The therapeutic use of a single molecule(s) for efficacious induction of bone regeneration may be ineffective owing to the various conditions prevailing in bone defect sites. Therefore, a combination therapy for stable bone regeneration has been developed. One of the RANKL-binding peptide was W9 (YCWSQYLCY). It was known to enhance osteoblast differentiation. Contrastingly, basic fibroblast growth factor (bFGF) promotes bone formation by enhancing osteoblast proliferation. This study was aimed at investigating the ability of co-administration of W9 and bFGF. Proliferation and differentiation assays were performed in vitro. A mesenchymal cell line was used in evaluating effects of the bFGF and W9 combination (bFGF/W9) on proliferation. One reason for using a mesenchymal stem cell line is that in primary osteoblasts W9 does not increase proliferation and only differentiation. Primary osteoblasts were used in evaluating the effect of bFGF/W9 on differentiation. W9 enhanced the bFGF-induced increase in proliferation and early osteoblast differentiation marker level. However, bFGF/W9 was not effective as a late osteoblast differentiation marker. For in vivo analyses, Wistar rats were used, and gelatin hydrogel containing bFGF with or without W9, was applied to the calvarial defect. The rats were sacrificed, and newly formed bones were analyzed radiographically and histologically. The bFGF/W9 group showed bone formation compared to other groups. Taken together, it was suggested that bFGF/W9 stimulates bone regeneration in post-operative bone defects of the maxillofacial region.
bone formation, bFGF, RANKL-binding peptide, combination therapy, rat calvarial defect model
For appropriate bone regeneration, the use of single molecule(s) in therapy may be ineffective because of the variety of conditions present at the bone defect sites. When the size of the bone defect exceeds larger than a critical size, a long period of time is required to regenerate bone if single molecule(s) is used. Additionally, there are concerns regarding the safety and practicality of growth factors, and side effects such as abnormal immune reactions occur [1,2]. Furthermore, various agents act not only osteogenesis but also angiogenesis, vasculogenesis and inflammation modulation [1,3-6]. So, it is considered desirable that two kinds of agents are used for bone regeneration [7]. Therefore, the effects of the co-administrations of bone forming agents such as bFGF, prostaglandin E2 agonist, BMP-2, TGF-β1, and bone-forming peptides has been investigated toward clinical applications [8-13]. Among those bone forming agents, a peptide drug with a molecular weight around 1,000 ~ 2,000 is expected as a suitable candidate for development of bone-anabolic drugs since it combines the merits of both low-molecular-weight drugs and antibody-drugs; high specificity to drug target, low adverse effects, feasibility for inhibiting protein-protein interactions, availability of synthesizing agent, and low production cost [14]. Especially the in vivo effects of bFGF on bone formation are apparent, its clinical usage have been studied intensively in animal experiment. Recently, W9 is found to promote bone formation by promoting osteoblast differentiation [15-17]. It was originally designed to mimic the critical binding site of tumor necrosis factor (TNF)-α on TNF type1 receptor, and binds the receptor activator of NF-κB ligand (RANKL), thereby inhibiting osteoclastogenesis [18]. On the other hand, bFGF is known to stimulate bone formation by promoting osteoblast proliferation [19,20]. Therefore, we hypothesize that co-administrations of bFGF and W9, the proliferation- and the differentiation-stimulators of osteoblasts, could be an appropriate combination for bone regeneration. In this study, we investigated the effects of co-administration of W9 and bFGF on cell proliferation, osteoblast differentiation and bone regeneration using rat calvarial defect model.
Reagents
W9 peptide (YCWSQYLCY) and the control peptide (CtrP; YCWSQNLCY) were purchased from the American Peptide Company (Sunnyvale, CA, USA). These peptides have similar size and structure, but CtrP binds to RANKL with a lower affinity than W9 [21]. In addition, bFGF was provided by Kaken Pharmaceutical Co. Ltd. (KAKEN, Tokyo, Japan). Gelatin hydrogel (GH) sheets (type pI5) and GH granules (type pI9) were used as bFGF and peptide carriers, respectively [22,23]. However, in the GH-W9 group, W9 was dropped onto GH sheets (type pI9) instead of GH granules to prevent outflowing from the defect site.
