Abstract
Background: Diverting ileostomies performed for ultralow rectal anastomoses are frequently reversed upon completion of adjuvant treatment, ensuring the integrity of the rectal anastomosis. Although low anterior resection syndrome has been frequently described in the literature after ileostomy reversal, the opposite clinical phenomenon is rarely discussed. Significant colonic dysmotility has been observed in several patients after ileostomy reversal. Therefore, we present a series of five patients and discuss colonic dysmotility’s potential contributing aetiologies, thus raising awareness of this unusual complication.
Case Summary: Five patients were diagnosed with this rare complication over a five-year period between 2016 and 2020. These patients developed symptoms of persistent abdominal distension by post-operative day five of ileostomy reversal despite frequent bowel movements in the postoperative period. Both small-bowel and large-bowel dilatations were observed on radiological examination. They were primarily managed conservatively, either with recurrent flatus tube insertions or endoscopic decompression for symptoms relief. The patients were eventually discharged between 18-29 d after ileostomy reversal and continued to remain well. Unfortunately, patient B developed colonic perforation which led to death.
Conclusion: Colonic dysmotility after ileostomy reversal is a rare occurrence. Timely diagnosis with early decompression is essential to prevent colonic perforation.
Introduction
Surgery for rectal cancer remains the mainstay of treatment, with pre-operative chemoradiotherapy as an important adjunct for locally advanced tumours. Advances in technology and surgical techniques have allowed surgeons to perform sphincter-saving surgery with primary anastomoses. However, rectal anastomoses are still associated with a significant leak rate of 3%-24% [1]. As such, many surgeons routinely perform a diverting stoma to mitigate these leaks. Nonetheless, stomas are not without complications, which may significantly impair patients’ quality of life [2].
While closure of ileostomies is considered a relatively minor surgical procedure, it has been associated with significant morbidity rates of 3%-30% [3]. Commonly encountered complications include intestinal obstruction, intra-abdominal sepsis, wound infections, fistulas, and anastomotic leaks. After ileostomy closure, some patients report varying symptoms of low anterior resection syndrome (LARS) [4], which has been well described. However, a literature review describing patients with significant colonic dysmotility leading to distension and even perforation has been limited despite extensive research efforts.
In this series, we present our single-institution experience of five patients who developed colonic dysmotility after ileostomy closure to raise awareness about this rare condition which can be potentially life-threatening if not recognised early.
Case Presentation
Chief complaints: Five patients who developed colonic distension after ileostomy closure following ultra-low anterior resections were identified between August 2016 and September 2020. These patients were Chinese men aged 49-70 years. At baseline, they had good functional performance with an Eastern Cooperative Oncology Group score of 1, and American Society of Anesthesiologists grades of either 1 or 2.
History of present illness: Within five days following ileostomy closure, all five patients developed nausea associated with a bloated abdomen after diet escalation, despite frequent bowel movements.
History of past illness: These patients previously underwent minimally invasive ultra-low anterior resection for mid- to low-rectal adenocarcinomas, with laparoscopic surgery being performed in patient B, while the Da Vinci XI robotic platform was used in the other four patients. Four patients were diagnosed with locally advanced tumours requiring neoadjuvant treatment with either short-course radiotherapy or long-course chemoradiation, while patient B underwent upfront surgery for early T2 tumours. Notably, intersphincteric resection was performed in patients B and C to achieve adequate distal margins. The colorectal anastomosis was primarily performed with a stapler device in an end-to-end fashion in all patients, except in patient A on whom an end-to-side anastomosis was performed. Defunctioning ileostomy was performed under the same conditions for all patients. Postoperatively, all patients except patient B required adjuvant chemotherapy for high-risk stage two or stage three colorectal cancers.
Prior to elective closure of the defunctioning ileostomy, patients were routinely scheduled for flexible sigmoidoscopy and gastrograffin enema to assess the integrity of the colorectal anastomosis. A small anastomotic sinus defect was identified in patient C on repeat endoscopy, revealing granulation tissue suggestive of healing. This area of concern was subsequently reinforced with transanal sutures placed during the ileostomy closure. There were no demonstrable anastomotic complications on flexible sigmoidoscopy or contrast studies in the remaining four patients. This was an important criterion for all patients to be eligible for ileostomy closure.
