Introduction: COVID-19 medical practice has varied widely across the world and several therapeutic interventions have been proposed, but there is no consensus on the best decisions.
Objective: To investigate the effect of a set of therapeutic interventions on length of stay, ICU admission, need for MV and mortality and to clarify the risk factors for COVID-19 outcomes.
Methods: Retrospective cohort of inpatients with RT-PCR positive for COVID-19 from March to July 2020. Multivariate models were used to assess risk for ICU admission, need for MV and hospital mortality. Logistic regression analysis was conducted to examine factors associated with the results.
Results: 459 patients were enrolled. For patients treated with AZM-Corticosteroid (46.8%) the risk for ICU admission was 0.17 (OR; 95%CI 0.05-0.57), for MV 0.16 (OR; 95%CI 0.04-0.74) and for mortality 0.16 (OR; 95%CI 0.03-0.91). AZM-Corticosteroid also decreased the length of stay in 1.5 day. AZM-Corticosteroid and anticoagulation when indicated (17.2%), also reduced the ICU stay in 1.5 and MV in 4 days. When included HCQ, the benefits were lost and the times increased. Age >65 years, presence of up one comorbidity, pulmonary involvement more than 50%, saturation <93%, lymphocytes <900 mm3, D-dimers >1,250 ng/mL and CRP >8.0 mg/dL at admission were clinical predictors for death.
Conclusion: AZM-Corticosteroids and anticoagulation represented a favorable combination for inpatients with COVID-19. HCQ did not yield benefits to combination therapy and we do not support its use for inpatients. These findings suggest that some clinical predictors may help to estimate a higher risk of poor evolution.
COVID-19; COVID-19 treatment; cohort studies; hydroxychloroquine; azithromycin
Doctors and hospitals have learned a lot about how best to treat people infected with the novel coronavirus disease 2019 (COVID-19) in the last months since the pandemic began. COVID-19 is an emerging health problem, in which a significant percentage of patients develop health conditions that require hospital care [1]. The practice has varied widely across the world and several therapeutic interventions have been proposed and methodological studies have been published, although far from overwhelming evidence they closely follow and analyze updates on this outbreak [2], but there is no consensus on the best decisions.
Therapeutic strategies using hydroxychloroquine (HCQ), antibiotics, corticosteroid, anticoagulants and others, in combination or not, were introduced to the clinical practice. However, there is not yet consensus about the best pharmacological combination to prove effectiveness and safety, incorporated in the usual care in the COVID-19 treatment. Despite that, recently a Brazilian guideline [3] recommended a number of therapeutic strategies in the management of COVID-19 patients based on available scientific evidence, discouraging the use of HCQ.
Our aim was to evaluate retrospectively the real-world medical practice in a reference hospital to clarify the risk factors and to investigate the effect of the main therapeutics’ interventions on length of hospital stay, need for admission to the intensive care unit (ICU) or mechanical ventilation (MV) and mortality during the COVID-19 outbreak in our center.
This is an observational retrospective analysis of patients (aged ≥18 years) with RT-PCR positive for COVID-19 obtained from nasal and pharyngeal swabs, admitted from March 15 to July 31, 2020 at Hospital Moinhos de Vento, a teaching private hospital, with 500 beds and reference for the treatment of patients with COVID-19 in the South of Brazil [4]. The institutional ethics committee approved this study.
Patients were assessed retrospectively for specific outcomes after receiving a set of therapeutic interventions. The interventions in different combinations, besides the usual care were: HCQ, azithromycin (AZM), corticosteroids (dexamethasone, hydrocortisone or methylprednisolone), tocilizumab, oseltamivir, convalescent plasma, therapeutic anticoagulation and different ways of improving oxygen without positive pressure (nasal catheter and Hudson mask) and with positive pressure (high flow nasal cannula (HFNC) Bi-level Airway Positive Pressure (BiPAP) and MV). Therapeutic anticoagulation was considered with intention-to-treat for patients with Deep Venous Thrombosis (DVT), Pulmonary Embolism (PE) or serum D-dimers >2,000 ng/mL. Obese people (body mass index ≥30), lymphopenia (lymphocytes <900 mm³), D-dimers >1,250 ng/mL and C-reactive protein (CRP) >8.0 mg/dL were considered abnormal values. The usual dose of corticosteroids used was ≤0.5–1 mg/kg per day of methylprednisolone or equivalent. The pulmonary impairment was assessed on a visual scale by two independent chest radiologists trained to interpreted COVID-19 patients.
