Abstract
Background: Combined Lung and Liver Transplantation (CLLT) is a lifesaving procedure for patients with coexisting end-stage lung and liver disease. CLLT is infrequently performed due to organ availability, dual organ allocation, limited guideline for candidate selection, and surgical complexity. This retrospective analysis of nineteen CLLT recipients, the largest known single-center cohort of these patients, focuses on complications and short- and long-term survival.
Methods: The charts of nineteen recipients and their donors who underwent combined CLLT at a tertiary care center from 2006 to 2021 were retrospectively chart were reviewed.
Results: Indications for lung transplant included cystic fibrosis (n=11), idiopathic pulmonary fibrosis (n=6), and alpha-1 antitrypsin deficiency (n=2). Liver transplant indications included cystic fibrosis (n=11), alpha-1 antitrypsin deficiency (n=2), alcohol-related cirrhosis (n=2), non-alcoholic steatohepatitis (NASH) (n=2), cryptogenic cirrhosis (n=2), and hepatopulmonary syndrome (n=1). Median recipient age at transplant was 30 years (IQR 25.0-55.5), and eight recipients (42.1%) were female. Mean Lung Allocation Score (LAS) at transplant was 56.4 (SD, 17.3) and mean Model for End-Stage Liver Disease (MELD)-Na score was 17.7 (SD, 8.3). Ten patients experienced acute lung rejection post-transplant, and one of whom was diagnosed with chronic allograft lung dysfunction (CLAD) six years after transplant. The most common post-operative complication was sepsis, with Pseudomonas aeruginosa as the most common infection (n=8). However, at time of current analysis, no incidences of graft loss or re-transplantation were reported. Overall survival probabilities were 84% at 1 year after transplant, 69% at 3 years, and 55% at 5 and 10 years.
Conclusions: This largest retrospective analysis of CLLT recipients to date demonstrates favorable survival outcomes with no incidences of re-transplantation, suggesting that CLLT is a viable option in selective recipients.
Keywords
Combined lung and liver transplantation, acute lung rejection, chronic allograft lung dysfunction
Abbreviations
AAT: Alpha-1 antitrypsin deficiency; AMR: Immune Mediated rejection; BMI: Body Mass Index; BOS: Bronchiolitis Obliterans Syndrome; CLAD: Chronic allograft lung function; CLLT: Combined Lung and Liver Transplantation; CPS: Child-Pugh Score; DSA: Donation Service Area; ECMO: Extracorporeal Membrane Oxygenation: FEV1: Forced Expiratory Volume in 1 second; FiO2: Fraction of inspired Oxygen; HCV: Hepatitis C Virus; HVPG: Hepatic Portal Venous Pressure Gradient; ICU: Intensive Care Unit; IQR: Interquartile Range; ISHLT: International Society for Heart and Lung Transplantation; LAS: Lung Allocation Score; MELD: Model for End-Stage Liver Disease; NASH: Non-alcholic steatohepatitis; OPTN: Organ Procurement Transplantation Network; RAS: Restrictive Allograft Syndrome; RRT: Renal Replacement Therapy; SD: Standard Deviation; TIPS: Transjugular Intrahepatic Portosystemic Shunt; TRALI: Transfusion-Related Acute Lung Injury
Introduction
Combined Lung and Liver Transplantation (CLLT) is a life-saving procedurefor patients with end-stage lung and liver disease. The most common indication for CLLT is cystic fibrosis followed by alpha-1 antitrypsin (AAT) deficiency and portopulmonary hypertension [1]. However, the procedure is infrequently performed due to organ availability, and the ethical issues associated with multi-organ allocation for one candidate,challenging surgical techniques, and limited information regarding appropriate recipient selection.
Based on Organ Procurement Transplantation Network (OPTN) data for January 1, 1988 to November 30, 2021, it comprises only 0.009% of 16,426 multi-organ transplantation in the United States [2]. The first CLLT was performed at the University of Illinois in 1994 and since then a total of 160 CLLT cases have been performed in the United States, per OPTN database [3]. Our center has performed 2,207 cases of single, double en-bloc, and bilateral sequential lung transplantations since 1990, and the 19 cases of CLLT, the largest number in the published literature. Due to theoverall limited experience in CLLT, the selection and management of this patient population is not yet standardized. We sought to describe our center’s experience with CLLT by defining indications, patient demographics, perioperative and post-transplant course and complications, including acute and chronic rejection, and early and latesurvival outcomes.
Methods
A retrospective medical chart review was performed using the electronic medical record at Cleveland Clinic for all patients older than 18 years who received CLLT due to end-stage lung and liver disease, from January 2007 to June 2022. Patients who received additional organ transplantation at the time of CLLT were excluded from analysis. Patients who underwent staged lung and liver transplantation were also excluded.
