The objective was to study the foamability of two proteins after ultrasound application. Soy protein and whey protein isolate were used as starting material. Ultrasound was used to analyze the foamability effect on the solutions relating with the bubble size change. The samples were sonicated at same conditions using an ultrasonic processor. Foam formation was measured by conductimetric and optical methods. Moreover, the evolution of the bubble size change was registered. The effect of ultrasound depended on the protein. Therefore, the use of soy or whey protein isolate will be decided by the functionality required.
Soy protein isolate, Whey proteins isolate, Foamability, Ultrasound
Soybean proteins are widely used in many foods as functional and nutritional ingredients [1]. These proteins are used in a wide range of food applications, including processed meat, nutritional beverages, infant formulas, and dairy product replacement. Glycinin and β-conglycinin, the major components of soybean protein, account for approximately 70% of the proteins in soybeans [2]. Most studies were done using native soy protein isolate, glycinin and β-conglycinin, which are of limited value for the understanding of commercially available soy isolates.
Whey protein concentrates and isolates are important food ingredients because of their desirable functional properties, such as gelation, foaming and emulsification. Whey proteins are a significant source of functional protein ingredients for many traditional and novel food products [3]. The main proteins in whey are β-lactoglobulin (β -lg), α-lactalbumin (α-lac) and bovine serum albumin (BSA) and they account for 70% of total whey proteins [4]. These proteins are responsible for the functional properties of whey proteins, such as solubility in water, viscosity, gelation, emulsification, foaming, colour, flavor and texture enhancement and offer numerous nutritional advantages to formulated products [5].
The effect of ultrasound is related to cavitation, heating, dynamic agitation, shear stresses, and turbulence [6]. It may cause physical changes producing aggregates through non-covalent bonds by cyclic generation and collapse of cavities depending of structural or aggregation protein state.
In the present work, effects of ultrasound of high intensity on the foamability of two different proteins at similar concentration and electrostatic charges were compared, and were studied at pH 7. Bubbling method is the unique system to form the foam that gives the precise liquid and gas used to form them, having thus, the exact density of foams obtained.
Soy protein and whey protein isolates were used as starting material. The foaming formation together with the bubble size change was analyzed.
Protein samples preparation
Soy protein isolate (SPI) was provided by Instituto de la Grasa, Seville, Spain and the complete description was published elsewhere [7].
Soluble SPI (SSPI) at pH 7 was used as starting material for the current work. Protein solution, at 4% w/w, was centrifuged for 1 hour at room temperature at 10,000 g. The protein content was determined in the soluble fraction by the Kjeldhal method (N x 6.25), resulting in 1.73.
Whey protein isolate (WPI) was provided by Milkaut, Argentina. The protein was used at 2 % wt/wt and adjusted further to pH7.
These final solutions were treated by high intensity ultrasound (HIUS).
Foam formation
The foams were made using a Foamscan instrument (Teclis-It Concept, Logessaigne, France). The foam is generated by blowing nitrogen gas at a flow of 45 mL/min through a porous glass filter of 0.2 µm at the button of a glass tube where 20 ml of the foaming aqueous solutions (25 ± 1ºC) is placed. In all experiments, the foam was allowed to reach a volume of 120 ml. The bubbling was then stopped and the evolution of the foam was analyzed by means of conductimetric and optical measurements. The Final Time of Foaming (FTF), the Total Gas Volume (TGV) and the Final liquid volume (FLV) were taken from the table results after each experiment. The generated foam rises along a thermostated square prism glass column, where the volume is followed by image analysis using a CCD camera. The evolution of the bubble size change in the foam was also determined by a second CCD camera set with a macro objective which allows to capture the variation of the air bubble size every 5 s. Thus, the images of initial obtained foam could be obtained to characterize them.
The following parameters were determined: Foam Expansion (FE), as the inverse of the Foam Maximum Density (MD) determined by (1), is a measure of the liquid retention in the foam; the Overall Foaming Capacity (OFC, mL/s) was determined from the slope of the foam volume curve up to the end of the bubbling. The Foam Capacity (FC), a measure of gas retention in the foam, was determined by (2).
FE = Vfoam (f) / (Vliq (i) - Vliq(f) (1)
FC = Vfoam (f) / Vgas(f) (2)
where Vliq(i) and Vliq(f) are the initial and the final liquid volumes; Vfoam(f) is the final foam volume and Vgas(f) is the final gas volume injected.