The primary endpoint was building up a COVID-19 predictor model based on individual-level data to estimate ICU admission, need for MV and hospital mortality. Additionally, three secondary endpoints were established: 1) the impact of these interventions on the length of stay in hospital, ICU and MV, 2) the likelihood of the different ways of noninvasive oxygen administration to prevent MV and 3) which clinical predictors are relevant to poor outcome (mortality).
Descriptive analysis was used to characterize the study population. Categorical variables were summarized using absolute frequencies and percentages, while continuous variables were analyzed using means and standard deviation (SD) or median and interquartile range (IQR). Different combinations of medications were administered and the one used in multivariable models was the use of AZM and corticosteroids with or without the use of therapeutic anticoagulation. Interaction term with HCQ was used to evaluate the behavior of the combined therapy of medications in the presence or absence of the use of HCQ.
Logistic regression analysis was conducted to examine factors associated with the outcomes, adjusting for all predictors presented in the model. Linear regression was applied to continuous outcomes. The model results are presented in odds ratio (OR) or beta (ß). For the multivariable analyses, a theoretical framework was structured according to literature. Statistical analysis was performed using SAS software (Statistical Analysis System, SAS Institute Inc., Cary, N.C.), version 9.4, and statistical significance was defined as p-value <0.05.
Between March 15 and July 31, 2020, 459 patients who had been admitted to Hospital Moinhos de Vento were identified and considered to meet the criteria.
Clinical characteristics of patients
The median age was 60.0 years (interquartile range [IQR], 45.0 to 72.0 years) being 262 (57.1%) males. The main clinical characteristics of patients are presented in Table 1 and 2. The median onset of symptoms before admission was 7.0 days (IQR, 4.0 to 9.5 days); 136 (29.6%) patients needed admission to the ICU and 97 (21.1%) required MV. The mean length of stay in hospital, MV and ICU was 13.9 (SD±16.1), 20.1 (SD±15.6) and 21.2 (SD±18.4) days, respectively.
Table 1. Clinical information at admission
Therapeutics’ interventions
Regarding pharmacologic therapies, HCQ-AZM was administered to 105 (23.2%) patients; HCQ-corticosteroid to 49 (10.7%) and AZM-corticosteroid to 212 (46.8%). Therapeutic anticoagulation was administered to 138 (31.8%) patients, being 36 (7.9%) combined with HCQ and 78 (17.2%) with AZM-Corticosteroid. The combination of HCQ-AZM-Corticosteroid and therapeutic anticoagulation occurred in 21 patients (4.6%).
For patients treated with AZM-Corticosteroid the risk for ICU admission 0.17 (OR; 95% CI 0.05 to 0.57), for MV 0.16 (OR; 95%CI 0.04 to 0.74) and mortality was 0.16 (OR; 95% CI 0.03 to 0.91). For those patients treated with HCQ-AZM-Corticosteroid, the association represented loss of benefit (Figure 1).
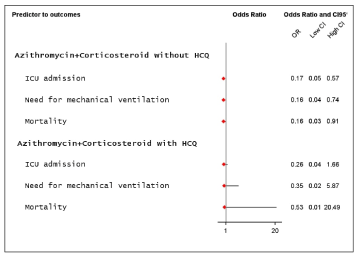
Figure 1. Risk predictors to primary endpoint - ICU admission, need for MV and hospital mortality
Tocilizumab (2.6%), convalescent plasma (16.5%) and oseltamivir (29.6%) were administered as adjunct therapy. Among patients admitted to the ICU, 57.6% (68) received vasopressor and supplemental oxygen without positive pressure was used in 56.9% (254). Positive non-invasive ventilation including HFNC and BiPAP were used in 23.9% (107) while MV in 21.1% (97). HFNC oxygen therapy was able to prevent the patient's progression to MV in 31.8% of cases.
Main outcomes and endpoints
Overall, 86.7% (398) of the patients were discharged alive and 4.5% (21) were still hospitalized by dataset freeze date. Of patients admitted to the ICU, 25.7% (35) died and when MV was required, the mortality increased to 34.0% (33). Among dead patients, most had median age of 83.3 years (IQR, 75.5 to 89.5 years) with the mean length of stay in hospital of 25.3 (SD±22.5) days, in ICU 22.8 (SD±18.7) and in MV 21.2 (SD±17.3). The main clinical predictors related to increased mortality, with >70% risk, were: age >65 years, presence of up one comorbidity, pulmonary involvement >50%, saturation <93%, lymphocyte <900mm3, D-dimers >1,250 ng/mL and CRP >8.0 mg/dL at admission, Oxygen requirement through BiPAP or HFNC, and ICU admission and MV required during hospitalization were also associated with a higher risk of death (Figure 2).