Personal and family history: These patients do not have significant past medical or family histories.
Physical examinations: Physical examination revealed abdominal distension and nausea without abdominal tenderness.
Laboratory examinations: Clinical information pertaining to patients' laboratory examinations was not gathered.
Imaging examinations: Abdominal radiography demonstrated dilatation of the small and large bowel up to the neorectum, suggestive of ileus (Figure 1). Computed tomography of the abdomen and pelvis revealed uniform small and large bowel dilatation up to the neorectum, without radiological evidence of distal mechanical obstruction as per clinical concern (Figure 2).
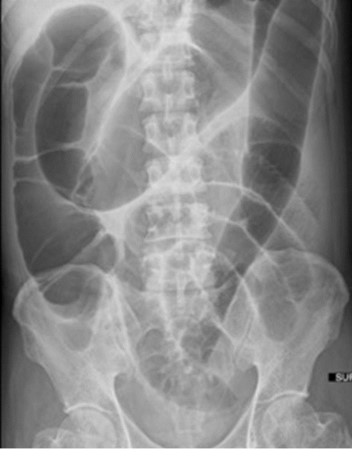
Figure 1: Abdominal X-ray showing small and large bowel dilatation of the neorectum.
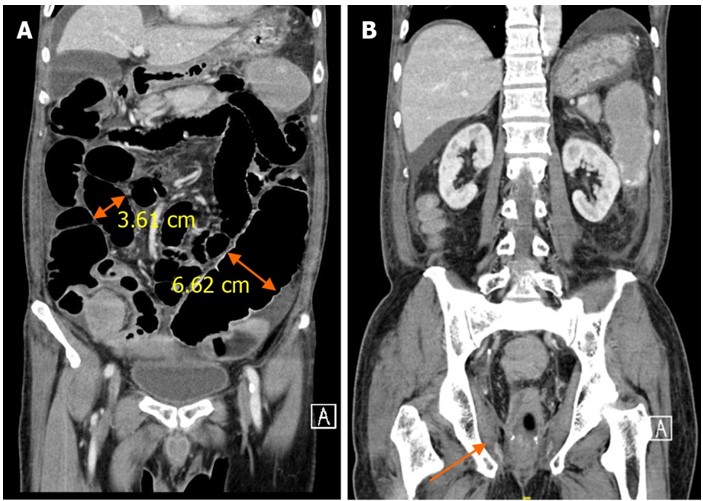
Figure 2: Computed tomography image of the abdomen and pelvis on coronal axis. A: Dilated small (3.61 cm) and large (6.62 cm) bowel loops; B: Smooth tapering of bowel towards the neorectum (highlighted by the arrow).
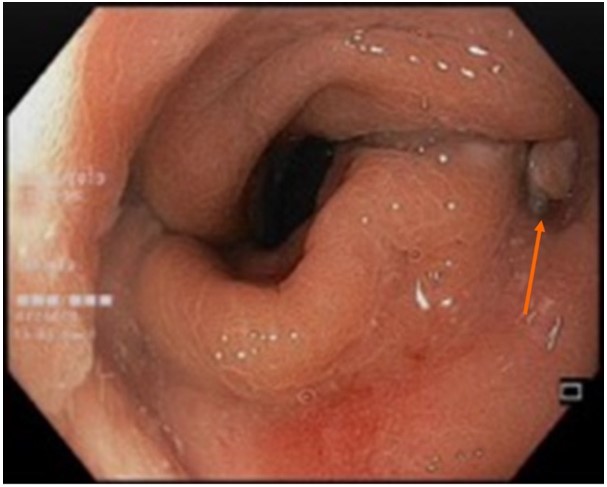
Figure 3: Endoscopic image of a small anastomotic sinus (pointed by the arrow).
Final Diagnosis
Given the concordant clinical and radiological findings, initially suggestive of postoperative ileus, the subsequent presence of frequent bowel movements pointed toward the possibility of colonic dysmotility and pseudo-obstruction, a distinct clinical entity altogether.