Information was collected on patients’ baseline demographic characteristics, pre-transplantation medical history, donor characteristics, intraoperative characteristics, post-operative management course and complications, including any infections, instances of rejection according to the International Society for Heart and Lung Transplantation (ISHLT) guideline [4,5] or the need for re-transplantation. (IRB#22-654)
Statistical analysis
Baseline characteristics and unadjusted outcomes were computed using descriptive statistics. Survival outcomes were represented using Kaplan-Meier curves. P-value of less than 0.05 were deemed statistically significant.
Results
Patient Selection and Characteristics
Nineteen patients underwent CLLT at our center (Table 1); 8 patients were female. The median age was 30 years [interquartile range (IQR) 25.0-55.5] and median body mass index (BMI) 20.43 [IQR 18.45-24.48].
Common indications for both liver and lung transplantations included cystic fibrosis (n=11) and AAT deficiency (n=2). Separate etiologies for lung transplantation included idiopathic pulmonary fibrosis (n=6), and chronic obstructive pulmonary disease (n=1) and for liver transplantation included alcoholic cirrhosis (n=2), nonalcoholic steatohepatitis (NASH) (n=2), cryptogenic cirrhosis (n=2), and hepato-pulmonary syndrome (n=1).
Table 1. Baseline Recipient Characteristics
Total number of patients |
19 |
Recipient age at transplant |
30.00[25.0,55.5] |
Recipient BMI at transplant |
20.43[18.5,24.5] |
Gender = Female |
8(42.1%) |
Liver transplantation indications |
N (%) |
Cystic fibrosis |
11(57.9) |
AATdeficiency |
2(10.5) |
Alcoholic cirrhosis |
2(10.5) |
NASH |
2(10.5) |
HCV |
0(0.0) |
HBV |
0(0.0) |
Cryptogeniccirrhosis |
2(10.5) |
Hepatopulmonary syndrome |
1(5.3) |
Lung Transplant Indications |
|
Cystic fibrosis |
11(57.9) |
AAT deficiency |
2(10.5) |
Idiopathic pulmonary fibrosis |
6(31.6) |
Hepato pulmonary syndrome |
0(0.0) |
COPD |
1(5.3) |
Others |
0(0.0) |
Waitlist time (days) |
160.0[63.0,382.0] |
Pre-transplant Past Medical History |
N (%) |
Hypertension |
1 (5.3) |
Type1diabetes |
8(42.1) |
Type2diabetes |
3(15.8) |
CKD |
0(0.0) |
Cardiovascular disease |
2(10.5) |
Hypercoagulable state |
1 (5.3) |
FEV1% at transplant |
28.0[17.0,42.4] |
FVC% at transplant |
41.0 [32.0-53.4] |
Six-minute walk test (m) |
291.7 (144.3) |
Oxygen requirement (FiO2) prior to transplant (%)
Resting
Exertion |
34.8 (17.0)
48.7 (27.8) |
LAS*attransplant |
56.4(17.3) |
MELD-Na at transplant |
17.7(8.3) |
Child-Pugh score at transplant |
7.05 (1.22) |
INR at transplant |
1.38(0.37) |
Total bilirubin at transplant |
1.46(1.12) |
Albumin at transplant |
3.14(0.62) |
Serum creatinine at transplant |
0.69(0.43) |
Recipient prior to admission status |
N (%) |
Home |
11(57.9) |
Regular nursing floor |
6(31.6) |
ICU |
2(10.5) |
Note: Values in [x,y] represent median[Q1,Q3], while values in (x) represent mean(standard deviation).
BMI: Body Mass Index, AAT: alpha 1 Antitrypsin, NASH: Nonalcoholic Steatohepatitis, HCV: Hepatitis C Virus, HBV: Hepatitis B Virus, COPD: chronic obstructive lung disease, CKD: chronic kidney disease, FEV1: Forced Expiratory Volume in the First Second, FVC: Forced Vital Capacity, FiO2: the Fraction of inspired Oxygen, LAS: Lung Allocation Score, MELD: Model For End-Stage Liver Disease, INR: International Normalized Ratio, ICU: Intensive Care Unit
*This study have had access to LAS only instead of CAS (lung composite allocation score), which is a new term as of 2023.
At time of transplant, median forced expiratory volume in 1 second (FEV1) was 28% (IQR 17.0-42.4). Prior to transplantation, mean fraction of inspired oxygen (FiO2) was 34.8% [standard deviation (SD), 17.0] at rest and 48.7% (SD, 27.8) on exertion. Mean LAS was 56.4 (SD, 17.3). Mean MELD-Na score and Child-Pugh score (CPS) were 17.7 (SD, 8.3) and 7.05 (SD, 1.22) respectively.
