Abstract
Background: Redo surgery aortic valve replacement is associated with increased risk compared with the initial operation. Transcatheter aortic valve implantation procedure with its less invasive nature has been expected to offer a safer treatment. However, benefit with transcatheter in this cohort remains unclear.
Objectives: The study undertook a systematic review and meta-analysis to compare the outcomes between transcatheter and surgery strategy in patients with previous cardiac surgery.
Methods: All studies reporting on the outcomes of aortic valve replacement with previous cardiac surgery were identified using an electronic search and pooled using established meta-analytical guidelines.
Key words
TAVI, SAVR, re-operation, meta-analysis
Results: A total of 6 eligible studies involving 617 patients were included (including 314 patients in transcatheter group, 303 patients in surgery group). There was no difference of mortality [7.3% vs. 6.9%, 95% confidence interval: 0.58 to 1.96] and stroke [6.9% vs. 7.3%, 95% confidence interval: 0.50 to 1.69] between transcatheter and surgical patients. Transcatheter patients were found to be associated with a significantly higher incidence of permanent pacemaker implantation [10.5% vs. 4.3%, 95% confidence interval: 1.32 to 4.78] and paravalvular aortic regurgitation [42.0% vs. 0.7%, 95% confidence interval: 18.34 to 116.28]. However, patients who underwent surgery were more likely to need rethoracotomy [0.23, 95% (0.08, 0.62)], and transfusion [16.7% vs. 7.5%, 95% confidence interval: 0.08 to 0.79], had longer procedural time [-152.01, 95% (-169.53, -134.48)] and ventilation time [-3.19, 95% (-4.35, -2.03)].
Conclusions: In patients with previous cardiac surgery, current data suggest a faster postoperative recovery after transcatheter, with mortality and stroke comparable with those underwent surgery, although paravalvular aortic regurgitation and permanent pacemaker implantation were more frequent after transcatheter approach.
Introduction
Transcatheter aortic valve implantation (TAVI) has now become an alternative opinion for patients suffering from severe aortic stenosis (AS), who were deemed unsuitable for surgical aortic valve replacement (SAVR) because of excessive surgery risk [1]. Recently, Svensson LG et al have proved that TAVI in inoperable AS patients, substantially reduced the risk of cardiovascular death [2]. Because TAVI remains a relatively new procedure, selection appropriate candidates for TAVI have a crucial influence on clinical outcomes.
A preferable strategy decided by the team should based on both quantitative tools (expected mortality >20% with the Logistic Euro Score and >10% with Society of Thoracic Surgeons (STS) SCORE) and risk factors that are not covered in scores but often seen in practice such as chest radiation, previous cardiac surgery, porcelain aorta, liver cirrhosis, etc [3].
Among these patients, a particular high-risk sub-group is patients with previous cardiac surgery in their medical anamnesis. Conventional redo cardiac surgery is still associated with a well described increase of risks [4,5]. However, although these patients presented high preoperative risk, they share similar outcomes to patients without previous cardiac surgery when they underwent TAVI [6,7]. So, it seems important to compare the impact of previous cardiac surgery on clinical outcomes between TAVI and SAVR. But up to now, scattered studies have drawn different conclusions [8,9]. The present systematic review and meta-analysis aims to compare outcomes of TAVI versus SAVR as a redo operation in patients with previous cardiac surgery, trying to provide evidence on a preferable strategy on this cohort.
Materials and Methods
Search strategy: We conducted a search on pub-med and web of knowledge from 2002 to September 2014 using following terms: aortic stenosis, aortic valve replacement, transcatheter aortic valve implantation, TAVI, transcatheter aortic valve replacement, TAVR, surgical aortic valve replacement, SAVR, conventional aortic valve replacement, reoperation, redo, previous cardiac surgery. Studies in the original research were considered.
Citations were screened at the title and abstract level and retrieved as a full report if they reported on outcome between TAVI and SAVR in patients with previous cardiac surgery. Limiting the search parameters to the English language was applied subsequently. The full texts and bibliography of all potential articles were further reviewed in detail to seek additional relevant studies. Major conference proceedings were also searched to retrieve unpublished studies until September 2014. Full text and references of all identified potential publications and conference proceedings were searched to select the reports for inclusion in the secondary analysis.