Cell culture
Proliferation and differentiation assays were conducted in vitro. In the proliferation assay, the mesenchymal cell line, C3H10T1/2 (a murine pluripotent mesenchymal cell line [RIKEN, Ibaraki, Japan]) was used to evaluate effects of combination therapy with bFGF/W9. These cells were seeded at a density of 5 × 103 cells/well in a 96-well plate, and cultured in basal medium eagle (BME; Life Technologies/Gibco, New York, USA) containing 10% fetal bovine serum (FBS; Hana-Nesco Bio, Brisbane, Australia), 100 U/ml penicillin, and 100 μg/ml streptomycin (Sigma-Aldrich) in the presence or absence of either 1 ng/ml bFGF, 200 μM W9, or 200 μM CtrP for 48 h. Cell Count Reagent SF (Nacalai Tesque, Inc., Kyoto, Japan) was added to each well, cells were incubated for 2 h, and the optical density of each well was measured at 450 nm using a microplate reader (iMarkTM Microplate Reader; Bio-Rad, Hercules, CA, USA).
The differentiation assay was performed as described previously [24]. Briefly, primary osteoblasts isolated from calvariae of one-day-old mice were seeded at a density of 5 × 104 cells/well in a 24-well plate (3.6 cm2/ each well, Sumitomo Bakelite Co., ltd.) with either 10 ng/ml bFGF, 200 μM W9, or 200 μM CtrP. The alkaline phosphatase (ALP) staining was performed on day 6, and the von Kossa staining was performed on day 21 of osteoblast culture, and the ALP- and von Kossa-positive areas were measured using an ImageJ analysis system [25].
Animals
Twenty 9-week-old male Wistar rats were used (weight: 270–310 g; Sankyo Labo Service, Tokyo, Japan). All experimental protocols were reviewed and approved by the animal research committee of the Tokyo Medical and Dental University (Tokyo, Japan; authorization number 0170324A). The rats were housed in individual stainless steel cages under controlled temperature conditions, with free access to standard rat feed and water.
Rat calvarial defect model
Before the procedures, rats were anesthetized with an intraperitoneal injection of pentobarbital sodium (30 mg/kg). The skin around the surgical area was shaved, and cleaned with 70% ethanol. Bleeding was suppressed by using local infiltration anesthesia (2% xylocaine, 1:80, 000 adrenaline).
Twenty rats were randomly divided into five groups (n = 4) based on materials incorporated on GH discs: 1) carrier alone; 2) bFGF (25 µg); 3) W9 (0.56 mg); 4) bFGF (25 µg) + W9 (0.56 mg); 5) bFGF (25 µg) + CtrP (0.56 mg). The GH disc containing each reagent(s) was placed on a calvarial critical-size defect (5 mm in diameter) on the right parietal bone, generated using a biopsy punch (Kai Industries, Gifu, Japan). However, bFGF and W9 have a different carrier type and were administered using 2 types of GH: the GH sheet with bFGF was placed in a previously generated defect, onto which a GH granule with W9 or CtrP was dropped. All rats were subcutaneously injected with calcein (10 mg/kg) and alizarin (30 mg/kg), on day 15 and 20, respectively. Twenty-eight days after surgery, all rats were sacrificed after overdose of pentobarbital (100 mg/kg). Subsequently, the cranial bone of rats was removed and fixed in PBS-buffered formaldehyde (3.7%) solution (pH 7.4) for 2 days at 4°C, washed with PBS for 1 day, and used for radiographic analysis.
Radiographic assessment of the calvarial defect
Three-dimensional reconstruction images of newly formed bones at the defective areas were obtained by micro-computed tomography (μCT) (Scan Xmate-E090; Comscan Tecno, Yokohama, Japan). Area of newly formed bone at the bone defects was calculated from μCT-reconstructed images by using an ImageJ analysis system [25].
Histological assessments and bone histomorphometry of the calvarial defect
For histological analysis, undecalcified frozen sections (5-μm thick) were prepared using a microtome (CM3050sIV; Leica Biosystems, Nussloch, Germany), as described previously [26]. Images of fluorescently labeled sections were used to confirm the form of bone formation. Bone histomorphometry was performed in a rectangular region of interest (3.88×5.50 mm2) of the whole defective site. Mineralizing surface (MS) and mineral apposition rate (MAR) were measured using a KS400 image analysis system, as previously described [24,27]. Local bone formation activity was defined as multiplication of MS and MAR.
Statistical analysis
Data are presented as mean ± standard deviation (SD). For both in vivo and in vitro studies, the statistical significance of differences among groups was assessed using one-way ANOVA. Fisher’s PLSD post hoc test was performed upon detection of significant F-values. P < 0.05 was considered to be statistically significant.
The bFGF/W9 combination promoted early osteoblast differentiation
Osteoblast culture was initiated with only bFGF, and W9 was administered after 3 and 5 days. ALP staining was performed on day 6 (Figure 1A). The ALP-positive area of the bFGF/ (after using W9) group was not significantly larger than that of the bFGF group. The ALP- and von Kossa-stained areas were measured respectively after 6 and 21 days to evaluate differentiation
of primary osteoblasts (Figures 1B & 1C). W9 increased the bFGF-induced ALP-positive area upon simultaneous addition of W9 and bFGF to the same well (Figure 1B). However, the bFGF/W9 combination group was not effective as per the von Kossa-positive area (Figure 1C). These additive effects were suppressed by CtrP, which binds RANKL with a lower affinity than W9.