Treatment
Patients fasted, and both nasogastric and flatus tubes were inserted for proximal and distal decompression. Flexible sigmoidoscopy was performed if bedside flatus tube insertion was unsuccessful in decompressing the dilated colon. Patients were also started on peripheral nutrition owing to prolonged ileus episodes. Stool bulking agents and prokinetic motility agents such as prucalopride were initiated to facilitate bowel motility and transit. However, while these medications failed to demonstrate drastic clinical improvements, colonic motility gradually returned in patients C, D, and E, with subsequent development of LARS to varying degrees. Patient A required surgical management with the creation of a diverting colostomy for persistent non-resolving symptoms, while patient B developed septic shock from a pinpoint bowel perforation and subsequently died.
Outcome And Follow-Up
Patients A, D, and E developed varying degrees of anastomotic complications at the previous colorectal anastomosis after ileostomy closure (Table 1), despite normal pre-operative results from pre-operative flexible sigmoidoscopy and contrast studies. These complications were primarily very small sinuses seen on endoscopy (Figure 3), without obvious pelvic collections on cross-sectional imaging. Nonetheless, these patients were primarily managed conservatively with flatus tube insertions, except for patient A, who required a diverting colostomy due to persistent colonic distension with subsequent development of a recto-prostatic fistula.
Table 1: Summary of patients’ baseline oncological history, presenting history, management, and outcomes.
|
Patient A |
Patient B |
Patient C |
Patient D |
Patient E |
Demographics |
54-year-old male ASA 1 |
70-year-old male ASA 2 |
69-year-old male ASA 2 |
67-year-old male ASA 1 |
49-year-old male ASA 1 |
Primary tumour |
Mid rectal tumour, 7 cm from anal verge |
Low rectal tumour, 3 cm from anal verge |
Low rectal tumour, 4 cm from anal verge |
Mid rectal tumour, 8 cm from anal verge |
Low rectal tumour, 4 cm from anal verge |
Neoadjuvant treatment |
Long course chemoradiation |
Short course radiation |
Long course chemoradiation |
Long course chemoradiation |
Long course chemoradiation |
Operative details |
Robotic ULAR |
Laparoscopic ULAR with ISR |
Robotic ULAR with ISR |
Robotic ULAR |
Robotic ULAR |
Adjuvant treatment |
Adjuvant chemotherapy |
Nil |
Adjuvant chemotherapy |
Adjuvant chemotherapy |
Adjuvant chemotherapy |
Time to reversal (mo) |
7 |
4 |
12 |
10 |
8.5 |
Summary of hospitalisation progress and outcome |
Medical therapy with stool bulking and prokinetic agents. Multiple endoscopic guided flatus tube insertions were attempted without resolution of symptoms. Eventually required defunctioning colostomy due to failure with conservative measures |
Medical therapy with stool bulking and prokinetic agents. Repeated bedside flatus tube insertions were required without resolution of symptoms. Developed septic shock from perforated caecum on post-operative day 12, complicated by cardiac arrest intra-operatively and subsequent demise |
Medical therapy with stool bulking and prokinetic agents. Repeated bedside flatus tube insertions were performed with resolution of symptoms. Discharged on post-operative day 29 |
Medical therapy with stool bulking and prokinetic agents. Repeated bedside flatus tube insertions were performed with resolution of symptoms. Discharged on post-operative day 18 |
Medical therapy with stool bulking and prokinetic agents. Repeated bedside flatus tube insertions were performed with resolution of symptoms. Discharged on post-operative day 22 |
Anastomotic complications |
Post-reversal sigmoidoscopy revealed multiple anastomotic sinuses with recto-prostatic fistula. Required subsequent trans-anal suture repair |
Not known; demised |
Small anastomotic sinus repaired trans-anally at the same sitting as ileostomy reversal.
Post-reversal endoscopy confirmed resolution of anastomotic dehiscence. |
Post reversal sigmoidoscopy noted a small perianastomotic sinuses at 3 and 9 o’clock positions
Required subsequent trans-anal suture repair |
Post reversal sigmoidoscopy noted a small anastomotic sinus at 12 o’clock. Resolved with conservative management |
Final staging1 |
ypT2N0 |
ypT2N0 |
ypT3N2a |
ypT3N1a |
ypT2N0 |
1In accordance with American Joint Committee on Cancer (AJCC) 8th edition. ypTNM: Extent of cancer after neoadjuvant therapy followed by planned post-treatment surgery [20].