Selection criteria: Included studies must meet the following inclusion criteria: (1) the studies must clearly describe the study design, country, year of publication, end point; (2) baseline characteristics of patients in each study must be present; (3) enrolled consecutive patients; (4) follow up time is also needed.
When 2 similar studies were reported from the same institution or author, the most recent publication was included in the analysis.
Studies were excluded if any of the following criteria applied: 1) duplicate publication, overlap of patients, subgroup studies (nonconsecutive) of a main study; 2) lack of data on main events, such as mortality, stroke, et al; 3) non-English reports.
Data extraction: Two investigators independently browsed the studies by title and abstract, finally making decision according to full text. Disagreements were discussed in a group. We extracted the following information from each study: first author, year of publication, study population characteristics, study size, study design, inclusion and exclusion criteria, time of follow-up, and survival data.
Data analysis: Pooled estimate of the odd ratio (OR) for binary outcome and the mean difference (MD) for continuous outcome were selected as the measurement of effect to analyze the results based on 95% confidence intervals (CI) and to report two-tailed P value. We examined heterogeneity across studies by the Cochran’s Q statistic and the I2 statistic. An I2<50% was considered low heterogeneity. We used Cochrane Collaboration meta-analysis software, Review Manager 5.2 to perform data analysis. And data was presented as mean ± SD, P value of 0.05 for any test or model was considered to be statistically significant.
Results
Study selection: Our literature search yielded 34 eligible articles. Figure 1 illustrates the PRISMA diagram of our search. A total of 6 studies [8-13] reporting on 617 patients (314 patients in TAVI group, 303 patients in SAVR group) met our inclusion criteria and were included in our analysis. In these studies, one study was a random clinical trial, four studies were retrospective studies, the other one study was a prospective observation. Table 1 showed the characteristics of included studies.
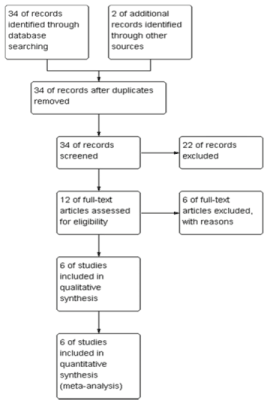
Figure 1. Flow chart depicting the selection process.
Table 1. Characteristics of Included Studies.
CABG: Coronary Artery Bypass Grafting, AVR: Aortic Valve Replacement, TAVI: Transcatheter Aortic Valve Implantation, CI: Confidence Interval, SAVR: Surgical Aortic Valve Replacement, TA: Trans-apical, TF: Trans-femoral, n: number, ns: not stated.
Preoperative data: Characteristics of the patient population are detailed in Table 2. Age distribution was similar in both groups, except the study from Stortecky S [12] that the TAVI patients were significantly older (78.2 ± 6 years in TAVI vs. 70.6 ± 8 years in SAVR, p<0.05). There was no gender-based differences in our involved studies except data from Jegaden O [11] (they did not provided gender information in their paper). The previous cardiac operations were mostly CABG (93%), other operations included: isolated SAVR (3.2%), CABG combined SAVR (3.8%). In TAVI group, 167 patients (53.2%) underwent transapical approach (TA), 144 patients (45.9%) underwent transformal approach (TF), 3 (0.9%) patients underwent trans-artery approach. Edwards balloon-expandable valve was used 100% of TAVI patients in four studies [8-11], while the two other studies [12,13] did not report the valve type.
Table 2. Characteristics of Baseline variables of patients in TAVI or SAVR.
Euro SCORE: European System for Cardiac Operative Risk Evaluation, COPD: Chronic Obstructive Pulmonary Disease, EF: Ejection Fraction, TAVI: Transcatheter Aortic Valve Implantation, SAVR: Surgical Aortic Valve Replacement, ns: not stated, *: P<0.05 vs. SAVR group.
Table 3. Clinical Outcomes in TAVI or SAVR Patients.
TAVI: Transcatheter Aortic Valve Implantation, SAVR: Surgical Aortic Valve Replacement, PPM: Permanent Pacemaker, PAR: Paravalvular aortic regurgitation, U: Unit, n: number, ns: not stated, *: P<0.05 vs. SAVR group.
