Struma ovarii is a monodermal teratoma consisting primarily of thyroid tissue. It represents less than 1.0% of ovarian tumors and has an intermediate risk of distant metastasis. It is frequently an incidental finding and hyperthyroidism symptoms are uncommon, making detection of the tumor difficult. Work-up of presumed cases includes pelvic ultrasound, radio imaging, and serum thyroglobulin monitoring. Treatment of benign and most malignant cases is cystectomy which has a favourable prognosis. However, biologically malignant teratomas often metastasize by the time of diagnosis, contributing to a poorer outcome. This report aims to highlight the importance of pathogenic cyst detection and appropriate management. We present a case report of a 35-year-old female who was admitted for abdominal pain. Her last menstrual period was 11 months ago, and visual disturbances were noted. Workup of the patient revealed a newly diagnosed 38 week pregnancy causing preeclampsia with severe features. Management with magnesium sulfate and a cesarean section was prompted. Intraoperatively, a 6cm complex right ovarian cyst was discovered. Cystectomy was performed and pathology was indicative of struma ovarii.
The teratoma was 6cm, consisted of both follicular and papillary thyroid carcinoma tissue, and had positive thyroid transcription factor-1 (TTF-1) immunostaining. The patient was found to have a normal TSH and lacked any clinical signs of thyroid hormone excess. Although incidental, this report illustrates the significance of immediate cystectomy and evaluation of cysts which are suspicious for malignancy. Workup and accurate diagnosis of struma ovarii includes analysis of histopathological cytoarchitecture and immunohistochemical markers such as TTF-1 immunostaining. It cannot be reliably diagnosed or ruled out by TSH and pelvic ultrasound. Prospective studies would investigate criteria for malignant cyst classification.
thyroid hormone, positive thyroid transcription factor-1, malignant cyst
Ovarian teratomas are the most common type of germ cell neoplasms and are pathologically classified into four categories: mature (dermoid cysts), immature (malignant), secondary neoplasms, and monodermal. Monodermal teratomas, such as the struma ovarii found in our patient, are highly specialized tumors in which one mature histologic cell type greatly predominates. These tumors can be benign or malignant. The most common of these tumors are struma ovarii and carcinoid tumors. Struma ovarii is a teratoma that predominantly consists of thyroid tissue. It is extremely rare, accounting for only 0.5% to 1.0% of all ovarian tumors [1-5]. The diagnosis of struma ovarii is made when greater than 50% of the tumor is composed of mature thyroid tissue. Grossly, a typical struma ovarii will resemble thyroid tissue in color and consistency. However, cystic variants can hinder accurate diagnosis. The tumor may show pathological features as well [2]. Clinical presentations of struma ovarii can vary based on the anatomic location of the tumor. It is often an incidental finding, and clinical features of hyperthyroidism are uncommon. Only 25% to 30% of women with struma ovarii present with thyrotoxicosis. Most clinical hyperthyroidism cases are in relation to a coexisting autoimmune hyperthyroidism disease, such as Graves’ disease. Much less commonly, in the absence of autoimmune hyperthyroidism, a struma ovarii tumor can produce thyrotoxicosis solely through the hormone secretions of the neoplasm itself. Therefore, a decreased TSH level and an elevated free T4 can help aid in the diagnosis, but absence of abnormal lab findings does not rule out struma ovarii. Typical findings on imaging are low uptake of radioiodine in the thyroid gland with notable and marked increased uptake in the pelvis [3]. As such, pelvic ultrasound and radioimaging should be obtained in hyperthyroidism cases suspected of struma ovarii. Similar to other ovarian neoplasms, struma ovarii may also manifest as ascites, an abdominal mass with peritoneal fluid, that may be bloody [1]. Rarely, patients will present with abnormal vaginal bleeding, as an effect of sex hormones altered by the tumor. Treatment of struma ovarii is typically surgical resection of the tumor [2].