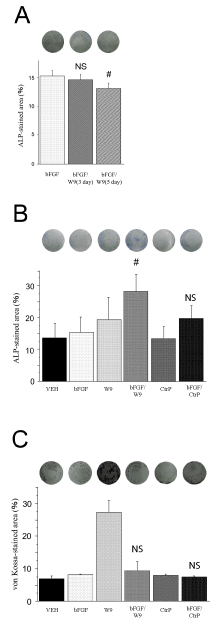
Figure 1. RANKL-binding peptide W9 enhanced early osteoblast differentiation. Primary osteoblasts were seeded with 5 × 104 cells/well. W9 and control peptide were used at a concentration of 200 μM, and bFGF was used at a concentration of 10 ng/ ml. (A) Representative image of experiments in which W9 was added into bFGF on days 3 and 5. The percentage of ALP-stained area was calculated in each well. The data are expressed as the means ± SD (n=3 for each group). (B) ALP-positive primary osteoblasts in culture on day 6. The osteoblasts were fixed and ALP staining was performed. Images of the ALP-staining cells were shown. The percentage of ALP-stained area was calculated in each well. (C) Cells were cultured for 21 days and von Kossa staining was performed. Images of the von Kossa-staining cells were shown. The percentage of von Kossa-stained area was calculated in each well. The data are expressed as the means ± SD (n=6 for each group). bFGF: bFGF group, bFGF/W9: bFGF and W9 group, CtrP: control peptide group, bFGF/CtrP: bFGF and the control peptide group, VEH: vehicle control group. NS: no significant difference. # P < 0.05 vs. bFGF; NS vs. bFGF.
In vitro assays suggested that W9 may promote bFGF-induced osteogenesis by promoting mesenchymal cell proliferation and early osteoblast differentiation.
W9 stimulated bFGF-induced cell proliferation
Mesenchymal cell line proliferation was evaluated in vitro. In our experiment, we used condition medium (CM), which constituted BME with 10% FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin in all groups. We performed two types of experiments—one, wherein bFGF and W9 were added simultaneously, and the other, wherein W9 was added one day later.
The bFGF/W9 combination group showed enhanced C3H10T1/2 cell proliferation on day 2 in the former experiment (Figure 2), but not in the latter. Furthermore, CtrP did not enhance bFGF-induced proliferation.
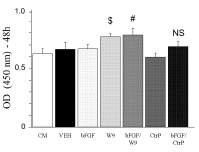
Figure 2. RANKL-binding peptide W9 enhanced bFGF-induced mesenchymal cell proliferation for 48h. C3H10T1/2 cells were seeded with 5 × 103 cells/well. Cell proliferation was assayed using Cell Count Reagents SF. W9 and Control peptide were used at a concentration of 200 μM, and bFGF was used at a concentration of 1 ng/ ml. The data are expressed as the means ± SD (n=5 for each group). CM: condition medium group. $ P < 0.05 vs. CM; # P < 0.05 vs. bFGF; NS: no significant difference.
These results suggested that these two agents are effective when used simultaneously. In this experiment, it was impossible to obtain a significant difference between the CM group and the bFGF group, but it was able to show the tendency of proliferation.
W9 stimulated bone formation at the rat calvarial defect site
Rats were sacrificed on day 28 post-operation, and local bone formation on a calvarial critical-size defect was first analyzed radiologically. Micro CT images revealed that the bFGF group showed local bone formation near the defect margins (Figure 3A). W9 group and carrier alone group did not show increased bone formation in defective areas. The bFGF/W9 combination
greatly enhanced marginal and bone formation in defective areas. However, CtrP seemed to have a less stimulatory effect on bFGF-induced bone formation. Newly formed bone area (%) at the defect site was calculated by using an ImageJ analysis system as previous described [25]. Newly formed bone area was significantly higher bFGF/W9 group than in the other experimental groups (Figure 3B).