All patients had cirrhosis on CT imaging, which was confirmed by liver biopsy in 12cases. Eighteen patients had portal hypertension, defined by splenomegaly, esophageal/gastric/rectal varices, as cites, hepatic encephalopathy, porto-systemic shunt, or hepatic portal venous pressure gradient (HVPG) >10mmHg. Ten patients had measured HVPG prior to transplantation with a median of 9.5 mmHg (IQR 5-11.75). Three patients had ascites on ultrasound, and two requiring recurrent paracentesis.Two patients received a Transjugular Intrahepatic Portosystemic Shunt (TIPS) procedure prior to surgery due to recurrent ascites and varices. Median of the mean pulmonary artery pressure was 20 mmHg (IQR 17.5-29.4).
Eleven patients (57.9%) were admitted from home at the time of the transplant. Six awaited transplant in the hospital. Two candidates were managed in the Intensive Care Unit (ICU); one patient required mechanical ventilation as a bridge to transplant, and the other patient required continuous renal replacement therapy for acute renal failure from hepatorenal syndrome.Median waitlist time was 160 days (IQR 63.0-382.0).
Baseline Donor Characteristics
Median donor age was 29 years (IQR 25.5-35.0). All donors were brain dead (Table 2). Median PaO2/FiO2 ratio (PF ratio) prior to procurement was 435 [IQR 361.5-479.5].United Network for Organ Sharing (UNOS) regions were identified as local(by Donation Service Area (DSA) until November 2017 and by 250-nautical mile radius afterwards) (n=8), regional (n=7), and national (n=4). One hepatitis C virus (HCV) positive donor was identified (genotype 1a), and the CLLT patient was treated with glecaprevir and pibrentasvir.
Table 2. Baseline Donor Characteristics
Donorage (years) |
29.0[25.5,35.0] |
Brain dead donor (BDD) or donoraftercardiac death(DCD) |
BDD 19 (100%)
DCD 0 (0%) |
PaO2/FiO2 ratio prior to procurement |
435.0[361.5,479.5] |
PaO2/FiO2ratio: the arterial partial pressure of oxygen (PaO2) divided by the inspired oxygen concentration (FiO2)
Intra-operative Characteristics and Post-operative Complications and Outcomes
All CLLT were performed with bilateral sequential lung transplantation, followed by liver transplantation (Table 3). Median total operative time from chest incision for lung transplant till abdominal closure for liver transplant was 827 minutes (IQR 729-919). Median total ischemic periods were 352 minutes for lung (IQR 296-409) and604 minutes for liver (IQR550-639). Median totalintraoperative fluid requirement excluding transfusion products or albumin, was 6250 ml. Complete transfusion data is described in Table 3. The chest was left open in three patients and the abdomen was left open in 6 patients. All patients had closure in the early post-operative period.
Table 3. Operative Characteristics and Complications and Outcomes
Transplant order: lung first |
19 (100%) |
Total operative time lung to liver closure (minutes) |
827.0[728.5,919.0] |
Total ischemic time (lungs) |
352.0 [296.0, 409.0] |
Total ischemic time (liver) |
604.0 [549.6, 638.5] |
Transfusions |
|
Intraoperative total amount fluid received (mL)
excluding transfusion product or albumin |
6250.0[5487.5,7250.0] |
RBCtransfused(unit) |
6.0[4.0,10.5] |
Plateletattimeoftransplant |
74.0[52.5,118.0] |
FFPtransfused(unit) |
3.0[1.0,5.0] |
Cryoprecipitatetransfused(unit) |
3.5[0.0,9.8] |
Albumintransfused(mL) |
2750.0[1937.5,3250.0] |
Nissenfundoplicationoranti-refluxprocedure |
0(0.0 %) |
Openchestpost-operatively |
3(15.8 %) |
Openabdomenpost-operatively |
6(31.6 %) |
Complications |
|
Surgical site (chest/abdomen) issues |
0 (0.0 %) |
Need for renal replacement therapy post-transplantation |
5 (27.8 %) |
Renal function recovered |
0 (0.0 %) |
Peri-operative outcomes |
|
ReturntoOR |
10(52.6) |
ReturntoICU(onlyduringtheindexadmission) |
6(33.3) |
NumberofreadmissiontoICU |
2.0[1.3,2.0] |
Lengthofstayaftersurgery(days) |
29.0[14.0,61.0] |
TotallengthofstayinICU(days) |
9.0[6.5,21.5] |
RBC: Red Blood Cells, FFP : Fresh Frozen Plasma, OR: Operating Room, ICU: Intensive Care Unit
Three patients required Extracorporeal Membrane Oxygenation (ECMO): one via veno-arterial cannulation and two via veno-venous cannulation. Five patients required renal Replacement Therapy (RRT) postoperatively, without renal function recovery. A total of 10 patients (52.6%) returned to the operating room: 9 patients required delayed chest and/or abdomen closure, 2 patients required re-exploration and washout of the chest due to bleeding, and 4 patients required exploratory laparotomy for portal vein thrombectomy (1), revision of vascular anastomosis (1), hematoma evacuation (1), and lysis of intestinal adhesions between small intestines and abdominal wall (1).
