Abstract
Primary hepatic neuroendocrine carcinoma (NEC) is uncommon and its coexistence with hepatocellular carcinoma (HCC) is extremely rare. This study reports the case of a 66-year-old male patient without symptoms at presentation in whom hepatitis C and a liver nodule were discovered during routine examination. The physical examination was unremarkable and no organomegaly or palpable mass was detected. The patient had no family history of cancer. Computed tomography of the abdomen showed a liver mass measuring 5.6 cm in diameter in segment VI/VII compatible with HCC. The patient underwent right liver resection. Thereafter, triple therapy (pegylated interferon: 180 µg/week + ribavirin: 1 g/day + telaprevir: 750 mg/tid) was started to treat hepatitis C and the patient achieved a sustained virological response. Two years after the initial diagnosis, a retroperitoneal mass was detected, and histological analysis was compatible with high-grade metastatic neuroendocrine tumor. Based on this new diagnosis, the pathological material of the previous liver resection was re-evaluated, including broad immunohistochemical analysis which demonstrated the presence of primary hepatic NEC combined with HCC in a non-cirrhotic liver. The patient died 6 years after the diagnosis. This study highlights the challenge of diagnosing this rare tumor considering the absence of symptoms, radiological clues, or even histological findings. In addition, rigorous histological examination including broad immunohistochemical analysis should always be performed. Despite the relatively long survival time for such severe illness, an earlier diagnosis of the combined tumor could have changed patient outcome.
key words
neuroendocrine carcinoma, hepatocellular carcinoma, diagnosis, treatment, hepatitis c, combined tumor
Background
The coexistence of histologically distinct primary tumors in the liver is very rare. Primary hepatic neuroendocrine carcinoma (NEC) is extremely uncommon and its coexistence with hepatocellular carcinoma (HCC) is even rarer [1]. Histologically distinct tumors can occur in the liver either as combined or as collision tumors. Combined tumors are a continuous mixture of components of both tumors that cannot be clearly separated. On the other hand, the less common collision tumors consist of two separate histological components without a transition area between them [2]. We report here a case of primary hepatic NEC coexisting with HCC in a liver with chronic hepatitis C.
Case
This was the case of an asymptomatic 66-year-old single retired man in whom genotype 1 chronic hepatitis C (anti-HCV and HCV RNA positive) and a liver nodule were discovered by abdominal ultrasound during routine examination in 2011. Physical examination performed on that occasion showed good general health, no signs of chronic liver disease, and no organomegaly or palpable masses. Regarding personal history, the patient had been an alcoholic for 20 years but abstinent since 2005, had used injectable illicit drugs in his youth, and had undergone inguinal hernia repair in 2006. The patient reported no transfusion of blood or blood derivatives, associated diseases, or smoking. He was using sildenafil for sexual dysfunction. In view of the ultrasound finding of a liver nodule, abdominal computed tomography (CT) was performed on 21 July 2011, which identified a liver mass measuring 5.6 cm in diameter in segment VI/VII. The mass exhibited the characteristic features of HCC: hypodense and mildly heterogenous in the non-contrast enhanced phase and hypervascularity followed by washout. No signs of cirrhosis or portal hypertension were observed (Figure 1A and 1B). Upper gastrointestinal endoscopy performed on 14 February 2012 revealed no esophageal varices. Laboratory tests performed at the time of diagnosis in 2011 showed the following serum levels: alanine aminotransferase=18 U/L; aspartate aminotransferase = 30 U/L; alkaline phosphatase = 57 U/L; gamma-glutamyltransferase = 98 U/L; international normalized ratio = 1.08; total bilirubin = 0.34 mg/dL; direct bilirubin = 0.19 mg/dL; albumin = 4.9 g/dL; creatinine = 1.0 mg/dL; hemoglobin = 13.2 g/dL; hematocrit = 38.5%; leukocytes = 7,020/µL; platelets = 258,000/µL; sodium = 34 mmol/L; alpha-fetoprotein (AFP) = 837.41 IU/mL. Tests for hepatitis B infection were negative.