Procedural Outcome
Procedure time: Compared with SAVR, the operative time was significantly reduced in TAVI from 3 studies [9,10,12] [-152.01, 95%CI (-169.53, -1 34.48)] (Figure 2A).
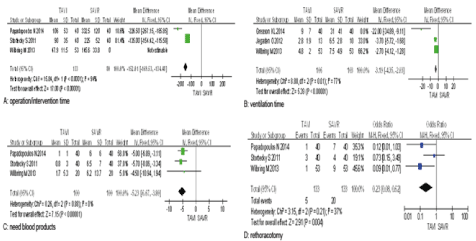
Figure 2. Forest plot of peri-procedural outcome.
- Showing the operation time of surgical aortic valve replacement and intervention time of transcatheter aortic valve implantation.
- Showing the ventilation time post operation/intervention.
- Showing the number of patients need blood products.
- Showing the number of patients requirement of rethoracotomy because of bleeding.
CI: Confidence Interval, SAVR: Surgical Aortic Valve Replacement, TAVI: Transcatheter Aortic Valve Implantation
Mechanical support and convention to surgery: One patient in TAVI group required temporary cardiopulmonary bypass (CPB) support because of hemodynamic instability in Papadopoulos N’ study [10]. Greason KL [8] proved that the need for CPB (n=4) and intra-aortic balloon pump (IABP) therapy (n=9) in TAVI group were significantly higher compared with no patient needed mechanical support in SAVR (p<0.0001).
An intra-ventricular migration of the prothesis occurred during the TA procedure in one patient (7.7%) of Jegaden O’ study [11], leading to implantation of a second valve and then a surgical removal of the first prothesis.
Concomitant revascularization: Two patients in TAVI group received percutaneous coronary angioplasty (PCI) compared with no CABG in SAVR group (p>0.05) in Papadopoulos N'study [10]. The rate of concomitant revascularization was 45% among patients undergoing SAVR vs. 25% in TAVI (p=0.06), and the number of patients receiving 2 or more bypass grafts (or multivessel percutaneous coronary intervention, respectively) was significantly higher in the SAVR group (p<0.05) in Stortecky S'study [12].
Post-operative Morbidity and Early Outcomes
Transfusion: Differences of postoperative transfusion were extracted from 3 studies [10-12] and meta-analysis of these data showed the pooled postoperative transfusion in TAVI was 7.5% (7/93) as compared with 16.7% (15/90) in SAVR, and reached statistical significance [0.25,95% CI(0.08,0.79)], as shown in Figure 2C. Major bleeding occurred in 12 patients (8.1%) in the TAVI group compared with 36 (25.7%) in the SAVR group (p <0.0001) in Greason KL'study [8]. There is evidence that patients who undergo SAVR are at higher risk for post-operative transfusion.
Intubation time: In 3 studies [9-11], the accurate ventilation time were reported. There results showed ventilation time in TAVI was significantly shorter compared with that in SAVR [-3.19, 95%CI (-4.35,-2.03)] (as shown in Figure 2B).
Rethoracotomy: Three studies [9,10,12] reported reoperation in their studies. Although Stortecky S et al. [12] did not found the difference of reexploration between TAVI and SAVR patients, both studies from Papadopoulos N [10] and Wilbring M [9] proved that reexploration was the most common postoperative complication in SAVR patients due to increased amount of bleeding. As a result, in our meta-analyses, compared with TAVI, reexploration was significantly increased in SAVR cohort [0.23, 95%CI (0.08, 0.62)] (Figure 2D).
Vascular complications: The differences of major vascular adverse events between TAVI and SAVR patients were only found in Greason KL’ study [8], their result showed that vascular complications occurred in 14 patients (9.6%) in the TAVI group in comparison with 5 (3.6%) in the SAVR group [2.65; 95% CI (0.98, 7.16); p=0.04)].