Six patients were readmitted to the Intensive Care Unit (ICU) during the index admission. Median length of hospital stay after surgery was 29 days (IQR 14.0-61.0). Median length of ICU stay was 9 days (IQR 6.5-21.5).
Infections during Initial Hospitalization for Transplantation
Ten patients had one or more infectionsduring initial hospitalization for transplantation, Intravenous antibiotics,consultations with Infectious Disease specialists and modification of ongoing treatment were required. Specific post-operative infectious complications are listed in Table 4.
Table 4. Infectious Complications during Initial Hospitalizations for Transplantation
Clostridium difficile |
3 (16.7 %) |
Pseudomonas aeruginosa |
8 (44.4 %) |
Fungal (eg. Candida) |
5 (27.8 %) |
Cytomegalovirus viremia |
3 (16.7 %) |
Post-Transplant Lymphoproliferative Disorder (PTLD) |
2 (11.1%) |
Peritonitis |
1 (5.6%) |
Post-transplant immunosuppression and rejection
A total of 10 patients (52.6%) experiencedacute rejection based on ISHLT classification of lung allograft rejection [4] (Table 5). Two of them had Antibody Mediated Rejection (AMR) [5] as well which were treated with plasmapheresis and intravenous immunoglobulin. Most of these patients (90%, n=9) developedacute rejections within the first year of transplantation.
Table 5. Post-transplant immunosuppression and rejection
ID |
PGD at T0, T72 |
Induction, type |
Maintenance |
Posttransplant rejection† and treatment |
CLAD |
Graft loss |
Re-transplantation |
Status
(as of 1.2023) |
Number of days of follow-up |
1 |
1, 0 |
N |
Tacrolimus,
Mycophenolate mofetil |
3 weeks A1B0: prednisone taper |
N |
N |
N |
Alive |
1626 |
2 |
0, 2 |
N |
Tacrolimus
Mycophenolate mofetil |
None |
N |
N |
N |
Alive |
4990 |
3 |
3, 3 |
N |
Tacrolimus |
None |
N |
N |
N |
Dead |
10 |
4 |
3, 3 |
Y, rATG |
Tacrolimus
Mycophenolate mofetil |
None |
N |
N |
N |
Dead |
221 |
5 |
1, 1 |
N |
Cyclosporin
Mycophenolate mofetil |
1 month: empiric pulse steroid concern for ACR;
1 month: De novo class II DQ2 s/p 5-day course of PLEX and IVIG |
N |
N |
N |
Dead |
848 |
6 |
1, 0 |
N |
Tacrolimus
Mycophenolate mofetil |
3 months A2B0: pulse and taper steroid |
N |
N |
N |
Alive |
1369 |
7 |
1, 1 |
N |
Tacrolimus
Mycophenolate mofetil |
None |
N |
N |
N |
Alive |
2678 |
8 |
0, 0 |
N |
Tacrolimus
Mycophenolate mofetil |
None |
N |
N |
N |
Alive |
5060 |
9 |
2, 1 |
N |
Tacrolimus
Mycophenolate mofetil |
None |
N |
N |
N |
Dead |
909 |
10 |
3, 1 |
N |
Tacrolimus
Mycophenolate mofetil |
9 months A1B0: steroids;
13 months A2B0: steroid pulse and taper |
Y
(BOS and RAS) |
N |
N |
Alive |
2443 |
11 |
N/A |
N |
Tacrolimus
Mycophenolate mofetil |
3 weeks A2B0: steroid;
6 weeks A2B0: not treated |
N |
N |
N |
Alive |
5840 |
12 |
1, N/A |
N |
None |
N |
N |
N |
N |
Dead |
605 |
13 |
1, 1 |
N |
Tacrolimus
Mycophenolate mofetil |
3 weeks A2B0: pred taper;
6 weeks A2B0: steroid pulse and taper |
N |
N |
N |
Alive |
1398 |
14 |
0, 1 |
Y, rATG |
Tacrolimus
Mycophenolate mofetil |
13 months A1B0: prednisone taper |
N |
N |
N |
Alive |
1204 |
15 |
3, 2 |
Y, rATG |
Tacrolimus |
8weeks A1B0,
3months A2B0,
4 months A1B0,
5months A1B0,
6 months A2B0,
7 months A1B0: all treated with steroids |
N |
N |
N |
Alive |
445 |
16 |
0, 1 |
N |
Tacrolimus
Mycophenolate mofetil |
3 months A2B0: steroid;
9 months A1B0: steroid;
11 months: treated with PLEX, IVIG and rituximab |
N |
N |
N |
Alive |
844 |
17 |
2, 1 |
N |
Tacrolimus
Mycophenolate mofetil |
3 weeks A2B1R: steroid |
N |
N |
N |
Alive |
880 |
18 |
0, 2 |
N |
Tacrolimus
Mycophenolate mofetil |
None |
N |
N |
N |
Alive |
708 |
19 |
0, 0 |
N |
Tacrolimus |
None |
N |
N |
N |
Alive |
1294 |
PGD: Primary lung Graft Dysfunction, T0: within 6 hours of reperfusion, T72: within 72 hours of reperfusion, rATG: rabbit Anti-Thymocyte Globulin, ACR: Acute Cellular Rejection, PLEX: plasma exchange, IVIG: Intravenous Immune Globumin; BOS: Bronchiolitis Obliterans Syndrome, RAS: Restrictive Allograft Syndrome
†Allograft rejection grading is based on International Society for Heart and Lung Transplantation (ISHLT) grading scheme for pulmonary allograft rejection [4].