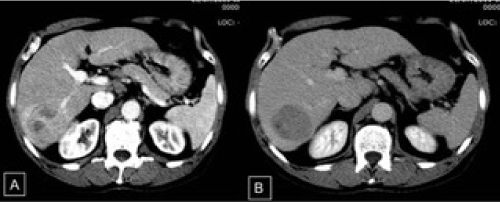
Figure 1. A: Intravenous contrast-enhanced computed tomography. In the VI/VII segment of the right hepatic lobe, note the hypervascular mass in the late arterial phase; B: heterogenous uptake and washout of the nodule in the equilibrium phase.
Based on the diagnosis of HCC beyond the Milan criteria, locoregional treatment consisting of transarterial chemoembolization (TACE) with doxorubicin plus lipiodol was chosen in an attempt to downstage the tumor. After TACE failure and considering the good clinical condition and preserved liver function of the patient, right hepatectomy was performed in October 2012. Histopathological examination of the surgical specimen confirmed the presence of HCC in a non-cirrhotic liver (Figure 2A and 2B).
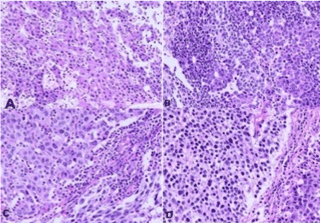
Figure 2. Histological sections of the combined tumor showing areas of moderately differentiated hepatocellular carcinoma with cells arranged in trabeculae. A: along poorly differentiated areas with a solid arrangement; B: Areas of a neuroendocrine tumor consisting of smaller cells, some of them containing oval nuclei, can be seen amidst the tumor; C and D: HE, 250X.
After hepatectomy in March 2014, the patient was started on triple therapy (pegylated interferon: 180 µg/week + ribavirin: 1 g/day + telaprevir: 750 mg/tid) for chronic hepatitis C. Treatment was discontinued after 7 months because of severe weight loss and anemia. However, a sustained virological response was achieved, i.e., no HCV RNA was detected 6 months after treatment discontinuation.
During periodic control examination after hepatectomy, serum AFP levels were persistently normal and imaging scans (CT) showed no findings suggestive of tumor recurrence in the liver or distant metastases. However, in August 2013, a control CT scan detected a solid nodule measuring 3.0 cm in diameter in the celiac chain, which was interpreted as lymphadenomegaly. The nodule exhibited progressive growth on subsequent scans (Figure 3A-3C). In view of the nonspecific imaging findings of the nodule and because serum AFP levels continued to be normal, the possibility of secondary implantation of HCC was less likely and an endoscopic ultrasound-guided biopsy of the lesion was therefore performed in November 2014. Histopathological examination revealed a small-cell malignant tumor. Immunohistochemistry was consistent with high-grade metastatic NEC. Since the imaging examination revealed no primary focus, the pathologist suggested a histopathological revision of the surgical specimen from the previous hepatectomy. This revision revealed an epithelial tumor that mainly consisted of blocks of large cells with pleomorphic nuclei and broad cytoplasm in a predominantly trabecular arrangement (Figure 2). Immunohistochemical analysis revealed the expression of HepPar-1 (Figure 4 and 4A), CK 8-18 (Figure 4D), and polyclonal CEA and CD10 in a canalicular pattern (Figure 4C), characterizing HCC. A tumor consisting of small to intermediate cells in a solid arrangement intermingled with HCC was detected. This tumor exhibited a high mitotic index and expressed the neuroendocrine markers chromogranin, synaptophysin and CD56, but was negative for HepPar-1 (Figure 5A-5C). The cell proliferation index, evaluated by immunoexpression of Ki-67, was higher than 20% (Figure 5D). These findings were compatible with poorly differentiated NEC. Thus, the final diagnostic conclusion of this revision was combined NEC-HCC.