A 35-year-old female presented to the emergency department with the chief complaint of generalized abdominal pain and a sensation of her water breaking. She admitted to abdominal pain with radiation to her right flank. Other than experiencing visual disturbances, her review of systems was unremarkable. Her last menstrual period was 11 months ago.
Upon admission, she was hypertensive with a blood pressure of 208/84 mmHg. All other vital signs were stable. Initial pregnancy test was positive. Transabdominal ultrasound revealed an intrauterine pregnancy measuring at 38 weeks and 0 days, and a fetal heart rate of 144 beats per minute. The patient was unaware of her pregnancy. She was also diagnosed with preeclampsia with severe features.
For immediate management, the patient was given magnesium sulfate for seizure prophylaxis. Due to the finding of suspected macrosomia and prior cesarean section, a trial of labor was not recommended. Repeat low transverse cesarean section was performed instead. During the procedure, the surgeon noted a 6cm complex right ovarian cyst. Due to the size and abnormal appearance, the cyst was excised in its entirety to avoid future torsion/rupture. The cyst was removed intact and sent to pathology for H&E and immunohistochemical staining. Histopathological results revealed predominant papillary carcinoma of thyroid tissue nature, consistent with struma ovarii (Figure 1).
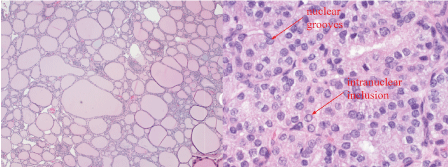
Figure 1. Section of ovarian cyst wall (10x left, 100x right). Nuclear crowding and overlapping, powdery chromatin, nuclear grooves and intranuclear inclusions consistent with papillary carcinoma of the thyroid.
Specimen was contained and surgical margins were clear of infiltrating thyroid tissue. The patient’s thyroid stimulating hormone (TSH) was found to be within normal range. One week postoperatively, a thyroid sonogram was unremarkable. The patient followed up with an oncology team. Future workup included a follow-up CT scan and pelvic sonogram as well as serial thyroid function studies.
Struma ovarii can occur at any age. Benign struma ovarii generally occur in women older than 40-years-old, while malignant struma ovarii typically peaks in the 5th and 6th decade of life [3]. However, our patient presented with a malignant struma ovarii at just 35 years old. Struma ovarii is usually an incidental finding, and less than 10% of women with struma ovarii present with symptoms of hyperthyroidism [3]. The teratoma found in our patient was found incidentally at the time of her cesarean delivery. She denied any previous symptoms of struma ovarii such as ascites, abnormal bleeding, palpitations, or classical clinical signs of thyroid hormone excess.
The average size of a struma ovarii tumor is 5 cm to 10 cm [4]. The patient’s tumor fell in this range, at 6 cm measured longitudinally. Struma ovarii tumors less than 4cm rarely metastasize and tumors greater than 4 cm have a propensity to be malignant. Malignant struma ovarii are extremely rare and account for less than 5% of the tumors [4].
The average size of a malignant struma ovarii tumor is 13 cm to 14 cm, but any mass greater than 5cm should be considered suspicious for malignant potential. The tumor in our patient was found to be a malignant papillary carcinoma. The ovarian cyst was reported to have non-invasive follicular architecture as well as some papillary features. This is consistent with a non-invasive follicular variant of papillary carcinoma. Papillary carcinoma is the most common type of thyroid cancer that arises in struma ovarii whereas follicular carcinoma is highly unlikely [5].
The mutation BRAF V600E is positive in 30% of papillary carcinomas with the classic papillary growth pattern and is occasionally seen in the follicular variant of papillary carcinoma [6]. Thyroid transcription factor-1 (TTF-1) is a nuclear transcription factor that is expressed in thyroid and respiratory epithelium. TTF-1 is specifically expressed in pulmonary and thyroid neoplasms. The teratoma of our patient was shown to be predominantly comprised of thyroid tissue, elucidated by positive TTF-1 immunostaining and folliculo-papillary architecture (Figure 2). CK19 and HBME1 which are highly discriminatory markers specific for papillary carcinomas and can also have positive immunostaining, aiding cytological diagnosis. Some struma ovarii may stain positive for epithelial marker PAX8 and lead to a misdiagnosis of ovarian epithelial tumor [5].