Follow Up Data
Mortality: The 30-day all-cause mortality was reported in all of the involved studies: 7.3% (23/314) in TAVI compared with 6.9% (21/303) in SAVR patients. As shown in Figure 3A, differences of 30-day mortality did not reach statistical significance [1.06, 95%CI (0.58, 1.96)]. There was no difference of 6 month mortally between these two groups from Stortecky S (12) (TAVI: 4/40 and SAVR: 4/40) and Wilbring M’ studies (TAVI: 9/53 and SAVR: 7/53) [9]. The similar result was found at 12 month follow up from Jegaden O (TAVI: 2/13 and SAVR: 0/10) [11] and 48 month follow up from Papadopoulos N’ studies (TAVI: 10/40 and SAVR: 11/40) [10]. While at 24 month follow up, Greason KL showed a higher mortality in TAVI cohort (TAVI: 59/148 and SAVR: 43/140, p=0.052) [8].
Only three studies [9-11] reported cause of death in 21 patients, so it is difficult to make a multivariable analysis identifying the risk factor of death because of small patient number. Among these three studies, only one patient died intra-operatively in TAVI procedure because of low cardiac output syndrome, and there is no report of death during operation in SAVR. It seemed that SVAR patients suffered more from respiratory failure (n=2) and right heart failure (n=2), while infection (n=4) and cancer (n=3) were more likely in TAVI patients at 30 day follow up. The spectrum of late follow up mortality are extra-cardiac reasons in both TAVI and SAVR patients.
Cerebralvascular events: We found total incidence of stroke was 6.9% (21/314) in TAVI compared with 7.3% (22/303) in SAVR patients 30 days after operation, but did not reach statistical significance [0.68, 95% CI (0.32, 1.44)] (as shown in Figure 3B). The presence of postoperative delirium was more frequently in SAVR patients (n=15, 28.3% vs. n=6, 11.5% in TAVI group; P= 0.046) in Wilbring M’ study [9].
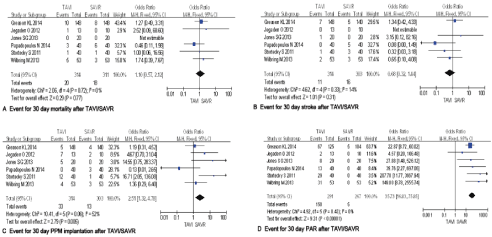
Figure 3. Forest plot of Follow up Data.
- Showing the 30-Day mortality after TAVI/SAVR.
- Showing the event of stroke 30-Day after TAVI/SAVR.
- Showing the event of PPM implantation 30-Day after TAVI/SAVR.
- Showing the event of PAR 30-Day after TAVI/SAVR.
CI: Confidence Interval, SAVR: Surgical Aortic Valve Replacement, TAVI: Transcatheter Aortic Valve Implantation, PAR: Paravalvular Aortic Regurgitation, PPM: Permanent Pacemaker.
Cardiac conduction abnormalities: Meta-analysis of postoperative PPM implantation data showed the pooled postoperative PPM in TAVI was 10.5% (33/314) as compared with 4.3% (13/303) in SAVR patients, and reached statistical significance [2.51,95% CI (1.32,4.78)], as shown in Figure 3C. There is evidence that patients who undergo catheter valve implantation are at higher risk for post-operative heart block.
PAR: The incidence of PAR was significantly higher in TAVI compared with SAVR patients [47.7% vs. 0.9%; 41.31, 95% CI (16.11, 105.94)]. PAR was prevalence of grade mild or moderate: Grade I/II of all 13 patients in Papadopoulos N’ study [10]; Grade II of all 2 patients in Jegaden O’ study [11]; Grade I of 24 patients and Grade II of 5 patients in Stortecky S ’ study [12]; Grade I of 18 patients, Grade I-II of 8 patients, Grade II of 5 patients in Wilbring M’ study (9); Grade I/II of all 8 patients in Jones SG’ study [13]. The incidence of PAR is presented in Figure 3D.
Echocardiographic data of cardiac function: The baseline left ventricular eject fraction (LVEF) were similar except Jegaden O’ study [11] (Table 2). Follow up echocardiography were carried out in three studies [8,10,12]. There was no difference of LVEF between TAVI and SAVR patients at 48 months in Papadopoulos N’ study [10] (56 ± 4 in TAVI group vs. 58 ± 2 in SAVR group), at 6 months in Stortecky S’ study [12] (49.9±1.6 in TAVI group vs. 56.3±1.0 in SAVR group), and at 1 month in Greason KL’ study (8) (52.9±11.8 in TAVI group vs 53.3±11.4 in SAVR group).