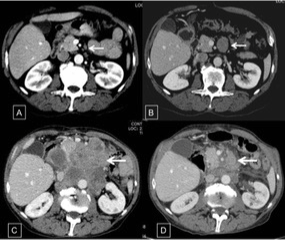
Figure 3.
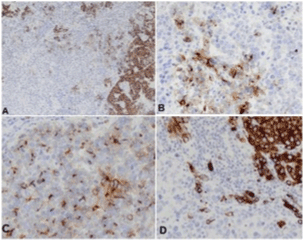
Figure 4. Immunohistochemical analysis showing areas of the tumor expressing HepPar-1. A: CD10; B: polyclonal CEA in a canalicular pattern; C: CK 8-18; D: characterizing the hepatocellular carcinoma component.
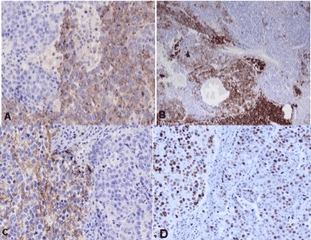
Figure 5. Immunohistochemical analysis demonstrating the neuroendocrine component of the tumor. A: Chromogranin; B: synaptophysin; C: CD56; D: Immunoexpression of Ki-67 in > 20% of tumor cells.
In February 2015, the patient was admitted with severe anemia (hemoglobin = 5 g/dL), poor general condition, melena, and an epigastric abdominal mass visible during physical examination. Upper gastrointestinal endoscopy revealed bulging in the lower wall of the first portion of the duodenum suggestive of extrinsic compression and the presence of a large ulceroinfiltrative lesion in the third duodenal portion, occupying 2/3 of the circumference and extending about 5 cm. The lesion was friable and bled on contact with the endoscope, with signs of recent bleeding. Electrocoagulation of the lesion with a bipolar catheter was performed and histopathological analysis revealed nonspecific chronic duodenitis without malignancy. Since bleeding was controlled and considering the improved clinical condition of the patient and the fact that this was an unresectable metastatic poorly differentiated NEC, the decision was made to start intravenous chemotherapy with etoposide (140 mg/m2 for 3 days) and cisplatin (60 mg/m2 on the first day and at intervals of 3 weeks). Six cycles of chemotherapy were performed. The patient showed improvement from ECOG 2 to 1, an increase in BMI from 18.31 to 21.37 kg/m2, and improvement of anemia. A control CT scan revealed a partial response of the component of the retroperitoneal ganglion mass (Figure 3D). However, the patient died in November 2016.
Discussion
Hepatocellular carcinoma is the most common primary liver cancer, followed by cholangiocarcinoma [3]. Primary NEC is rare in the liver. Hepatic neuroendocrine tumors (NETs) are generally of metastatic origin. The presence of combined or collision tumors in the liver is also rare and hepatocellular-cholangiocarcinoma is the most common combined tumor [4,5]. The coexistence of NEC and HCC is extremely rare and only few reports are available in the literature [6]. Since this type of tumor is uncommon, the present case report was prompted by the intriguing and interesting features of its clinical presentation since the initial diagnosis was only HCC while, surprisingly, the final diagnosis was combined NEC-HCC.
Considering the presence of hepatitis C and of a hyper vascular hepatic nodule with washout, associated with a marked increase in AFP even in the absence of cirrhosis, the diagnosis of HCC could be made safely in the present case and was confirmed by histopathological analysis of the resected lesion. On the other hand, there are no typical imaging features of primary hepatic NEC and imaging findings vary widely in the few cases reported in the literature. In some of these cases, NEC resembled HCC, exhibiting hypervascularization, washout and interspersed calcifications [7,8].