ASA: American Society of Anaesthesiologists; ULAR: Ultralow anterior resection; ISR: Inter-sphincteric resection
Patient B developed septic shock due to a caecal perforation. He was initially managed with nasogastric decompression and required repeated flatus tube insertions at the bedside. Although he developed a mild persistent abdominal distension despite regular bowel movements, his abdomen remained soft and without signs of peritonism on physical examination. However, the patient developed septic shock on postoperative day 12, and chest radiography revealed intraperitoneal free air. Thus, he was brought to the operating theatre for emergency laparotomy, during which a pinpoint perforation over the mid-ascending colon was identified. Unfortunately, the patient developed cardiac arrest intraoperatively and resuscitation efforts were unsuccessful.
Management principles: While ileus is not an uncommon complication after ileostomy reversal, these patients differ clinically as they were able to evacuate their bowels regularly despite their physical and radiological findings being similar to those observed in postoperative ileus. Therefore, it is important to recognise this condition soon and initiate early treatment.
Decompression of the bowel is essential to manage this condition. Mechanical obstruction is typically absent, as these patients would have recently undergone endoscopic and radiological investigations to assess anastomosis prior to reversal. Decompression of the large bowel may be performed with either bedside insertion of a flatus tube or endoscopic insertion of a guided tube, if required. This helps relieving colonic distension and reduces the risk of perforation while allowing the bowel to rest and regain motility. In patients with persistent symptoms, despite conservative management, a defunctioning stoma may be required.
Lastly, we suggest re-assessment of the anastomosis as small sinus defects may be present, contributing to prolonged periods of ileus and colonic dysmotility, although these may not be present in pre-operative investigations. In our series, we noticed an association between anastomotic complications and the development of colonic dysmotility, and most of them were not detected prior to ileostomy reversal.
Discussion
LARS has been frequently described in the literature as a constellation of symptoms, including frequent bowel movements and incontinence. However, colonic dysmotility with bowel dilatation proximal to the previous colorectal anastomosis is rarely encountered in patients who have previously undergone ultralow anterior resection. It manifests after defunctioning ileostomy closure, meant to restore colonic function after a period of prolonged disuse. In this case series, the patients collectively presented with persistent colonic distension proximal to the neorectum, despite the absence of distal obstruction in radiographic images. Notably, these patients had persistent abdominal distension and were unable to empty their bowels effectively, despite frequent loose bowel movements. Recent reports of dysmotility leading to pseudo-obstruction in this subset of patients are scarce. Nonetheless, repeated decompression is essential; when this fails, there is a risk of colonic perforation, as seen in patient B.
Defunctioning stomas are commonly performed for various medical conditions. It is well established that faecal diversion leads to atrophic and inflammatory changes in the colon, which responds to treatment with glutamine and short-chain fatty acids [5] which are utilised by colonocytes. Patients with prolonged faecal diversion are at risk of diversion colitis and chronic inflammation, with studies suggesting a resultant increase in nitrate-reducing bacteria and the production of toxic nitric oxide by pathogenic bacteria [4]. Although restoration of intestinal continuity appears to be the most effective treatment, Szczepkowski, et al. [6] demonstrated persistent inflammation-related histological changes several years after restoration. In our series, only patients C and D underwent biopsies during endoscopy because of erythematous mucosa, suggestive of possible inflammation. However, these biopsies showed regenerative changes, without significant inflammatory signs.
Multiple studies on bowel dysfunction after sphincter-preserving surgery for rectal cancer have been conducted. However, these studies primarily emphasised LARS-type symptoms affecting patients’ quality of life. Meanwhile, studies on impaired colonic motility causing acute colonic pseudo-obstruction after rectal surgery have been limited.
Nowakowski, et al. [7] identified protective ileostomies as a risk factor for the development of LARS and suggested early ileostomy closure within six months. This suggests that the risk of colonic dysfunction may increase with a longer period of disuse. In our series, four out of five patients underwent reversal ileostomy beyond 6 mo because of the need to complete adjuvant chemotherapy.