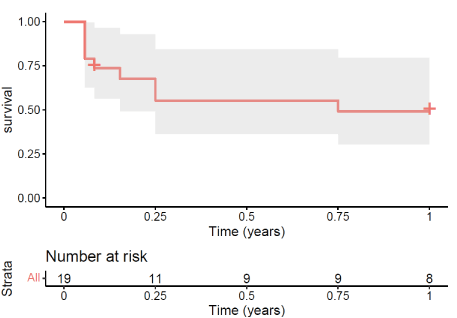
Probability of recipient freedom from all rejection was 55.26% (95% Confidence Interval [CI], 36.20%-84.36%) at 0.5 years after transplantation (Figure 1). One patient (5.3%) developed Chronic Lung Allograft Dysfunction (CLAD) [6] with mixed Bronchiolitis Obliterans Syndrome (BOS) and Restrictive Allograft Syndrome (RAS) phenotype 6 years after transplantation. One patient (5.3%) had biopsy-proven mild liver rejection postoperative day 4 and received a steroid therapy.
Three patients (15.8%) received induction therapy with rabbit anti-thymocyte globulin]. Eighteen patients (94.7%) received maintenance therapy with tacrolimus. One patient was switched to cyclosporine due to altered mental status, presumed to be an adverse effect of tacrolimus. No acute or chronic graft loss was attributable as a cause of death, and no re-transplantationwas performed for neither lung nor liver.
Patient Survival Analysis
Mean survival after transplant probabilities were 84% at 1-year after transplant, 69% at 3 years, and 55% at both 5 and 10 years (Figure 2). At the time of analysis, 14 of 19 patients (73.7%) were alive; 3 of these patients survivedfor more than 10 years after transplant. Five patients died. Three were CF patients who died during index admission: 2 patients due to septic shock and multiorgan failure, and one patient due to right thalamic intracranial hemorrhage and intraventricular hemorrhage. In the other 2 patients, the causes of death were sepsis and cardiogenic shock, respectively.
Figure 1. Freedom from All Rejection during the First Post-Transplant Year
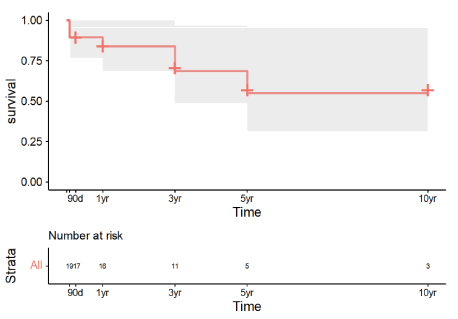
Figure 2. Lung and Liver Transplant Survival Probability in Patients after combined liver and lung transplantation
Discussion
This analysis is one of the largest retrospective studies of CLLT recipients in the United States. Previous single-center studieshave reported 1-year survival rates from 56 to 92% and 3-year survival rates from 62 to79% [1,7-16]. Most of these studies presented data from three-years of follow up. Overall, our center’s CLLT patient population received ten years’ follow up and demonstrated higher long-termsurvival compared to previously published case series.Similar to lung-alone transplant outcomes performed between 2008 and 2015, according to OPTN database, our CLLT survival outcomes were 87.2% at 1-year, 68.7% at 3-years, and 53.5% at 5-years [17].
Candidate selection for combined lung and liver transplantation
Definitive criteria for CLLT patient selection have not yet been determined. Current guidelines recommend against CLLT in patients with albumin < 2g/dL, prothrombin time international normalized ratio (INR) >1.8, severe ascites or encephalopathy. One patient who underwent CLLT despite high INR (2.6) and low albumin (1.7) and had an uncomplicated post-operative course and remains alive, 3 years and 10 months after transplant. Yi et al selected CLLT candidates who qualified for lung transplant after multidisciplinary board review, had both biopsy-proven cirrhosis and a ≥ 10 mmHg portal gradient [11]. In all previously published case series, patients were selected after multidisciplinary discussion. Five patients had good liver function (MELD-Na <17 and CPS ≤ 6) but underwent CLLT due to the presence of cirrhosis. Two patients with high MELD-Na score and low CPS had lower mortality (0/2 patients expired) compared to 9 patients with low MELD-Na and high CPS (1/9 patients expired). These results may indicate CPS is a better predictor of survival outcomes, but the number of patients is too small to be conclusive. At our institution, CLLT candidates were selected after lung and liver multidisciplinary committee review.The decision to CLLT was made after careful evaluation of isolated lung and isolated liver transplantation.