High-intensity ultrasound (HIUS) treatment
Protein solutions were sonicated for 20 min using an ultrasonic processor Vibra Cell Sonics, model VCX at a frequency of 20 kHz and an amplitude of 20%, which were constant. A 13 mm high grade titanium alloy probe threaded to a 3 mm tapered microtip was used to sonicate 10 ml of the solutions. Samples contained into glass test tubes were immersed into a glycerine-jacketed at 0.5ºC to dissipate most of the heat produced during sonication treatments (Polystat, Cole-Parmer).
Statistical analysis
All the experiments were performed in duplicate or triplicate. The model goodness-of-fit was evaluated by the coefficient of determination (R2) and the analysis of variance (ANOVA), using Statgraphics Plus 3.0. software.
Efficiency in foam formation
Principles of the technique were explained in a previous publication [8]. In the Table 1 it can be seen the Final Time of Foaming (FTF), the Total Gas Volume (TGV) incorporated into the foam and the Final liquid volume (FLV), corresponding to the remaining liquid after foam formation; for untreated (Control) and HIUS treated systems. Thus, we can assume a better efficiency in foam formation when the Final Time of Foaming is low, also the Total Gas Volume incorporated and high the liquid incorporated, that it means, a low remaining FLV [9].
Table 1. Final Time of Foaming (FTF), Total Gas Volume (TGV) and Final Liquid volume (FLV) for untreated proteins (SSPI and WPI) and HIUS treated.
Protein |
FTF(±10 s.) |
TGV(±5 cm3) |
FLV(±3 cm3) |
SSPI |
174 |
127 |
11.55 |
SSPI HIUS |
194 |
138 |
6.85 |
WPI |
174 |
127 |
20 |
WPI HIUS |
161 |
134 |
25 |
The Table 1 shows for SSPI that ultrasound provoked an increment of FTF, indicating that the formation of foam is more difficult after the treatment. However, at the same time it is observed that at the end of the formation, greater amount of gas and liquid were incorporated by the TGV increase and FLV decease. This means that the delay in the formation of the foam, by the HIUS effect, allows to include a greater quantity of protein at the final formed foam, which, could be very beneficial for the later stability as same way.
For WPI, a different effect was found. HIUS provoked a decreased of the FTF, it indicates a greater velocity in the formation of the foam, however, it can be seen a similar gas amount incorporated by comparing with the untreated WPI, and less liquid quantity.
This means that the greater speed in reaching the set foam height, prevents to incorporate liquid and consequently, more protein to the foam formed.
The advantage of a higher rate of foam formation or a greater amount incorporation of protein at the dispersed system will depend on the needs and functional application in the food industry.
There is no much literature dealing with simple and direct comparative HIUS treatments effect on the bubbling foaming properties by using different source protein. It is said in general that HIUS effects promote foaming changes attributed to partial denaturation of proteins affecting the solubility and then, the ability to form the [9].
Nicorescu et al., (2011), [10] studied the effect of thermal treatments applied in typical industrial conditions on the foaming properties with whipping method of whey protein isolate and egg white proteins. The results showed that the maximum overrun achieved with whey protein solutions was significantly higher than for egg protein solutions although their protein content was lower. Thus, it is suppose that the degree of protein aggregation after treatment would be a cause, more than the initial amount of protein in the solution.
Effects of HIUS on other parameters for formation and stability of foams
In Figure 1 and 2 (a-d) it can be seen the HIUS effect for SSPI and WPI on FE, FC, OFC and MD.
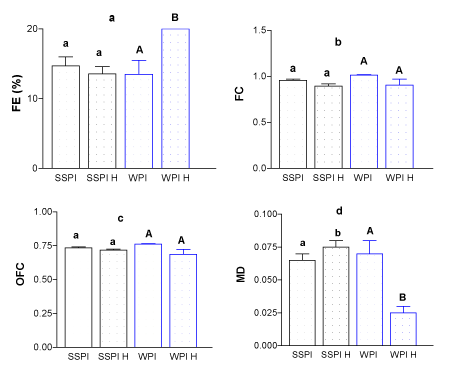
Figure 1. (a) FE% (b) FC (c) OFC and (d) MD for soy soluble protein isolate (SSPI) and whey protein isolate (WPI) at similar concentrations, pH 7.
It can be seen, in general terms, that there were no changes in FE, FC, and OFC, for SSPI, however, there was and increment of MD as a consequence of HIUS treatment.
These results partially coincide with the parameters described above. It was found that the foaming time was prolonged to form the foam but this led to the incorporation of more liquid. In this case it is reflected only in the MD, which characterizes the density of the foam obtained at the end of its formation. In the Table 2, it can be seen the Initial Aspect of the foam obtained for each protein. A foam formed with a greater liquid quantity clearly it can be seen for SSPI after HIUS in the Table 2. The foam presents smaller bubbles compared to the foam obtained without the treatment.