Discussion
TAVI has achieved a widely practice in recent years, nowadays studies have focused on identification subgroups that would benefit from this procedure. Redo cardiac surgery is technically challenging regarding the surgical approach, myocardial protection, calcified aortic root, and specially in case of patent arterial grafts. In such patients, a less invasive treatment may be a desirable alternative.
The salient findings from this meta-analysis are as follows: TAVI patients had a faster postoperative recovery (less procedure time and intubation time) and less post-operative morbidity (reduced rethoracotomy and transfusion). Both surgery and transcatheter strategies shared similar mortality and Cerebralvascular events, although PAR and PPM were more frequent after TAVI.
Mortality of TAVI or SAVR as a redo operation:
A variety of information, mainly on the effectiveness and safety of TAVI compared with SAVR as a redo operation, has been published in recent years, but their conclusions remained controversy. Jegaden O et al. [11] reported 1-year survival is similar after SAVR and TF-TAVI (100%) and lower after TA-TAVI (78%). Greason KL et al. [8] found that although the mortality difference between TAVI and SAVR group did not reach statistical significance at 1 year follow up (p=0.19), it increased at 2 years (p=0.052). These studies implied SAVR may be a preferred choice in this cohort. But Drews et al. [14] reported that previous heart surgery was not a risk factor in TA-TAVI. The study of Papadopoulos N et al. [10] suggested a trend towards a higher 30-day mortality following SAVR as compared to TAVI patients (p=0.06). So, it may be difficult for clinicians making their decisions when they facing these patients.
Our meta-analysis did not identify statistically difference with respect to 30-day mortality in TAVI compared with SAVR patients (p=0.84). This result is important, but several issues still need further investigation: 1. The number of patients receiving TA-TAVI (n=167) or TF-TAVI (n=143) was not different in our analysis, but TF-TAVI has been shown a low risk for 30-day mortality against TA procedure [15]. However, we could not make analyses between TA-TAVI, TF-TAVI and SAVR separately because of some small sample size studies are involved in our analyses. 2. Although Stortecky S [12] found all-cause mortality was similar in both groups, the TAVI patients were older (78.5±6 vs. 70.6±8 years, p<0.001) and presented higher logistic Euro SCORE (33.5±17 vs. 20.2±14, p<0.001). So, we do not certain what will be happen with aspect to mortality if they made a propensity-score matched analysis. 3. The most concerned arterial grafts injury during SAVR was not reported in any of these studies. Respiratory failure (n=2) and right heart failure (n=2) were reported only in SAVR, whereas electromechanics dissociation (n=2) and low cardiac output syndrome (n=1) were reported only in TAVI patients. This result implies that although total mortality is similar between these two groups, risk factor for individual group is different and need further understanding. 4. We can only show 30-day mortality in this analysis, long-term follow-up of the 2 cohorts in randomized clinical trials will be critical to understanding the results.
Neurological events were similar between TAVI and SAVR patients:
Studies of PARTNER (Placement of Aortic Transcatheter Valves) trials have raised major safety concerns with TAVI, reporting 30-day stroke/transient ischemic attack (TIA) rates of 6.7% and 5.5%, respectively, in TAVI patients [16,17]. Previous studies showed strokes are more common post-TAVI than after alternative management strategies, with the highest reported incidence for any cardiac procedure: 30-day stroke rates of 3.3%, 2.4% and 1–2% have been reported for populations undergoing TAVI, isolated SAVR, and balloon valvuloplasty, respectively [18-20]. Furthermore, 30-day mortality was regarded 3.5-fold higher in patients with stroke compared to those without stroke (25.5±21.9% vs. 6.9±4.2%) [18].