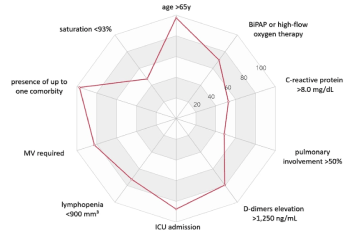
Figure 2. Clinical predictors in mortality
Considering the secondary endpoints, AZM-Corticosteroid decreased the mean length of hospital stay in 1.5 day (ß -1.5 95%CI -4.9 to 1.9), while HCQ alone increased in 6.9 days (ß 6.9 95%CI 3.6 to 10.3). AZM-Corticosteroid and therapeutic anticoagulation combination reduced the ICU length stay in 1.5 day (ß -1.5 95%CI -9.7 to 6.7) and MV in 4 days (ß -4.0 95%CI -13.4 to 5.3), however, this effect was not observed when HCQ was associated (Figure 3). For the AZM-Corticosteroid and therapeutic anticoagulation the mean length of ICU stay was 15.9 days, but when HCQ was included, again this time was increased to 40.3 days (Figure 3). There was a trend to more time in MV in obese patients. All models were adjusting for sex, age, obesity, pulmonary involvement, D-dimers, CRP, oxygen support without positive pressure (nasal catheter and Hudson mask) and length of hospital stay. Regarding HCQ have been used or not, the comparability of the groups was verified in relation to the clinical predictors for mortality, where there was no difference was observed among those who received the drug or not between those treated or not with HCQ.
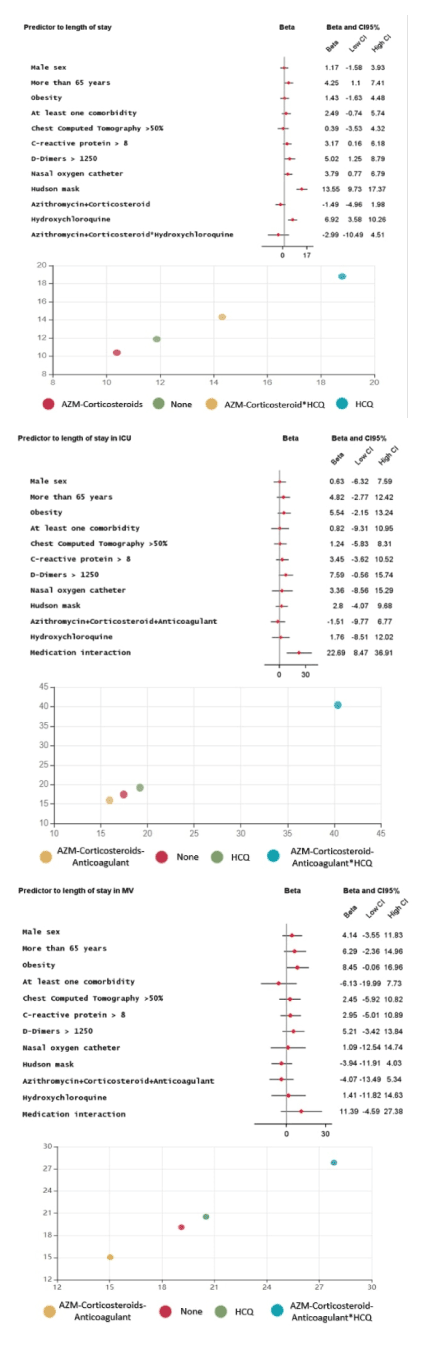
Figure 3. Medication interaction is the behavior of the combination AZM-Corticosteroids-Anticoagulant in the presence or absence of HCQ. At the bottom, the mean length of stay for each outcome according to the combination of treatment use.