Besides the negative effects of diversion on colonic function, prolonged muscular inactivity of the pelvic floor and sphincter complex may further contribute to impaired emptying, resulting in pseudo-obstructions. In addition, pelvic dissection in patients with ultralow anterior resections also frequently results in neural injury. Some studies have postulated that abnormal neural regeneration may also contribute to colonic dysfunction [8]. The colonic wall forming the neorectum is also thinner, with reduced contraction ability, compared to a normal rectal wall [9]. The ascending fibres of the pelvic plexus and descending fibres of the inferior mesenteric plexus supply the descending colon; however, these are sacrificed during transection of the colon and inferior mesenteric artery [4]. These factors may predispose patients to impaired colonic function and possible pseudo-obstruction.
Another contributing factor may be radiotherapy. All patients in this series received pre-operative radiotherapy. Previous studies have shown that radiation leads to fibrosis, which in turn may lead to impaired neorectal function. Bregendahl, et al. [10] demonstrated neorectal hyposensitivity in patients who received neoadjuvant radiation, possibly because of impaired afferent nerve function. Ihnát, et al. [11] demonstrated that radiotherapy significantly impairs the functional outcomes of patients in manometry studies.
A recently published Japanese case of megacolon after ileostomy reversal described a patient who developed colitis after ileostomy reversal for low anterior resection. Eventually, total colectomy was necessary because of persistent constipation and colonic distension. Histology revealed isolated hypoganglionosis secondary to acquired isolated hypoganglionosis (AIHG). This condition is rare, and there is no consensus on its diagnostic criteria [12] and exact mechanism [13,14]. Nonetheless, it requires a histological diagnosis demonstrating reduced ganglion cells, degeneration and ganglionosis of myenteric ganglion cells [15,16], and decreased activity of acetylcholinesterase in the lamina propria [15]. Recently, immunohistochemical staining has been proposed to facilitate AIHG diagnosis [17,18]. It is possible that ongoing colitis may have damaged the ganglion cells within the Auerbach’s plexus, resulting in hypoganglionosis, giving rise to dysmotility. While this case presented in an alarmingly similar fashion to that of our patients, none of the histologies from our series were conclusive for hypoganglionosis.
Finally, pelvic sepsis and inflammation could similarly contribute to the development of our patients’ symptoms, as four out of the five of them developed anastomotic complications, ranging from a small sinus to a leak with collection. Bittorf, et al. [19, 20] reported no difference in functional outcomes after rectal anastomotic leakage; these mainly focused on LARS-type symptoms. Given that most patients in this series eventually developed anastomotic complications, we postulate that inflammation and pelvic sepsis may lead to worsening fibrosis and decreased neorectum compliance, which might have contributed to the insurgence of pseudo-obstructions. In addition, these anastomotic complications were not apparent before defunctioning ileostomy reversal even with routine pre-operative endoscopy and contrast studies.
This case series is not without its limitations. First, as a case series from a single tertiary institute, the small number of patients observed may not be sufficient to represent or identify the disease characteristics and aetiologies fully. Second, as a retrospective series, information and recall biases were invariably present. Nonetheless, within the limitations of our case series, we seek to address clinicians and inform them about this clinical scenario, thus enabling further discussion on optimal management principles.
Conclusion
Colonic pseudo-obstruction after ileostomy closure in patients who have previously undergone low rectal cancer surgery is a rare but important complication. The pre-operative radiation treatment and anastomotic complications may contribute to its occurrence. Early recognition of this complication is essential to perform decompression timely, which is necessary to prevent colonic perforation. Furthermore, it is important to recognise that although these patients frequently have loose bowel movements, this may falsely reassure clinicians against the need to perform colonic decompression.
Author contributions
Tan MNA and Tham HY organised the information, performed the literature review, and drafted the manuscript; How KY and Wong KY provided guidance and supervised and validated the content of the manuscript; all authors have read and approved the final manuscript.
Informed consent statement
Informed consent was waived by the ethics board owing to the use of anonymous, de-identified data.
Conflict-of-interest statement
The authors declare that they have no conflicts of interest.