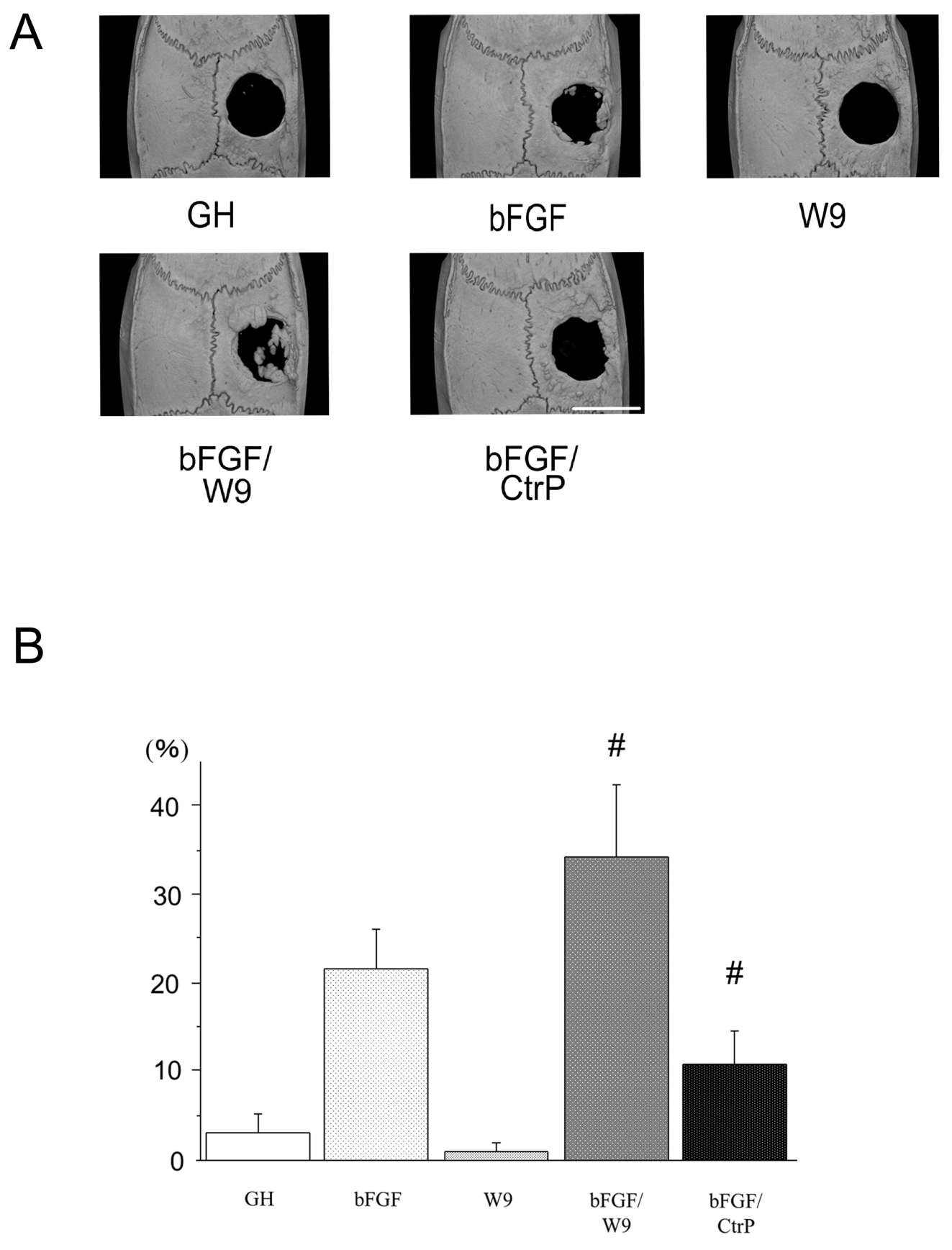
Figure 3. RANKL-binding peptide W9 promotes bFGF-induced bone formation in a rat calvarial defect model. (A) Micro-computed tomography (μCT) reconstruction images of the whole mount of calvariae. Scale bar represents 5 mm. (B) The percentage of newly formed bone area in defect was shown. The data are expressed as the means ± SD (n=4 for each group). # P < 0.05 vs. bFGF.
The local bone formation was histologically analyzed. To visualize sequential changes in local bone formation at the calvarial defect site, fluorescent dyes—calcein and alizarin—were subcutaneously injected on day 15 and 20 post-operation, respectively. The results of HE staining were similar to the result of fluorescent labeling, whereby HE-stained area for bFGF/W9 group was only detected ectopically-like bone formation in all groups (Figures 4A & 4B). In addition, local bone formation activity, which indicates the total calcified area that is produced in the region of interest in a single day, also showed similar results to those of the mineralizing surface (Figure 4C).