Surgical considerations in combined liver and lung transplantation
While data have been published on liver-first CLLT, all patients in this case series underwent lung-first transplantation. In general, both lungs and liver can be safely transplanted with the acceptable donor ischemic time. Immediate postoperative management of CLLT can be challenging as liver transplantation and lung transplantation have opposing goals regarding volume resuscitation [18-20]. For volume resuscitation, liver transplant requires aggressive colloid resuscitation due to perioperative low systemic vascular resistance and the high likelihood of reperfusion syndrome with reactive oxygen species release from an ischemic liver. However, excess volume administration puttransplanted lungs at higher risk of hypervolemia and Transfusion-Related Acute Lung Injury (TRALI).
Although there are no absolute guidelines that specify which organ should be transplanted first, most centers report a lung-first approach, since lungs are thought to be more sensitive to ischemia than the liver. Some studies report success with a liver-first approach and hypothesized potential benefit from absorption of donor-specific HLA antibodies, correction of coagulopathy prior to lung transplantation, decreased reperfusion injury to transplanted lungs, and decreased in biliary stricture from shorter liver ischemic time [21,22]. Freischlag, et al. reported a patient who received a liver-first CLLT and had numerous initial complications, including biliary stricture, immune thrombocytopenic purpura, and hemoperitoneum [14]. However, further studies are needed to define whether the liver-first approach has advantages over the lung-first approach.
Outcomes of combined lung and liver transplant recipients
Our institution transplants organs from donors who have tested positive for the Hepatitis C virus (HCV), only with recipient patients’ consents. One donor was HCV-positiveand the recipient was successfully treated with glecaprevir/pibrentasvir after transplantation. Patients with AAT deficiency received chronic augmentation therapy with an alpha-1 proteinase inhibitor until the day of transplantation, as well as postoperatively to preserve function of the newly transplanted organs.
Sepsis was the most common cause of mortality in this study. Patients with cystic fibrosis, who comprise the majority of our CLLT patients, are especially vulnerable to infections postoperatively. Patients with cystic fibrosis undergoing transplantation have demonstrated worse outcomes when colonized with microorganisms such as Pseudomonas aeruginosa or Burkholderiacepacia [23,24]. The infectious diseasespecialists determine appropriate antibiotics peri- and post-operatively.Our center’s mortality for CLLT cystic fibrosis patients (3/11, 27.2%) was similar compared to overall mortality for all CLLT patients (5/19, 26.3%). Of the 3 cystic fibrosis patients who died, 2 patients had sepsis as cause of death. Aris et al. reports that cystic fibrosis patients colonized with pan drug-resistant microorganisms had similar outcomes as patients colonized with sensitive bacteria [25]. Five of our patients (26.3%) had fungal infection during index hospitalization.Majority of our CLLT population was cystic fibrosis patients with pre-existing fungal colonization. Therefore, we provide at least 3-month fungal prophylaxis post-transplantation. We also recommend such infections should not be contraindications for multiorgan transplantation.
Five of our CLLT patients (26.3%) had an irreversible kidney injury requiring RRT. Although acute kidney injury post-lung transplantation needing renal replacement therapy is a known complication, the rate of irreversible kidney injury on our CLLT population is higher than lung transplantation alone patients (5-13%) [26]. This can be explained by the prolonged nature of multiorgan transplantation surgery associated with higher bleeding risk and frequent hypotension episodes. Also, cystic fibrosis has been demonstrated to be independent factor for AKI after lung transplantation due to nephrotoxic drug exposure such as antibiotics, diabetes, and abnormal calcium metabolism [27]. Of note, one patient was on continuous renal replacement therapy while waiting for CLLT. At that time, kidney co-transplantation was not considered for this patient due to patient’s critical illness and organ availability.
Cystic fibrosis patients comprised the majority of our CLLT cohort (57.9%). However, the severity of CF has been declining with CF transmembrane conductance regulator (CFTR) modulator therapy. Changesin overall indications and patient outcomes for CLLT are anticipated. Regardless, the need for CLLT will continue to exist given the increased recognition of short telomere syndrome with high prevalence of idiopathic pulmonary fibrosis and end-stage liver disease [28].