The clinical manifestations of NETs depend on the location of the tumor. Some NETs of the gastrointestinal tract, especially those associated with liver metastases, can manifest as carcinoid syndrome which is characterized by diarrhea, abdominal pain, and facial flushing [9]. In these cases, the investigation of gastrin, glucagon, VIP and chromogranin in blood or of serotonin metabolites such as 5-hydroxyindolacetic acid in 24-h urine can assist in the diagnosis [10]. Mapping by the infusion of octreotide (Octreoscan) or positron emission tomography with gallium 68 (DOTAG) also permits the diagnosis of NETs [11]. On the other hand, poorly differentiated NETs are rarely functional and are therefore clinically inapparent. The present patient had no symptoms suggestive of carcinoid syndrome.
Computed tomography permitted the detection of a nonspecific lesion located in the celiac chain that exhibited rapid and progressive growth and diagnostic conclusion of this case was possible after histopathological evaluation. It should be noted that, when HCC with poorly differentiated areas is detected, the investigation of neuroendocrine markers is essential to exclude this component. In addition, the use of an immunohistochemistry panel is important for diagnosis since the presence or absence of reactivity to some markers indicates the type of tumor [12].
The histogenesis of the neuroendocrine component in combined tumors is controversial. Two hypotheses have been proposed: 1) transformation of stem cells with potential divergent differentiation, and 2) neuroendocrine differentiation of poorly differentiated cells of HCC [13]. No pathogenic mechanism of the tumor could be suggested in the present case.
At present, NETs are classified by the World Health Organization [14] and by the European Neuroendocrine Tumor Society [15] according to the degree of differentiation. This classification is important for the decision of disease management and for prognostic assessment. When respectable, NETs can be treated surgically with an excellent prognosis. The patient studied here underwent resection of the primary tumor, but metastases appeared later. Therefore, systemic chemotherapy was the only option because of the presence of unresectable metastases in the celiac chain.
Well-differentiated or moderately differentiated, functional or non-functional, metastatic NETs are treated with somatostatin analogs (octreotide or lanreotide) [16]. Sunitinib and everolimus can also be used. These drugs increase progression-free survival in most cases [17]. A partial response is observed in a smaller proportion of cases. Poorly differentiated tumors, as is the case of the present patient, respond better to combination chemotherapy with cisplatin and etoposide. The response ranges from 40-60%, but median survival is only 11-20 months [18]. Second-line regimens with temozolomide, capecitabine or the combination of irinotecan plus fluorouracil and folinic acid can be prescribed for patients who show progression of the disease after initial treatment. A response is observed in approximately 30% of cases [19]. Regarding the combination of NEC-HCC, the results are uncertain because of the small number of cases reported, but both tumors are known to have a poor prognosis [20]. All reported cases of NEC-HCC developed metastases to the lymph nodes and/or other organs. More cases need to be studied for a better understanding of these tumors.
In conclusion, the present case of combined NEC-HCC is rare and only few cases have been reported in the literature. Intriguing features should be highlighted, such as the sustained virological response of hepatitis C to triple therapy for only 7 months, the occurrence of HCC in a non-cirrhotic liver, the retrospective diagnosis of combined metastatic NEC-HCC, and the favorable response to systemic chemotherapy, with interesting survival in such a severe case.
References
- Ishida M, Seki K, Tatsuzawa A, Katayama K, Hirose K, et al. (2003) Primary hepatic neuroendocrine carcinoma coexisting with hepatocellular carcinoma in hepatitis C liver cirrhosis: report of a case. Surg Today 33: 214-218. [Crossref]
- Garcia MT, Bejarano PA, Yssa M, Buitrago E, Livingstone A (2006) Tumor of the liver (hepatocellular and high-grade neuroendocrine carcinoma): a case report and review of the literature. Virchows Arch 449: 376-381. [Crossref]
- Altekruse SF, Petrick JL, Rolin AI, Cuccinelli JE, Zou Z, et al. (2015) Geographic variation of intrahepatic cholangiocarcinoma, extrahepatic cholangiocarcinoma, and hepatocellular carcinoma in the United States. PLoS One10: e0120574. [Crossref]
- Jarnagin WR, Weber S, Tickoo SK, Koea JB, Obiekwe S, et al. (2002) Combined hepatocellular and cholangiocarcinoma: demographic, clinical, and prognostic factors. Cancer 94: 2040-2046. [Crossref]
- Goodman ZD, Ishak KG, Langloss JM, Sesterhenn IA, Rabin L (1985) Combined hepatocellular-cholangiocarcinoma. A histologic and immunohistochemical study. Cancer 55: 124-135. [Crossref]
- Wang LM, An SL, Wu JX (2014) Diagnosis and therapy of primary hepatic neuroendocrine carcinoma: clinical analysis of 10 cases. Asian Pac J Cancer Prev 15: 2541-2546. [Crossref]
- Huang J, Yu JQ, Sun JY (2014) Computer tomography and magnetic resonance image manifestations of primary hepatic neuroendocrine cell carcinomas. Asian Pac J Cancer Prev 15: 2759-2764.