Our study sought to identify the clinical predictors for COVID-19 that resulted in risk for worst outcomes and the effect of a set of therapeutic interventions on length of stay, ICU admission, need for MV and mortality. The study was conducted based in a real world of care at a Brazilian private hospital. Our major findings suggest: 1) there was a benefit with the combination of AZM-Corticosteroid to reduce the risk of ICU admission, need for MV and mortality; 2) the combination of AZM-Corticosteroid and therapeutic anticoagulation when indicated, reduced the mean length of stay in ICU and MV; 3) the introduction of HCQ to the AZM-Corticosteroid combination increased the mean length hospital stay; 4) the use of HFNC prevented in one third the patient's progression to MV and 5) clinical predictors related to higher mortality at admission, included: age >65 years, presence of up one comorbidity, pulmonary involvement >50%, saturation <93%, lymphopenia, D-dimers and CRP altered.
Observational and Randomized Clinical Trials (RCT) have been published, evaluating the effects of several drugs in terms of potency, efficiency or efficacy in clinical management [3,5]. Predictive factors and clinical characteristics that may influence COVID-19 severity have already been demonstrated in the literature and multivariable models have been used to identify high-risk individuals [6,7].
Although some treatments are promising, it is thought to be early to clearly state that there is a definitive treatment. The Solidarity Trial Consortium [8], funded by the World Health Organization showed that antiviral drugs including remdesivir, HCQ, lopinavir, and interferon regimens had little or no effect on hospitalized patients with COVID-19, as indicated by overall mortality, initiation of ventilation, and duration of hospital stay. Also, another promising intervention with convalescent plasma, validated in a RCT showed no significant differences in clinical status or overall mortality between patients treated or received placebo [9].
Although controversial, the use of corticosteroid seems to have clinical potential on mortality reduction and need for intubation, provided it is adequate for the treatment regimen and individual clinical characteristics [10-12]. The Randomized Evaluation of COVID-19 Therapy (RECOVERY trial) showed that survival was significantly higher among patients treated with dexamethasone, especially for those requiring invasive intubation [13]. Our data reinforce these findings and highlight the corticosteroid therapeutic effectiveness, especially in reducing the risk of mortality, ICU admission and need for MV when combined with AZM.
Therapeutic or prophylactic anticoagulation when indicated has proven to be an important strategy to the COVID-19 treatment framework, reducing intubation and mortality [14]. We also observed some benefits related to the reduction of ICU and MV length of stay in patients who used anticoagulant.
Some studies published at the beginning of the pandemic, with limited evidence, highlighted the benefits of using HCQ combined or not with AZM in reducing mortality and total length of stay [15,16]. However, in an open-label, multicenter, randomized, controlled trial conducted by the Coalition COVID-19 Brazil I, among inpatients with mild-to-moderate COVID-19, the use of HCQ, alone or with AZM, did not improve clinical status at 15 days as compared with standard care [17]. Self et al. [18] reported similar ineffectiveness in HCQ treatment on the 14th day of hospitalization.
Rosenberg (2020) [19] and Magagnoli (2020) [20] also performed a protocol using HCQ combined or not with AZM and found no reduction in mortality risk and need for MV. Also, they reported an increase in overall mortality for patients treated with HCQ alone. In the same direction, we observed that whenever HCQ was included in the model, the protective benefit of the association of AZM-Corticosteroids loses significance and becomes a risk factor for a worse prognosis. We showed that patients treated with HCQ have a longer hospitalization compared to patients not treated, a finding previously discussed by Kalligeros et al. [21]. Interestingly also, when we analyzed the clinical predictors influence under the use of HCQ, there was no significant difference between those treated or not with HCQ. We assume that other factors, such drug interactions may be involved in these findings. Besides that, oseltamivir, convalescent plasma, vasopressor and tocilizumab when evaluated alone or combined with HCQ, AZM and corticosteroids showed no benefit.
The use of HFNC showed a trend toward reduction in the intubation rate and no difference in mortality, findings similar to those reviewed by Lin (2020) [22]. Geng (2020) presented HFNC as a favorable option to avoid intubation through adequate monitoring of the respiratory function of COVID patients [23].
Clinical predictors associated with mortality included individuals older than >65 years, with up to one comorbidity, pulmonary involvement more than 50%, saturation <93%, lymphopenia, elevated D-dimers and CRP at admission. Oxygen requirement through BiPAP or HFNC, ICU admission and MV required during hospitalization were also risk markers. A recent publication showed that patients with leukocytosis and CRP altered on arrival were associated with poor prognosis and may predict the severity of COVID-19 [24]. Other reports also found the increase of biochemical and inflammatory markers - D-dimers, ferritin, lactate dehydrogenase, and CRP - when no anticoagulant was used in treatment [14].