In this study, 47.4% patients developed acute rejection within the first year, an outcome similar to lung transplantation alone (~50%) [29]. Surveillance transbronchial lung biopsies were performed during the first year to monitor for rejection. Donor-specific antigens were routinely assessed for 10 days postoperatively and with each surveillance bronchoscopy. No patients in this study developed graft loss or needed re-transplantation with median follow-up of 3.3 years and with the longest follow-up over 10 years.
Ethics of performing combined lung and liver transplantation
An important ethical issue in multiorgan transplantation is the equity of allocating multiple scarce and life-saving organs to one person, when they could potentially save more individual lives. OPTN emphasizes on optimal balance between equity (fairness in organ procurement and allocation system) and utility (maximization of net benefit to the community) and concluded that multi-organ transplantation is life-saving therapy for patients who do not have an alternative. However, it must be limited in situations when the expected survival of the multi-organ transplant candidate is poor or the need for second organ is unclear [30]. Comparing heart-lung recipients and bypassed liver transplant waitlist candidates, Goldberg, et al. found that waitlist candidates who had delayed transplantations did not have excess mortality compared to matched multiorgan transplant control groups, which may reduce concerns about inequity [31]. More data are needed to compare outcomes in CLLT patients and bypassed isolated lung or liver transplant patients. Careful selection of candidates under an established system, such as multidisciplinary committee review and standardized selection criteria, will minimize potential ethical issues regarding multi-organ transplantation.
Conclusion
We report one of the largest published retrospective analysis of patients with CLLTpublished to dateover 17 years since 2007 with longer follow-up. Survival outcomes with CLLT compared favorably to survival with isolated lung transplantations. Our data provide the longest follow-up data up to 10 years. Although further study is needed regarding the lung-first vs. liver-first approach, our data confirmed that the lung-first approach was viable option for appropriately selected patients.
Authorship
Jee Young You, MD participated in writing of paper, performance of the research, data collection and analysis. Katie Shen, MD participated in writing of paper, performance of the research, and data collection. Atul C. Mehta, MD participated in research design, writing of the paper, and data analysis. William Carey, MD, Jamak Modaresi Esfeh, MD, Koji Hashimoto, MD, Kenneth McCurry, MD, and James Yun, MD participated in research design, revision of the manuscript, and performance of research. Yifan Wang, MPH and Xiaofeng Wang, PhD contributed to analytic tools and data analysis. Marie Budev, DO participated in research design, writing of the paper, revision of the paper, guiding the course of research progress, performance of the research, and data analysis.
Disclosure
The authors declare no conflict of interest.
Funding
No funding resource to declare.
References
- Grannas G, Neipp M, Hoeper MM, Gottlieb J, Lück R, et al. (2008) Indications for and outcomes after combined lung and liver transplantation: a single-center experience on 13 consecutive cases. Transplantation 85: 524-31. [Crossref]
- OPTN (2021) Multiple Organ Transplants in the U.S. by Recipient ABO. U.S. Multi-Organ Transplants Performed.
- OPTN (2021) Multiple Organ Transplants in the U.S. by Center. U.S. Multiple Organ Transplants Performed.
- Stewart S, Fishbein MC, Snell GI, Berry GJ, Boehler A, et al. (2007) Revision of the 1996 Working Formulation for the Standardization of Nomenclature in the Diagnosis of Lung Rejection. J Heart Lung Transplant 26: 1229-1242. [Crossref]
- Levine DJ, Glanville AR, Aboyoun C, Belperio J, Benden C, et al. (2016) Antibody-mediated rejection of the lung: A consensus report of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant 35: 397-406. [Crossref]
- Verleden GM, Glanville AR, Lease ED, Fisher AJ, Calabrese F, et al. (2019) Chronic lung allograft dysfunction: Definition, diagnostic criteria, and approaches to treatment-A consensus report from the Pulmonary Council of the ISHLT. J Heart Lung Transplant 38: 493-503. [Crossref]
- Couetil JP, Houssin DP, Soubrane O, Chevalier PG, Dousset BE, et al. (1995) Combined lung and liver transplantation in patients with cystic fibrosis. A 4 1/2-year experience. J Thorac Cardiovasc Surg 110: 1415-1422. [Crossref]