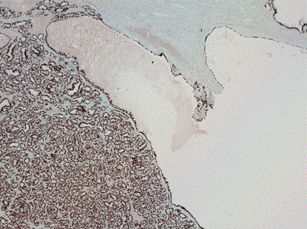
Figure 2. TTF-1 immunohistochemichal stain of the specimen. TTF-1 is positive in thyroid tissue (99% are positive).
The treatment of benign struma ovarii is surgical cystectomy or unilateral salpingo-oophorectomy. There are no standard guidelines for the treatment of malignant struma ovarii due to its scarcity. Radical treatment is rarely necessary since only 5% of struma ovarii metastasize. Even tumors diagnosed as malignant struma ovarii on histologic grounds are rarely aggressive or infiltrating and do not typically require aggressive treatment. Biologically malignant strumas frequently spread beyond the ovary at the time of diagnosis. The most common site of metastasis is locally to the peritoneum or omentum, but distant metastasis to bone [7], lymph nodes [8], lung [7], liver [9], spinal cord [10] and brain [11] are uncommon but well documented in the literature. If metastasis is found, treatment recommendations differ depending on the case, but include hysterectomy, bilateral salpingo-oophorectomy, thyroidectomy, radioactive iodine therapy, and long-term monitoring of serum thyroglobulin.
- Wu M, Hu F, Huang X, Tan Z, Lei C, et al. (2018) Extensive peritoneal implant metastases of malignant struma ovarii treated by thyroidectomy and 131I therapy: A case report. Medicine (Baltimore) 97: e13867. [Crossref]
- Fletcher CDM (2021) Diagnostic Histopathology of Tumors. (Fifth edn), Elsevier, Philadelphia, USA.
- Gild ML, Heath L, Paik JY, Clifton-Bligh RJ, Robinson BG (2020) Malignant struma ovarii with a robust response to radioactive iodine. Endocrinol Diabetes Metab Case Rep: 19-0130. [Crossref]
- Wee JY, Li X, Chern BS, Chua IS (2015) Struma ovarii: management and follow-up of a rare ovarian tumour. Singapore Med J 56: 35-39. [Crossref]
- Hamazaki S, Okino T, Tsukayama C, Okada S (2002) Expression of thyroid transcription factor-1 in strumal carcinoid and struma ovarii: an immunohistochemical study. Pathol Int 52: 458-462. [Crossref]
- Kebebew E, Weng J, Bauer J, Ranvier G, Clark OH, et al. (2007) The prevalence and prognostic value of BRAF mutation in thyroid cancer. Ann Surg 246: 466-471. [Crossref]
- Steinman RA, De Castro IO, Shrayyef M, Chengazi V, Giampoli E, et al. (2013) Two cases of malignant struma ovarii with metastasis to pelvic bone. Gynecol Obstet Invest 75: 139-144. [Crossref]
- Hatami M, Breining D, Owers RL, Del Priore G, Goldberg GL (2008) Malignant struma ovarii--a case report and review of the literature. Gynecol Obstet Invest 65: 104-107. [Crossref]
- Konez O, Hanelin LG, Jenison EL, Goyal M, Randolph W et al. (2000) Functioning liver metastases on an I-131 whole-body scan: a case of malignant struma ovarii. Clin Nucl Med 25: 465-496. [Crossref]
- McDougall IR, Krasne D, Hanbery JW, Collins JA (1989) Metastatic malignant struma ovarii presenting as paraparesis from a spinal metastasis. J Nucl Med 30: 407-411. [Crossref]
- Tokuda Y, Hatayama T, Sakoda K (1993) Metastasis of malignant struma ovarii to the cranial vault during pregnancy. Neurosurgery 33: 515-518. [Crossref]


