RM (2011) Skin ageing. Journal of the European Academy of Dermatology and Venereology 25: 873-884. [Crossref]
- Prockop DJ (2013) Protein/Enzyme Structure Function and Degradation: Collagen. Encyclopedia of Biological Chemistry (Second Edition) 1: 482-487.
- Montagna W, Kirchner S, and Carlisle K (1989) Histology of sun-damaged human skin. Journal of the American Academy of Dermatology 21: 907-918.
- Wagermaier W, Fratzl P (2012) Collagen. In Polymer science: A comprehensive reference. Elsevier 35-55.
- Matsumoto H, Ohara H, Itoh K, Nakamura Y, Takahashi S, et al. (2006) Clinical effect of fish type I collagen hydrolysate on skin properties. ITE Letters 7: 386-390.
- Asserin J, Lati E, Shioya T, Prawitt J (2015) The effect of oral collagen peptide supplementation on skin moisture and the dermal collagen network: evidence from an ex vivo model and randomized, placebo-controlled clinical trials. Journal of cosmetic dermatology 4: 291-301. [Crossref]
- Sanchez A, Blanco M, Correa B, Perez-Martin R I, Sotelo CG, et al. (2018) Effect of Fish Collagen Hydrolysates on Type I Collagen mRNA Levels of Human Dermal Fibroblast Culture. Marine drugs 16: 144. [Crossref]
- Nakyinsige K, Man YB, Sazili AQ (2012) Halal authenticity issues in meat and meat products. Meat Science 91: 207-214. [Crossref]
- Scott MR, Will R, Ironside J, Nguyen HO, Tremblay P, et al. (1999) Compelling transgenetic evidence for transmission of bovine spongiform encephalopathy prions to humans. Proceedings of the National Academy of Sciences of the USA 96: 15137-15142.
- Davinelli S, Nielsen ME, Scapagnini G (2018) Astaxanthin in Skin Health, Repair, and Disease: A Comprehensive Review. Nutrients 10: 522. [Crossref]
- Camera E, Mastrofrancesco A, Fabbri C, Daubrawa F, Picardo M, et al. (2009) Astaxanthin, canthaxanthin and beta-carotene differently affect UVA-induced oxidative damage and expression of oxidative stress-responsive enzymes. Experimental dermatology 18: 222-231. [Crossref]
- De Oliveira JR., Camargo S, and de Oliveira LD (2019) Rosmarinus officinalis L. (rosemary) as therapeutic and prophylactic agent. Journal of biomedical science 26: 5.
- Parrado C, Mascaraque M, Gilaberte Y, Juarranz A, Gonzalez S (2016) Fernblock (Polypodium leucotomos Extract): Molecular Mechanisms and Pleiotropic Effects in Light-Related Skin Conditions, Photoaging and Skin Cancers, a Review. International journal of molecular sciences 17: 1026. [Crossref]
- Zattra E, Coleman C, Arad S, Helms E, Levine D, et al. (2009) Polypodium leucotomos extract decreases UV-induced Cox-2 expression and inflammation, enhances DNA repair, and decreases mutagenesis in hairless mice. The American Journal of Pathology 175: 1952-1961. [Crossref]
- Truchuelo MT, Jiménez N, Mascaraque M, Lucena S, Días IJ, et al. (2016) Pilot study to assess the effects of a new oral photoprotector against infrared-visible radiations. The Journal of investigative dermatology 136: S106.
- Middelkamp-Hup MA, Pathak MA, Parrado C, Garcia-Caballero T, Rius-Díaz F, et al. (2004) Oally administered Polypodium leucotomos extract decreases psoralen-UVA induced phototoxicity, pigmentation, and damage of human skin. Journal of the American Academy of Dermatology 50: 41-49. [Crossref]
- Philips N, Conte J, Chen YJ, Natrajan P, Taw M, et al. (2009) Beneficial regulation of matrix metalloproteinases and their inhibitors, fibrillar collagens and transforming growth factor by Polypodium leucotomos, directly or in dermal fibroblasts, ultraviolet radiated fibroblasts, and melanoma cells. Archives of Dermatological Research 301: 487-495.
- Polonini H, Dijkers E, Ferreira AO (2021) Beauty from within: A Review of the Science behind YulivTM Collagen Drink: An Anti-Aging Nutraceutical. Journal of Cosmetics, Dermatological Sciences and Applications 11: 263-278.
- Martini N (2019) Collagen supplements. Journal of Primary Health Care 11: 385-386.
- Choi FD, Sung CT, Juhasz ML, Mesinkovsk NA (2019) Oral Collagen Supplementation: A Systematic Review of Dermatological Applications. Journal of drugs in dermatology 18: 9-16. [Crossref]
- Oesser S, Schunk M, Proksch E (2020) Positive effect of fish-derived Bioactive Collagen Peptides on skin health. International Journal on Nutraceuticals, Functional Foods and Novel Foods.
- Calleja-Agius J, Brincat M, Borg M (2013) Skin connective tissue and ageing. Best practice & research. Clinical Obstetrics & Gynaecology 27: 727-740. [Crossref]