- Arnon R, Annunziato RA, Miloh T, Padilla M, Sogawa H, et al. (2011) Liver and combined lung and liver transplantation for cystic fibrosis: analysis of the UNOS database. Pediatr Transplant 15: 254-264. [Crossref]
- Praseedom RK, McNeil KD, Watson CJ, Alexander GJ, Calne RY, et al, (2001) Combined transplantation of the heart, lung, and liver. Lancet 358: 812-813. [Crossref]
- Barshes NR, DiBardino DJ, McKenzie ED, Lee TC, Stayer SA, et al. (2005) Combined lung and liver transplantation: the United States experience. Transplantation 80: 1161-1167. [Crossref]
- Yi SG, Burroughs SG, Loebe M, Scheinin S, Seethamraju H, et al. (2014) Combined lung and liver transplantation: analysis of a single-center experience. Liver Transpl 20: 46-53. [Crossref]
- Ceulemans LJ, Monbaliu D, Verslype C, van der Merwe S, Laleman W, et al. (2014) Combined liver and lung transplantation with extended normothermic lung preservation in a patient with end-stage emphysema complicated by drug-induced acute liver failure. Am J Transplant 14: 2412-2416. [Crossref]
- Wolf JH, Sulewski ME, Cassuto JR, Levine MH, Naji A, et al. (2013) Simultaneous thoracic and abdominal transplantation: can we justify two organs for one recipient? Am J Transplant 13: 1806-1816. [Crossref]
- Freischlag KW, Messina J, Ezekian B, Mulvihill MS, Barbas A, et al. (2018) Single-Center Long-Term Analysis of Combined Liver-Lung Transplant Outcomes. Transplant Direct 4: 349. [Crossref]
- Han JL, Beal EW, Mumtaz K, Washburn K, Black SM (2019) Combined liver-lung transplantation: Indications, outcomes, current experience and ethical Issues. Transplant Rev 33: 99-106. [Crossref]
- Connor AA, Huang HJ, Mobley CM, Graviss EA, Nguyen DT, et al. (2023) Progress in Combined Liver-lung Transplantation at a Single Center. Transplant Direct 9: 1482. [Crossref]
- OPTN (2017) Organ Procurement and Transplantation Network Lung Kaplan-Meier Patient Survival Rates For Transplants Performed: 2008-2015.
- Adams B, Chiem D, Wang C, Neelankavil J (2013) Combined bilateral lung-liver transplantation complicated by intraoperative right ventricular dysfunction and postoperative hepatic artery thrombosis. J Cardiothorac Vasc Anesth 27: 1343-1346. [Crossref]
- Baez B, Castillo M (2008) Anesthetic considerations for lung transplantation. Semin Cardiothorac Vasc Anesth 12: 122-127. [Crossref]
- Wray CL (2017) Advances in the Anesthetic Management of Solid Organ Transplantation. Adv Anesth 35: 95-117. [Crossref]
- Salman J, Grannas G, Ius F, Sommer W, Siemeni T, et al. (2018) The liver-first approach for combined lung and liver transplantation. Eur J Cardiothorac Surg 54: 1122-1127. [Crossref]
- Ceulemans LJ, Strypstein S, Neyrinck A, Verleden S, Ruttens D, et al. (2016) Combined liver-thoracic transplantation: single-center experience with introduction of the 'Liver-first' principle. Transpl Int 29: 715-726. [Crossref]
- Botha P, Archer L, Anderson RL, Lordan J, Dark JH, et al. (2008) Pseudomonas aeruginosa colonization of the allograft after lung transplantation and the risk of bronchiolitis obliterans syndrome. Transplantation 85: 771-774. [Crossref]
- Boussaud V, Guillemain R, Grenet D, Coley N, Souilamas R, et al. (2008) Clinical outcome following lung transplantation in patients with cystic fibrosis colonised with Burkholderia cepacia complex: results from two French centres. Thorax 63: 732-737. [Crossref]
- Aris RM, Gilligan PH, Neuringer IP, Gott KK, Rea J, et al. (1997) The effects of panresistant bacteria in cystic fibrosis patients on lung transplant outcome. Am J Respir Crit Care Med 155: 1699-1704. [Crossref]
- Jing L, Chen W, Guo L, Zhao L, Liang C, et al. (2021) Acute kidney injury after lung transplantation: a narrative review. Ann Transl Med 9: 717. [Crossref]
- Schindler R, Radke C, Paul K, Frei U (2001) Renal problems after lung transplantation of cystic fibrosis patients. Nephrol Dial Transplant 16: 1324-1328. [Crossref]
- Gorgy AI, Jonassaint NL, Stanley SE, Koteish A, DeZern AE, et al. (2015) Hepatopulmonary syndrome is a frequent cause of dyspnea in the short telomere disorders. Chest 148: 1019-1026. [Crossref]
- Martinu T, Howell DN, Palmer SM (2010) Acute cellular rejection and humoral sensitization in lung transplant recipients. Semin Respir Crit Care Med 31: 179-188. [Crossref]
- Holm AM, Fedson S, Courtwright A, Olland A, Bryce K, et al. (2022) International society for heart and lung transplantation statement on transplant ethics. J Heart Lung Transplant 41: 1307-1308. [Crossref]
- Goldberg DS, Reese PP, Amaral S, Abt PL (2014) Reframing the impact of combined heart-liver allocation on liver transplant wait-list candidates. Liver Transpl 20: 1356-1364. [Crossref]