The weakness of our study is related to the fact that it is observational, unicentric and retrospective however our results are in line with other RCTs that recommended the association of corticosteroids to the set of treatment and advise against HCQ use in patients with COVID-19. The reduced HCQ treatment efficiency when included to the set of drugs can be speculate through the pharmacological interaction with others drugs triggering for example an increase in its serum concentration, prolongation of the QT interval in the ECG and possibly triggering episodes of ventricular tachycardia [25-28]. It is known that longer QTc can cause life-threatening arrhythmias especially in critically ill patients, however monitoring of ECG and drug serum level was not uniformly standardized, given the retrospective nature of the study. Finally, the results of our study should be evaluated considering individual clinical characteristics in a real world and clinicians should carefully weigh the risks and benefits when considering any therapeutic scheme out of the randomized clinical trial setting.
In this retrospective cohort study, AZM-Corticosteroids and therapeutic anticoagulation, when indicated, represented a favorable combination for patients hospitalized with COVID-19, reducing mortality, length of hospitalization and the risk of MV. HCQ did not yield benefits to combination therapy and we do not support its use for inpatients. HFNC oxygen therapy was able to reduce the risk of MV support. Individuals older than >65 years, with presence of up one comorbidity, pulmonary involvement more than 50%, saturation <93%, lymphopenia, D-dimers and CRP elevated on arrival, and Oxygen requirement through BiPAP or HFNC, ICU admission and MV during hospitalization represented the set of clinical predictors for worse prognosis.
M.M.P, G.N.B and L.A.N had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. M.A.F, M.B, I.W.Z, A.B.G, R.D.G, R.G.R, M.M.P, G.N.B and L.A.N contributed substantially to the study design. M.A.F, M.B, I.W.Z, A.B.G, R.D.G and R.G.R collected data. M.A.F, M.B, A.B.G performed data analysis and interpretation. M.A.F, M.B, I.W.Z, A.B.G, M.M.P, G.N.B and L.A.N drafted and revised the manuscript. All authors approved the final draft of the manuscript for publication.
The authors declare that they have no competing interests.
- Verity R, Okell LC, Dorigatti I, Winskill P, Whittaker C, et al. (2020) Estimates of the severity of coronavirus disease 2019: a model-based analysis. Lancet Infect Dis 20: 669-677. [Crossref]
- Infectious Diseases Society of America Guidelines on the Treatment and Management of Patients with COVID-19.
- Falavigna M, Colpani V, Stein C, Azevedo L, Bagattini A, et al. (2020) Guidelines for the pharmacological treatment of COVID-19. The task-force/consensus guideline of the Brazilian Association of Intensive Care Medicine, the Brazilian Society of Infectious Diseases and the Brazilian Society of Pulmonology and Tisiology. Rev Bras Ter Intensiva 232: 166-196. [Crossref]
- Polanczyk CA, Rohsig V, Bastos GN, Zavascki A, Nasi L, et al. (2020) Getting Ready for the Covid-19 Pandemic: Experience of a Brazilian Hospital. N Engl J Med
- Wise J, Coombes R (2020) Covid-19: The inside story of the RECOVERY trial. BMJ 370: m2670. [Crossref]
- Barda N, Riesel D, Akriv A, Levi J, Finkel U, et al. (2020) Developing a COVID-19 mortality risk prediction model when individual-level data are not available. Nat Commun 11: 4439.
- Zhao Z, Chen A, Hou W, Graham J, Li H, et al. (2020) Prediction model and risk scores of ICU admission and mortality in COVID-19. PloS one 15: e0236618. [Crossref]
- WHO Solidarity Trial Consortium, Pan H, Peto R, Henao-Restrepo AM, Preziosi MP, et al. (2020) Repurposed Antiviral Drugs for Covid-19 - Interim WHO Solidarity Trial Results. N Engl J Med p. 384. [Crossref]
- Simonovich VA, Burgos Pratx LD, Scibona P, Beruto MV, Vallone M, et al. (2020) PlasmAr Study Group. A Randomized Trial of Convalescent Plasma in Covid-19 Severe Pneumonia. N Engl J Med p. 384.
- Fang X, Mei Q, Yang T, Tong F, Geng S, et al. (2020) Low-dose corticosteroid therapy does not delay viral clearance in patients with COVID-19. J Infect 81: 147-178. [Crossref]
- Shang L, Zhao J, Hu Y, Du R, Cao B (2020) On the use of corticosteroids for 2019-nCoV pneumonia. Lancet 395: 683-684. [Crossref]
- World Health Organization. Corticosteroids for COVID-19 guideline.