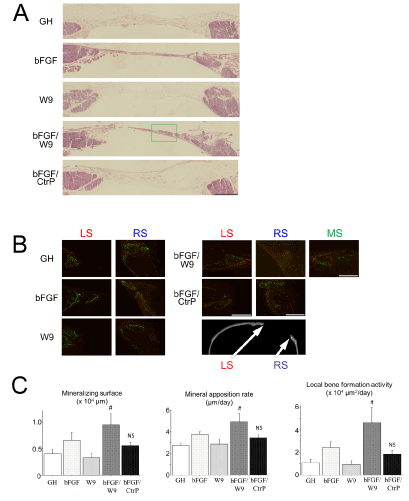
Figure 4: RANKL-binding peptide W9 could promote bFGF-induced bone formation in vivo. All sections were prepared to pass through the center. (A) Representative HE staining images of decalcified frozen sections of defect in the right side of calvaria. Scale bar represents 1 mm. (B) Representations of the fluorescently stained decalcified frozen sections of the calvarial defect. The panels indicate higher magnification views of the LS, RS and green frame (denoted as MS; middle side). Marginal bones are depicted as the red side (LS) and the blue side (RS). Center bone is only depicted in MS of the FGF/W9 group. Calcein labeling (administered on day 15) is shown in green, and alizarin labeling (administered on day 20) is shown in red. Representations of the staining are shown. Scale bar represents 500 µm. (C) Quantitative analyses of bone formation activity were performed using standard bone histomorphometric measurement techniques based on the calcein- and alizarin red-labeled surface in the ROI (as described in Materials and methods section), centering on the area of regenerated bone. Local bone formation activity was calculated as (mineralizing surface) × (mineral apposition rate). The data are expressed as the means ± standard deviation for each group (n=4). # P < 0.05 vs. bFGF.
W9-induced augmentation of cell proliferation may be involved in stimulation of early osteoblast differentiation by simultaneous addition of bFGF and W9
We have investigated the combined effects of bFGF and the RANKL-binding peptide W9, which are known to stimulate osteoblast proliferation and differentiation, respectively [15-17,28,29]. We first hypothesized that the bFGF works as an inhibitor of osteoblast differentiation induced by W9 when two agents were added to the culture media at the same time since proliferation stimulator usually inhibits differentiation [30]. Although this was the reason why we decided to add W9 to the osteoblast culture several days after the bFGF stimulation, our differentiation assay revealed that the simultaneous addition of bFGF and W9 got the better augmentation of early osteoblast differentiation compared to the condition that W9 was added later (Figure 1). This result is consistent with the results that bFGF increases proliferation and early differentiation activity and suppresses late differentiation [31]. Then, we thought W9 might affect to cell proliferation as well as differentiation. Actually, we found at the first time that W9 stimulated cell proliferation in the mesenchymal cell line (Figure 2). W9 has been already shown to stimulate mTORC1 signaling in ST-2 osteoblastic cell line [17], and mTORC1 signaling is important in mesenchymal cell proliferation [32]. W9 might promote cell proliferation through mTORC1 signaling. Since the control peptide of W9, which has a lower affinity to RANKL compared to W9, did not stimulate mesenchymal cell proliferation, suggesting that the affinity to RANKL might be involved in the stimulatory mechanism of W9 on cell proliferation. Further studies are necessary to clarify the stimulatory mechanism of W9 on cell proliferation.
W9 promotes bFGF-induced bone formation
In this study, we have shown that the administration of W9 with bFGF promotes bone formation in critical-sized (5 mm in diameter) rat calvarial defect. The reason for using this experimental model is that rat calvaria is similar to human mandible as both develop through intramembranous bone formation and both demonstrate limited innate regenerative potential [33,34]. It has been reported that 0.3% (30 μg) bFGF increases bone formation in the same model, as that used in this study, using absorbable collagen sponge carrier, but bone formation was not observed in the whole defect [35].