But recently studies have found great reduce of stroke in TAVI patients. Papadopoulos N et al. [10] reported 0% stroke rate in their series of TAVI group, which is significantly lower in contrast to SAVR group at 4 years follow up (15% vs. 0%, p = 0.03). Such a low rate of neurologic complications is in accordance with previously published data reporting an incidence of stroke of 0 to 1% after TAVI [6]. Ducroq et al. [21] explored the early and mid-term outcome of 54 patients undergoing TAVI after previous CABG, reporting excellent results with an early stroke rate of 0%. Additionally, the presence of postoperative delirium was less in TAVI patients (28.3% vs. 11.5% in TAVI group; P= 0.046) [9].
Our meta-analysis did not find statistically difference of stroke in TAVI compared with SAVR patients (p=0.66). The excellent result of stroke with TAVI procedure may be a complex of improved patient selection, modification of TAVI procedure, refinements in catheter technique, more experienced doctors and progress in valve design. Although approximate doubling on the rate of neurologic events between TAVI and SAVR still remains a concern, the significant improvement of neurologic complications as shown above, in certainly promises the greatest potential for optimization of TAVI in this cohort high risk patients.
Procedural outcome and post-operation recovery:
Redo cardiac surgery is associated with an increase of morbidity and mortality in comparison with the initial surgery [6], especially in patients with previous CABG [12,21]. TAVI procedure with its less invasive nature has been expected to offer a safer treatment solution for such patients as pictured by elimination of the need for mediastinal reentry, avoiding CABG-grafts injury, and abolishment of extracorporal circulation.
As expected, peri-procedural hazards reflected the inherent differences between an open operation and a transcatheter procedure. Owing to the greater blood loss as a consequence of adhesiolysis, a larger wound surface, and the necessity of extracorporeal circulation with systemic heparinization, SAVR resulted in a higher need for blood products, even rethoracotomy due to bleeding. While in TAVI group, patients had lower postoperative chest tube drainage and lower transfusion compared with those in SAVR group. It is obviously that operation/intervention time is shorter with TAVI procedure, together with less bleeding; one could expect a fast recovery in TAVI patients. Although we did not compare Intensive Care Unit (ICU) stay and hospital stay time in our analyses because of incomplete data, Stortecky S [12] observed a trend of shorter hospital stay (11±7 in TAVI vs. 15±14 in SAVR, p=0.065), Wilbring M et al. [9] found less patients stayed in ICU within 24 hours (19 in TAVI vs. 34 in SAVR, p=0.00216) in TAVI group. COPD is popular before operation either in TAVI or SAVR patients, ranged from 7.5%-45.3%; however, a longer postoperative ventilation time was observed in SAVR compared with that in TAVI patients. It is regarded that preoperative COPD and postoperative mechanical ventilation time were independent risk factors for re-intubation, which is often associated with significant increase of morbidity and mortality [22]. Above all, the theoretical benefit of TAVI has been clinically shown as minimize trauma. However, concerns should be raised on circulatory instability during TAVI, although only one report from Greason KL et al. [8] showed more mechanical support was applied in TAVI patients.
PAR and NYHA functional class
A design limitation of transcatheter aortic valves has been PAR, which results from incomplete circumferential apposition of the prosthesis with the annulus. There is a significant number of TAVI patients in this trial, experienced more PAR than that in SAVR group (p<0.001), although it is also worth mentioning that most of these PAR was mild or moderate (Grade I/II). Papadopoulos N et al. [10] found no association of PAR with the 2-year outcome of death of any cause (p=0.011); Doss M et al. [23] showed a good survival rate in midterm outcomes, even 32% of their TAVI patients still with an unreleased PAR. But Kodali et al. [24] reported that PAR (mild or greater) was associated with increased late mortality (HR 2.11; 95% CI: 1.43, 3.10; p<0.001).
In our results, we did not find mortality difference between TAVI and SAVR patients, but there still remains controversy between PAR and mortality, and there are some limitations of our study. First, the incidence of PAR ranged from 15.4% (9) to 72.5% [10] according to our data, and there is apparent different among these involved studies. The reason may be multiple, but center expertise and the optimization of the TAVI technique should impact the incidence of PVR. Furthermore, we cannot provide the exact number of patients who were pure AS or AS combined with aortic valve insufficiency at baseline. As PVR is most impactful on patients without previous aortic regurgitation, further studies, such as more detailed sub-group analyses may be helpful to identify the relation between PVR and cardiac function. Finally, more accurate methods for annulus measurements, advanced deployment techniques and new-generation valves aim to have much lower rates of PVR may not involve in this analysis, because some of the studies were started at early as 2005.