- Nakanishi C, Sato K, Ito Y, Abe T, Akada T, et al. (2012) Combined hepatocellular carcinoma and neuroendocrine carcinoma with sarcomatous change of the liver after transarterial chemoembolization. Hepatol Res 42: 1141-1145. [Crossref]
- Gregersen T, Brock C, Haase AM, Laurberg S, Drewes AM, et al. (2016) Rectal Mechano-sensory Function in Patients with Carcinoid Diarrhea. J Neurogastroenterol Motil 22: 264-271. [Crossref]
- Gravante G, De Liguori Carino N, Overton J, Manzia TM, Orlando G (2008) Primary carcinoids of the liver: a review of symptoms, diagnosis and treatments. Dig Surg 25: 364-368. [Crossref]
- Sorbye H, Strosberg J, Baudin E, Klimstra DS, Yao JC (2014) Gastroenteropancreatic high-grade neuroendocrine carcinoma. Cancer 120: 2814-2823. [Crossref]
- Pilichowska M, Kimura N, Ouchi A, Lin H, Mizuno Y, et al. (1999) Primary hepatic carcinoid and neuroendocrine carcinoma: clinicopathological and immunohistochemical study of five cases. Pathol Int 49: 318-324. [Crossref]
- Yamaguchi R, Nakashima O, Ogata T, Hanada K, Kumabe T, et al. (2004) Hepatocellular carcinoma with an unusual neuroendocrine component. Pathol Int 54: 861-865. [Crossref]
- Kloppel G (2011) Classification and pathology of gastroenteropancreatic neuroendocrine neoplasms. Endocr Relat Cancer 18: S1-S16. [Crossref]
- Arnold R, Chen YJ, Costa F, Falconi M, Gross D, et al. (2009) ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: follow-up and documentation. Neuroendocrinology 90: 227-233. [Crossref]
- Massironi S, Zilli A, Conte D (2015) Somatostatin analogs for gastric carcinoids: For many, but not all. World J Gastroenterol 21: 6785-6793. [Crossref]
- Stevenson R, Libutti SK, Saif MW (2013) Novel agents in gastroenteropancreatic neuroendocrine tumors. JOP 14: 152-154. [Crossref]
- Moertel CG, Kvols LK, O'Connell MJ, Rubin J (1991) Treatment of neuroendocrine carcinomas with combined etoposide and cisplatin. Evidence of major therapeutic activity in the anaplastic variants of these neoplasms. Cancer 68: 227-232. [Crossref]
- Peixoto RD, Noonan KL, Pavlovich P, Kennecke HF, Lim HJ (2014) Outcomes of patients treated with capecitabine and temozolamide for advanced pancreatic neuroendocrine tumors (PNETs) and non-PNETs. J Gastrointest Oncol 5: 247-252. [Crossref]
- Yang CS, Wen MC, Jan YJ, Wang J, Wu CC (2009) Combined primary neuroendocrine carcinoma and hepatocellular carcinoma of the liver. J Chin Med Assoc 72: 430-433. [Crossref]