Therefore, we used the GH carrier in in vivo studies. The GH carrier holds the agent through molecular interactions, such as hydrogen binding, hydrophobic binding, and Coulomb’s force, and the agent is released slowly as the GH is degraded [36]. This carrier is known to achieve controlled release of bFGF [37] and peptide drugs [16,29]. Therefore, the GH has good affinity with drug regardless of hydrophilicity or hydrophobicity. Since the isoelectric points of bFGF and W9 are different, we have used two different types of GH carriers, pI5 and pI9, respectively [16,29,38]. 25 μg bFGF, which was used in in vivo experiments, was decided to be appropriate for bone formation in this model. In a previous study, the skull defect in rabbits was filled with newly regenerated bone and was almost closed 12 weeks after implantation, after treatment with 100 μg bFGF-incorporated GH [36]. Additionally, it is reported that bone regeneration induced by bFGF-incorporated GH was not initiated from the central portion of the defect but from the edge [37]. First, to combine bFGF/W9, we assessed the amount of bone formation occurring with minimal quantity of bFGF. After determining the dose of bFGF, the dose of W9 was determined with reference to previous reports [16,17]. Combination of 25 μg bFGF and 0.56 mg W9 (bFGF/W9 group) showed significant enhancement of bone formation compared with that of other groups. Newly formed bone was scarcely recognized at twice the dose in in vivo experiments, and administration of the same amount resulted in bone formation more than that by bFGF alone. Next, to confirm the difference in RANKL binding capacity, we used CtrP, which is the peptide containing W9 mutation, and results revealed that they bind with a lower affinity to RANKL than to W9. The bFGF/CtrP group resulted in significant suppression of bone formation compared to that in the bFGF/W9 group. However, bone formation was scarcely observed in GH carrier and GH + W9 groups. From these results, W9 might promote bFGF-induced osteogenesis in a RANKL-affinity dependent manner. Until now, bone formation was not expected to occur due to bFGF in experiments using the rat calvarial defect model. However, promotion of osteogenic effect by bFGF was confirmed by the concomitant use of W9. The results of this experiment revealed bone formation in the edges and the ectopic area. A previous study reported that bFGF alone does not induce ectopic bone formation (16, 80 and 400 ng; and 2, 10, and 50 μg bFGF), but induces the formation by addition of 2 μg bFGF in rat calf muscle [39]. Briefly, bone formation requires BMP-2. In the dog periodontal model, bFGF promoted the expression of BMP-2 to induce osteoblastic differentiation and bone formation [40]. Considering these results, our data suggested that BMP-2 was expressed when bFGF was used, and BMP-2-like action was enhanced by using W9 resulting in bone formation. This is consistent with the report that the RANKL-binding peptide enhances the action of BMP-2, compared with past mouse calvarial defect experiments. Moreover, as mentioned above, it is thought that it can support against the late differentiation effect of bFGF can’t be expected. Further, it is necessary to confirm the mechanism of bone formation and BMP-2 expression when bFGF and W9 are combined.
Perspectives of combination therapy with two different agents
We used two types of agents bFGF and W9 with a controlled release. The dual controlled release system is one of the attractive methods employed for bone regeneration [7]. In this study, osteoblast proliferation was carried out by bFGF, and W9 might have enhanced bFGF-induced osteoblast differentiation. However, more complicated reactions must be occurring during the process. If two types of agents are used, synergistic effect is just a possibility, and if they are used at high concentrations, there is a possibility of inhibition of bone regeneration. It is difficult to decide a ratio, but good bone regeneration can be expected when the ratio is appropriate.
The synergistic effects of two different agents on proliferation might be one of the reason why the combination of W9 and bFGF could stimulate bone regeneration compared to the administrations of W9 or FGF alone. Since bFGF stimulate proliferation through MAPK pathway and W9 might be related to the mTORC1 activation on proliferation as described earlier, the different stimulatory mechanisms could generate additively effects for local bone formation. As suggested in this study (Figure 1), the RANKL-binding peptide could stimulate osteoblast differentiation under the RANKL-affinity dependent mechanism [15,41]. The combination therapy of W9 and bFGF also exerts its effects under the RANKL-affinity dependent mechanism (Figure 4), suggesting that two different stimulatory mechanism might produce additively effects on osteoblast differentiation and bone formation.
Although we need to clarify the signaling crosstalk or the stimulatory mechanisms on bone formation induced by two different ligands, our results clearly showed the beneficial effects of the combination therapy with two different bone forming agents.
The results of this study indicate that W9 peptide can promote bFGF-triggered osteogenesis by stimulating mesenchymal cell proliferation and enhancing early differentiation of osteoblasts in vitro. In addition, this peptide also stimulated bFGF-induced bone formation in a rat critical-sized
calvarial defect (5 mm in diameter) in vivo. Therefore, combination therapy of bFGF and the RANKL-binding peptide W9 could be expected to stimulate bone augmentation at the post-operative bone defect in the maxillofacial region.
The authors are grateful to the faculty and staff of the Division of Oral and Maxillofacial Surgery, Department of Oral Health Sciences, and the Division of Pharmacology, Department of Biomatrix, Graduate School of Medical and Dental Sciences, Tokyo Medical and Dental University for their technical assistance and helpful discussion. We also thank Ms. Mikiko Kurihara (Kaken Pharmaceutical Co.,Ltd, KAKEN) for providing bFGF and Mr. Junichiro Jo (Insititute for Frontier Medical Science, Kyoto University) for providing the gelatin hydrogel.
No competing financial interests exist.