Table 2. Initial Foam Aspect for untreated proteins (SSPI and WPI) and HIUS treated solutions.
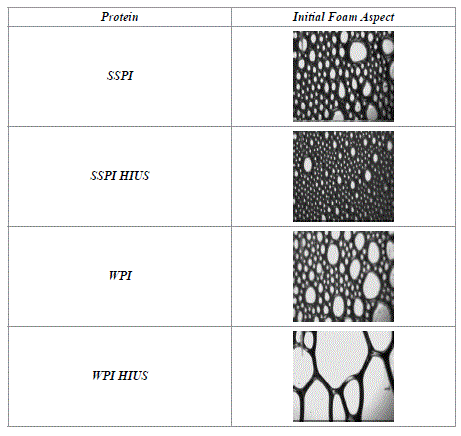
In the other hand for WPI solution, the Figure 1 2021 Copyright OAT. All rights reserved FC, OFC but a decrease of MD after HIUS. In the same way, also relates very well to the data obtained previously (Table 1). It was possible to see that the time of foam formation was improved and less liquid was incorporated. The corresponding images in the Initial Aspect (Table 2), showed foams with greater size bubbles, by comparing with untreated WPI solution. This confirms that HIUS causes greater incorporation of liquid on the SSPI solutions and less incorporation in the WPI, seeing also in the obtained images.
There is a lack of simple and direct comparison of ultrasound effects on different proteins focusing on foamability by bubbling method.
Two different source of protein were used to study the effect of this technology on foam production. Soluble soy protein (SSPI) and whey protein isolates (WPI) were prepare at pH7 at similar concentration conditions.
The observed results indicated that the velocity of foam formation as well as the incorporated protein solution in the foam after HIUS, would depend on the protein structure. No changes between untreated HIUS proteins were found on the analyzed parameters. However, when HIUS was applied, for SSPI an increment of foaming time and density increase was observed, whereas, a decrease of foaming time and lower foam density was found for WPI. In addition, the recorded bubbles images of the corresponding obtained foams with the second camera showed smaller bubbles for HIUS treated SSPI, and bigger ones for WPI, confirming the above attained results.
Therefore, the use of SSPI or WPI will be decided precisely by the industrial functionality required.
This research was supported by Consejo Nacional de Investigaciones Científicas y Técnicas de la República Argentina (CONICET), Universidad de Buenos Aires and Departamento de Ingeniería Química, Facultad de Química, Universidad de Sevilla.
The authors declare no conflict of interest.
- Vohra P, Kratzer FH (1991) Evaluation of soybean meal determines adequacy of heat treatment. Feedstuffs, February 25, 22-28.
- Nielsen NC (1985) Structure of soy proteins. In: A. A. a. W. Altschul, H.L., New York: Academic Press. New protein foods 5: 27-58.
- Mishra S, Mann B, Joshi VK (2001) Functional improvement of whey protein concentrate on interaction with pectin. Food Hydrocolloids 15: 9-15.
- Cayot P, Lorient D (1997) Structure-function relationships of whey proteins. In: Dekker, M. (Ed.), Food Proteins and their Applications, New York, USA.
- Krešic G, Lelas V, Jambrak AR, Herceg Z, Brncˇic SR (2008) Influence of novel food processing technologies on the rheological and thermophysical properties of whey proteins. Journal of Food Engineering 87: 64-73.
- Knorr D, Zenker M, Heinz V, Lee D (2004) Applications and potential of ultrasonics in food processing. Trends in Food Science & Technology 15: 261-266.
- Martinez KD, Sanchez CC (2014) New view to obtain dryer food foams with different polysaccharides and soy protein by high ultrasound. International Journal of Carbohydrate Chemistry, 6.
- Martinez KD, Sanchez CC (2017) Whey protein isolate and celluloses derivates interactions improved by ultrasound of high intensity. Frontiers in Nanoscience and Nanotechnology 3: 4.
- Higuera-Barraza OA, Del Toro-Sanchez CL, Ruiz-Cruz S, Márquez-Ríos E (2016) Effects of high-energy ultrasound on the functional properties of proteins. Ultrasonics Sonochemistry 31: 558-562.
- Nicorescu I, Vial C, Talansier E, Lechevalier V, Loisel C, et al. (2011) Comparative effect of thermal treatment on the physicochemical properties of whey and egg white protein foams. Food Hydrocolloids 25: 797-808.