Study Limitations:
This is a subgroup analyses comparing outcomes between TAVI and SAVR in patients with previous cardiac surgery, although the peri-operative benefit was found in transcatheter strategy, the results must be interpreted with caution because of some limitations. First, most studies involved in this paper are single-center experience that could only provide low volumes of patients. Second, there was only one randomized trial analyzed a subgroup of PARTNER cohort A patients. Nevertheless, despite these limitations, our results provided valuable insights into the risk of re-aortic valve implantation after previous cardiac surgery.
Conclusion
Our findings demonstrated TAVI technique resulted in similar with aspect to mortality compared with SAVR, and an approximate incidence of stroke between these two groups of patients. PAR was common complications in TAVI patients because of inherent of the procedure. However, patients in TAVI group had a faster post-operation recovery.
Some clinicians convinced TAVI may be particularly beneficial and hypothesized it should have a more liberally indication in future guidelines in patient with previous cardiac surgery. But their studies were single center experience with small patient population. In this article, a total of 617 patients were analyzed, demonstrating less trauma related complications and faster postoperative recovery in TAVI patients. Although additional critical designed randomized clinical trials and improved risk stratification is required, our encouraging results promises the greatest potential for optimization of TAVI in this patient cohort.
Authorship contributions
On behalf of all of the authors, I declare the contributions of each author as following: Xiaoping Wu is in charge of acquisition of data, Xiaojing Wu is responsible for interpretation of data, Lijuan lin wrote this manuscript and Qi Zhou designed this analysis. There is no financial support by any companies.
Acknowledgments
No.
Funding Information
No.
Competing Interests
All of the authors declare that there are no competing interests.
References
- Walther T, Schuler G, Borger MA, Kempfer Jörg t, Seeburger J, et al. (2010) Transapical aortic valve implantation in 100 consecutive patients: comparison to propensity-matched conventional aortic valve replacement. Eur Heart J 31: 1398-1403. [Crossref]
- Svensson LG, Blackstone EH, Rajeswaran J, Brozzi N, Leon MB, et al. (2014) Comprehensive analysis of mortality among patients undergoing TAVR: results of the PARTNER trial. J Am Coll Cardiol 64: 158-168. [Crossref]
- Vahanian A, Alfieri O, Al-Attar N, Antunes M, Bax J, et al. (2008) Transcatheter valve implantation for patients with aortic stenosis: a position statement from the European Association of Cardio-Thoracic Surgery (EACTS) and the European Society of Cardiology (ESC), in collaboration with the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J 29: 1463-1470. [Crossref]
- Jones JM, O’kane H, Gladstone DJ, Sarsam MA, Campalani G, et al. (2001) Repeat heart valve surgery: risk factors for operative mortality. J Thorac Cardiovasc Surg 122: 913-918. [Crossref]
- Fukunaga N, Okada Y, Konishi Y, Murashita T, Kanemitsu H, et al. (2014) Redo valvular surgery in elderly patients aged > 75 years. J Heart Valve Dis 23: 228-234. [Crossref]
- D’Onofrio A, Rubino P, Fusari M, Musumeci F, Rinaldi M, et al. (2012) On behalf of the I-TA investigators: Impact of previous cardiac operations on patients undergoing transapical aortic valve implantation: results from the Italian Registry of Transapical Aortic Valve Implantation. Eur J Cardiothorac Surg 42: 480-485. [Crossref]
- Minha S, Magalhaes MA, Barbash IM, Ben-Dor I, Dvir D, et al. (2014) Impact of previous coronary artery bypass grafting on patients undergoing transcatheter aortic valve implantation for aortic stenosis. Am J Cardiol 113: 1222-1227.