- Arboleya L, Castañeda S (2013) Osteoimmunology: the study of the relationship between the immune system and bone tissue. Reumatol Clin 9: 303-315. [Crossref]
- Hwang CJ, Vaccaro AR, Lawrence JP, Hong J, Schellekens H, et al. (2009) Immunogenicity of bone morphogenetic proteins. J Neurosurg Spine 10: 443-451. [Crossref]
- Lind M, Bünger C (2001) Factors stimulating bone formation. Eur Spine J 2: S102-109. [Crossref]
- Saran U, Gemini Piperni S, Chatterjee S (2014) Role of angiogenesis in bone repair. Arch Biochem Biophys 561: 109-117. [Crossref]
- Tsiridis E, Upadhyay N, Giannoudis P (2007) Molecular aspects of fracture healing: which are the important molecules? Injury 1: S11-S25. [Crossref]
- Dimitriou R, Tsiridis E, Giannoudis PV (2005) Current concepts of molecular aspects of bone healing. Injury 36: 1392-1404. [Crossref]
- Kim YH, Tabata Y (2015) Dual-controlled release system of drugs for bone regeneration. Adv Drug Deliv Rev 94: 28-40. [Crossref]
- Kubota K, Iseki S, Kuroda S, Oida S, Iimura T, et al. (2002) Synergistic effectof fibroblast growth factor-4inectopicbone formation induced by bone morphogenetic protein-2. Bone 31: 465-471.
- Tachi K, Takami M, Sato H, Mochizuki A, Zhao B, et al. (2011) Enhancement of bone morphogenetic protein-2-induced ectopic bone formation by transforming growth factor-beta1. Tissue Eng1 Part A 17: 597- 606. [Crossref]
- Fiorellini JP, Howell TH, Cochran D, Malmquist J, Lilly LC, et al. (2005) Randomized study evaluating recombinant human bone morphogenetic protein-2 for extraction socket augmentation. J Periodontol 76: 605-613. [Crossref]
- Nakamura Y, Tensho K, Nakaya H, Nawata M, Okabe T, et al. (2005) Low dose fibroblast growth factor-2 (FGF-2) enhances bone morphogenetic protein-2 (BMP-2)-induced ectopic bone formation in mice. Bone 36: 399-407. [Crossref]
- Arai Y, Aoki K, Shimizu Y, Tabata Y, Ono T, et al. (2016) Peptide-induced de novo bone formation after tooth extraction prevents alveolar bone loss in a murine tooth extraction model. Eur J Pharmacol 782: 89-97. [Crossref]
- Toyoda H, Terai H, Sasaoka R., Oda K, Takaoka, K (2005) Augmentation of bone morphogenetic protein-induced bone mass by local delivery of a prostaglandin E EP4 receptor agonist. Bone 37: 555-562. [Crossref]
- Aoki K, Alles N, Soysa N, Ohya K (2012) Peptide-based delivery to bone. Adv Drug Deliv Rev 64: 1220-1238. [Crossref]
- Furuya Y, Inagaki A, Khan M, Mori K, Penninger JM, et al. (2013) Stimulation of bone formation in cortical bone of mice treated with a receptor activator of nuclear factor-kappa B ligand (RANKL)-binding peptide that possesses osteoclastogenesis inhibitory activity. J Biol Chem 288: 5562-5571. [Crossref]
- Khan AAM, Alles N, Soysa NS, Mamun MAA, Nagano K, et al. (2013) The local administration of TNF-a and RANKL antagonist peptide promotes BMP-2-induced bone formation. J Oral Biosci 55: 47-54.
- Sugamori Y, Mise-Omata S, Maeda C, Aoki S, Tabata Y, et al. (2016) Peptide drugs accelerate BMP-2-induced calvarial bone regeneration and stimulate osteoblast differentiation through mTORC1 signaling. Bioessays 38: 717-725. [Crossref]
- Aoki K1, Saito H, Itzstein C, Ishiguro M, Shibata T, et al. (2006) A TNF receptor loop peptide mimic blocks RANK ligand-induced signaling, bone resorption, and bone loss. J Clin Invest 116: 1525-1534. [Crossref]
- Yamachika E, Tsujigiwa H, Matsubara M, Hirata Y, Kita K, et al. (2012) Basic fibroblast growth factor supports expansion of mouse compact bone-derived mesenchymal stem cells (MSCs) and regeneration of bone from MSC in vivo. J Mol Histol 43: 223-233. [Crossref]
- Marie PJ (2003) Fibroblast growth factor signaling controlling osteoblast differentiation. Gene 316: 23-32. [Crossref]
- Cheng X, Kinosaki M, Takami M, Choi Y, Zhang HT, et al. (2004) Disabling of receptor activator of nuclear factor-kappa B (RANK) receptor complex by novel osteoprotegerin-like peptidomimetics restores bone loss in vivo. J Biol Chem 279: 8269-8277. [Crossref]