References
- Pisarska M, Gajewska N, Małczak P, Wysocki M, Witowski J, et al. (2018) Defunctioning ileostomy reduces leakage rate in rectal cancer surgery - systematic review and meta-analysis. Oncotarget 9: 20816-20825. [Crossref]
- Hanna MH, Vinci A, Pigazzi A (2015) Diverting ileostomy in colorectal surgery: when is it necessary? Langenbecks Arch Surg 400: 145-152. [Crossref]
- Sharma A, Deeb AP, Rickles AS, Iannuzzi JC, Monson JR, et al. (2013) Closure of defunctioning loop ileostomy is associated with considerable morbidity. Colorectal Dis 15: 458-462. [Crossref]
- Iizuka I, Koda K, Seike K, Shimizu K, Takami Y, et al. (2004) Defecatory malfunction caused by motility disorder of the neorectum after anterior resection for rectal cancer. Am J Surg 188: 176-180. [Crossref]
- Pathak M, Srinivas M, Shariff A (2017) Prevention of Histological Changes after Colonic Diversion in Rats: An Experimental Study. J Neonatal Surg 6: 26. [Crossref]
- Szczepkowski M, Banasiewicz T, Kobus A (2017) Diversion colitis 25 years later: the phenomenon of the disease. Int J Colorectal Dis 32: 1191-1196. [Crossref]
- Nowakowski MM, Rubinkiewicz M, Gajewska N, Torbicz G, Wysocki M, et al. (2018) Defunctioning ileostomy and mechanical bowel preparation may contribute to development of low anterior resection syndrome. Wideochir Inne Tech Maloinwazyjne 13: 306-314. [Crossref]
- Park YY, Yang SY, Han YD, Cho MS, Hur H, et al. (2019) Predictive Factors for Bowel Dysfunction After Sphincter-Preserving Surgery for Rectal Cancer: A Single-Center Cross-sectional Study. Dis Colon Rectum 62: 925-933. [Crossref]
- Cheong C, Oh SY, Choi SJ, Suh KW (2019) Ultralow Anterior Resection and Coloanal Anastomosis for Low-Lying Rectal Cancer: An Appraisal Based on Bowel Function. Dig Surg 36: 409-417. [Crossref]
- Bregendahl S, Emmertsen KJ, Fassov J, Krogh K, Zhao J, et al. (2013) Neorectal hyposensitivity after neoadjuvant therapy for rectal cancer. Radiother Oncol 108: 331-336. [Crossref]
- Ihnát P, Slívová I, Tulinsky L, Ihnát Rudinská L, et al. (2018) Anorectal dysfunction after laparoscopic low anterior rectal resection for rectal cancer with and without radiotherapy (manometry study). J Surg Oncol 117: 710-716. [Crossref]
- Orset G, Lebreton E, Assouline A, Giordano P, Denis F, et al. (1991) The axial orientation of the phalanges following the curling up of the fingers. Ann Chir Main Memb Super 10: 101-107. [Crossref]
- Aldossary MY, Privitera A, Elzamzami O, Alturki N, Sabr K (2018) A Rare Case of Adult-Onset Rectosigmoid Hypoganglionosis. Am J Case Rep 19: 557-561. [Crossref]
- Qadir I, Salick MM, Barakzai A, Zafar H (2011) Isolated adult hypoganglionosis presenting as sigmoid volvulus: a case report. J Med Case Rep 5: 445. [Crossref]
- Schärli AF, Sossai R (1998) Hypoganglionosis. Semin Pediatr Surg 7: 187-191. [Crossref]
- Watanabe Y, Kanamori Y, Uchida K, Taguchi T (2013) Isolated hypoganglionosis: results of a nationwide survey in Japan. Pediatr Surg Int 29: 1127-1130. [Crossref]
- Yoshimaru K, Taguchi T, Obata S, Takemoto J, Takahashi Y, et al. (2017) Immunostaining for Hu C/D and CD56 is useful for a definitive histopathological diagnosis of congenital and acquired isolated hypoganglionosis. Virchows Arch 470: 679-685. [Crossref]
- Park SH, Min H, Chi JG, Park KW, Yang HR, et al. (2005) Immunohistochemical studies of pediatric intestinal pseudo-obstruction: bcl2, a valuable biomarker to detect immature enteric ganglion cells. Am J Surg Pathol 29: 1017-1024.
- Bittorf B, Stadelmaier U, Merkel S, Hohenberger W, Matzel KE (2003) Does anastomotic leakage affect functional outcome after rectal resection for cancer? Langenbecks Arch Surg 387: 406-410. [Crossref]
- Brierley JD, Greene FL, Sobin LH, Wittekind C (2006) The "y" symbol: an important classification tool for neoadjuvant cancer treatment. Cancer 106: 2526-2527.