- Greason KL, Mathew V, Suri RM, Holmes DR, Rihal CS, et al. (2014) Transcatheter versus surgical aortic valve replacement in patients with prior coronary artery bypass graft operation: a PARTNER trial subgroup analysis. Ann Thorac Surg 98: 1-7. [Crossref]
- Wilbring M, Tugtekin SM, Alexiou K, Simonis G, Matschke K, et al. (2013) Transapical transcatheter aortic valve implantation vs conventional aortic valve replacement in high-risk patientswith previous cardiac surgery: a propensity-score analysis. Eur J Cardiothorac Surg 44: 42-47. [Crossref]
- Papadopoulos N, Schiler N, Fichtlscherer s, Lehmann R, Weber CF, et al. (2014) Propensity matched analysis of longterm outcomes following transcatheter based aortic valve implantation versus class aortic valve replacement in patients with previous cardiac surgery. J Cardiothorac Surg 9: 99-108. [Crossref]
- Jegaden O, Lapeze J, Farhart F, de Gevigney G (2012) Aortic valve stenosis after previous coronary bypass: transcatheter valve implantation or aortic valve replacement? J Cardiothorac Surg 29: 47-49. [Crossref]
- Stortecky S, brinks H, wenaweser P, C Huber,T Pilgrim, et al. (2011) Transcatheter based aortic valve implantation or surgical aortic valve replacement as redo procedure after prior coronary artery bypass grafting. Ann thorac Surg 92: 1324-1330. [Crossref]
- Jones SG, Abdulkareem NR, Williams F, Brecker S, Jahangiri M (2013) Outcomes of transcatheter aortic valve implantation compared to surgical aortic valvereplacement following previous surgery. J Heart Valve Dis 22: 204-208. [Crossref]
- Drews T, Pasic M, Buz S, Axel U, Stephan D, et al. (2011) Transapical aortic valve implantation after previous heart surgery. Eur J Cardiothorac Surg 39: 625-630. [Crossref]
- Li X, kong M, Jiang D, Dong A (2013) Comparison 30-day clinical complications between transfemoral versus transapical aortic valve replacement for aortic stenosis: a meta-analysis review. J Cardiothorac Surg 3: 168-174. [Crossref]
- Leon MB, Smith CR, Mack M, Miller DC, Moses JW, et al. (2010) Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 363: 1597-1607. [Crossref]
- Smith CR, Leon MB, Mack MJ, Miller DC, Moses JW, et al. (2011) Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 364: 2187-2198. [Crossref]
- Eggebrecht H, Schmermund A, Voigtlander T, Kahlert P, Erbel R, et al. (2012) Risk of stroke after transcatheter aortic valve implantation (TAVI): a meta-analysis of 10,037 published patients. Eurointervention 8: 129-138. [Crossref]
- Vasques F, Messori A, Lucenteforte E, Biancari F (2012) Immediate and late outcome of patients aged 80 years and older undergoing isolated aortic valve replacement: A systematic review and meta-analysis of 48 studies. Am Heart J 163: 477-485. [Crossref]
- O'Brien SM, Shahian DM, Filardo G, Ferraris VA, Haan CK, et al. (2009) The Society of Thoracic Surgeons Quality Measurement Task Force: The Society of Thoracic Surgeons 2008 Cardiac Surgery Risk Models: Part 2‚ Isolated Valve Surgery. Ann Thorac Surg 88: S23-S42. [Crossref]
- Ducrocq G, Al-Attar N, Himbert D, Messika-Zeitoun D, Iung B, et al. (2012) Early and mid-term outcomes in patients undergoing transcatheter aortic valve implantation after previous coronary artery bypass grafting. Eur J Cardiothorac Surg 41: 499-504. [Crossref]
- Jian L, Sheng S, Min Y, Zhongxiang Y (2013) Risk factors for endotracheal re-intubation following coronary artery bypass grafting. J Cardiothorac Surg 9: 208-214. [Crossref]
- Doss M, Buhr EB, Martens S, Moritz A, Zierer A (2012) Transcatheter-based aortic valve implantations at midterm: what happened to our initial patients? Ann Thorac Surg 94: 1400-1406. [Crossref]
- Kodali SK, Williams MR, Smith CR, Svensson LG, Webb JG, et al. (2012) Two-year outcomes after transcatheter or surgical aortic-valve replacement. N Engl J Med 366: 1686-1695. [Crossref]