- Furuya H, Tabata Y, Kaneko K (2014) Bone regeneration for murine femur fracture by gelatin hydrogels incorporating basic fibroblast growth factor with different release profiles. Tissue Eng Part A 20: 1531-1541. [Crossref]
- Uehara T, Mise-Omata S, Matsui M, Tabata Y, Murali R, et al. (2016) Delivery of RANKL-binding peptide OP3-4 promotes BMP-2-induced maxillary bone regeneration. J Dent Res 95: 665-672. [Crossref]
- Kato G, Shimizu Y, Arai Y, Suzuki N, Sugamori Y, et al. (2015) The inhibitory effects of a RANKL-binding peptide on articular and periarticular bone loss in a murine model of collagen-induced arthritis: a bone histomorphometric study. Arthritis Res Ther 17: 14. [Crossref]
- Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9: 671-675. [Crossref]
- Kawamoto T (2003) Use of a new adhesive film for the preparation of multi-purpose fresh-frozen sections from hard tissues, whole-animals, insects and plants. Arch Histol Cytol 66: 123-143. [Crossref]
- Dempster DW, Compston JE, Drezner MK, Glorieux FH, Kanis JA, et al. (2013) Standardized nomenclature, symbols, and units for bone histomorphometry: a 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res 28: 2-17. [Crossref]
- Kawaguchi H, Oka H, Jingushi S, Izumi T, Fukunaga M, et al. (2010) A local application of recombinant human fibroblast growth factor 2 for tibial shaft fractures: A randomized, placebo-controlled trial. J Bone Miner Res 25: 2735-2743. [Crossref]
- Mamun MAA, Khan AAM, Alles N, Matsui M, Tabata Y, et al. (2013) Gelatin hydrogel carrier with the W9-peptide elicits synergistic effects on BMP-2-induced bone regeneration. J Oral Biosci 55: 217-223.
- Kalajzic I, Kalajzic Z, Hurley MM, Lichtler AC, Rowe DW (2003) Stage specific inhibition of osteoblast lineage differentiation by FGF2 and noggin. J Cell Biochem 88: 1168-1176. [Crossref]
- Fakhry A, Ratisoontorn C, Vedhachalam C, Salhab I, Koyama E, et al. (2005) Effects of FGF-2/-9 in calvarial bone cell cultures: differentiation stage-dependent mitogenic effect, inverse regulation of BMP-2 and noggin, and enhancement of osteogenic potential. Bone 36:254-266. [Crossref]
- Rodgers JT, King KY, Brett JO, Cromie MJ, Charville GW, et al. (2014) mTORC1 controls the adaptive transition of quiescent stem cells from G0 to G(Alert). Nature 510: 393-396. [Crossref]
- Prolo DJ, Pedrotti PW, Burres KP, Oklund S (1982) Superior osteogenesis in transplanted allogeneic canine skull following chemical sterilization. Clin Orthop Relat Res 168: 230-242. [Crossref]
- Yun JI, Wikesjo UM, Borke JL, Bisch FC, Lewis JE, et al. (2010) Effect of systemic parathyroid hormone (1-34) and a beta-tricalcium phosphate biomaterial on local bone formation in a critical-size rat calvarial defect model. J Clin Periodontol 37: 419-426. [Crossref]
- Kigami R1, Sato S, Tsuchiya N, Yoshimakai T, Arai Y, et al. (2013) FGF-2 angiogenesis in bone regeneration within critical-sized bone defects in rat calvaria. Implant Dent 22: 422-427. [Crossref]
- Ikada Y1, Tabata Y (1998) Protein release from gelatin matrices. Adv Drug Deliv Rev 31: 287-301. [Crossref]
- Tabata Y, Yamada K, Miyamoto S, Nagata I, Kikuchi H, et al. (1998) Bone regeneration by basic fibroblast growth factor complexed with biodegradable hydrogels. Biomaterials 19: 807-815. [Crossref]
- Kimura A, Kabasawa Y, Tabata Y, Aoki K, Ohya K, et al. (2014) Gelatin hydrogel as a carrier of recombinant human fibroblast growth factor-2 during rat mandibular distraction. J Oral Maxillofac Surg 72: 2015-2031. [Crossref]
- Fujimura K, Bessho K, Okubo Y, Kusumoto K, Segami N, et al. (2002) The effect of fibroblast growth factor-2 on the osteoinductive activity of recombinant human bone morphogenetic protein-2 in rat muscle. Arch Oral Biol 47: 577-584. [Crossref]
- Nagayasu-Tanaka T, Anzai J, Takaki S, Shiraishi N, Terashima A, et al. (2015) Action mechanism of fibroblast growth factor-2 (FGF-2) in the promotion of periodontal regeneration in beagle dogs. PLoS One 10: e0131870. [Crossref]
- Sims NA, Romas E (2015) Is RANKL inhibition both anti-resorptive and anabolic in rheumatoid arthritis? Arthritis Res Ther 17: 328. [Crossref]