- De Backer D, Azoulay E, Vincent JL (2020) Corticosteroids in severe COVID-19: a critical view of the evidence. Crit Care 24: 627. [Crossref]
- Nadkarni GN, Lala A, Bagiella E, Chang H, Moreno P, et al. (2020) Anticoagulation, Bleeding, Mortality, and Pathology in Hospitalized Patients With COVID-19. J Am Coll Cardiol 76: 1815-1826. [Crossref]
- TanriverdI E, ÇÖrtÜk M, Yildirim BZ, Chousein E, Turan D, et al. (2020) The use of hydroxychloroquine plus azithromycin and early hospital admission are beneficial in Covid-19 patients: Turkey experience with real-life data. Turk J Med Sci p. 51.
- Arshad S, Kilgore P, Chaudhry ZS, Jacobsen G, Wang D, et al. (2020) Treatment with hydroxychloroquine, azithromycin, and combination in patients hospitalized with COVID-19. Int J Infect Dis 97: 396-403. [Crossref]
- Cavalcanti AB, Zampieri FG, Rosa RG, Azevedo LCP, Veiga VC, et al. (2020) Coalition Covid-19 Brazil I Investigators. Hydroxychloroquine with or without Azithromycin in Mild-to-Moderate Covid-19. N Engl J Med. 383: 2041-2052.
- Self WH, Semler MW, Leither LM, Casey J, Angus D, et al. (2020) Effect of Hydroxychloroquine on Clinical Status at 14 Days in Hospitalized Patients With COVID-19: A Randomized Clinical Trial. JAMA p. 324. [Crossref]
- Rosenberg ES, Dufort EM, Udo T, Wilberschied L, Kumar J, et al. (2020) Association of Treatment With Hydroxychloroquine or Azithromycin With In-Hospital Mortality in Patients With COVID-19 in New York State. JAMA 323: 2493–2502. [Crossref]
- Magagnoli J, Narendran S, Pereira F, Cummings T, Hardin J, et al. (2020) Outcomes of hydroxychloroquine usage in United States veterans hospitalized with Covid-19. medRxiv
- Kalligeros M, Shehadeh F, Atalla E, Mylona E, Aung S, et al. (2020) Hydroxychloroquine use in hospitalised patients with COVID-19: An observational matched cohort study. J Glob Antimicrob Resist 22: 842-844. [Crossref]
- Lin SM, Liu KX, Lin ZH, Lin PH (2017) Does high-flow nasal cannula oxygen improve outcome in acute hypoxemic respiratory failure? A systematic review and meta-analysis. Respir Med 131: 58-64. [Crossref]
- Geng S, Mei Q, Zhu C, Yang T, Yang Y, et al. (2020) High flow nasal cannula is a good treatment option for COVID-19. Heart Lung 49: 444-445. [Crossref]
- Yamada T, Wakabayashi M, Yamaji T, Chopra N, Mikami T, et al. (2020) Value of leukocytosis and elevated C-reactive protein in predicting severe coronavirus 2019 (COVID-19): A systematic review and meta-analysis. Clinica Chimica Acta 509: 235-243. [Crossref]
- Huang HD, Jneid H, Aziz M, Ravi V, Sharma P, et al. (2020) Safety and Effectiveness of Hydroxychloroquine and Azithromycin Combination Therapy for Treatment of Hospitalized Patients with COVID-19: A Propensity-Matched Study. Cardiol Ther 9: 523-534. [Crossref]
- Mercuro NJ, Yen CF, Shim DJ, Maher T, McCoy C, et al. (2020) Risk of QT Interval Prolongation Associated With Use of Hydroxychloroquine With or Without Concomitant Azithromycin Among Hospitalized Patients Testing Positive for Coronavirus Disease 2019 (COVID-19). JAMA Cardiol 5: 1036-1041. [Crossref]
- Chorin E, Wadhwani L, Magnani S, Dai M, Shulman E, et al. (2020) QT interval prolongation and torsade de pointes in patients with COVID-19 treated with hydroxychloroquine/azithromycin. Heart Rhythm 17: 1425-1433. [Crossref]
- Saleh M, Gabriels J, Chang D, Kim BS, Mansoor A, et al. (2020) Effect of Chloroquine, Hydroxychloroquine, and Azithromycin on the Corrected QT Interval in Patients With SARS-CoV-2 Infection. Circ Arrhythm Electrophysiol 13: e008662.



